Isolation, Characterization, and Anti-Allergic Evaluation of Phytochemicals from Wikstroemia trichotoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation
2.3. Cell Culture
2.4. IL-4 mRNA Expression in RBL-2H3 Cells
2.5. β-Hexosaminidase Release in RBL-2H3 Cells
2.6. Statistical Analysis
3. Results
3.1. Isolation of Compounds from W. trichotoma Extract and the Structural Elucidation of Compounds 1 and 2
3.2. Evaluation of the Effects of Compounds (1–42) on IL-4 Expression in RBL-2H3 Cells
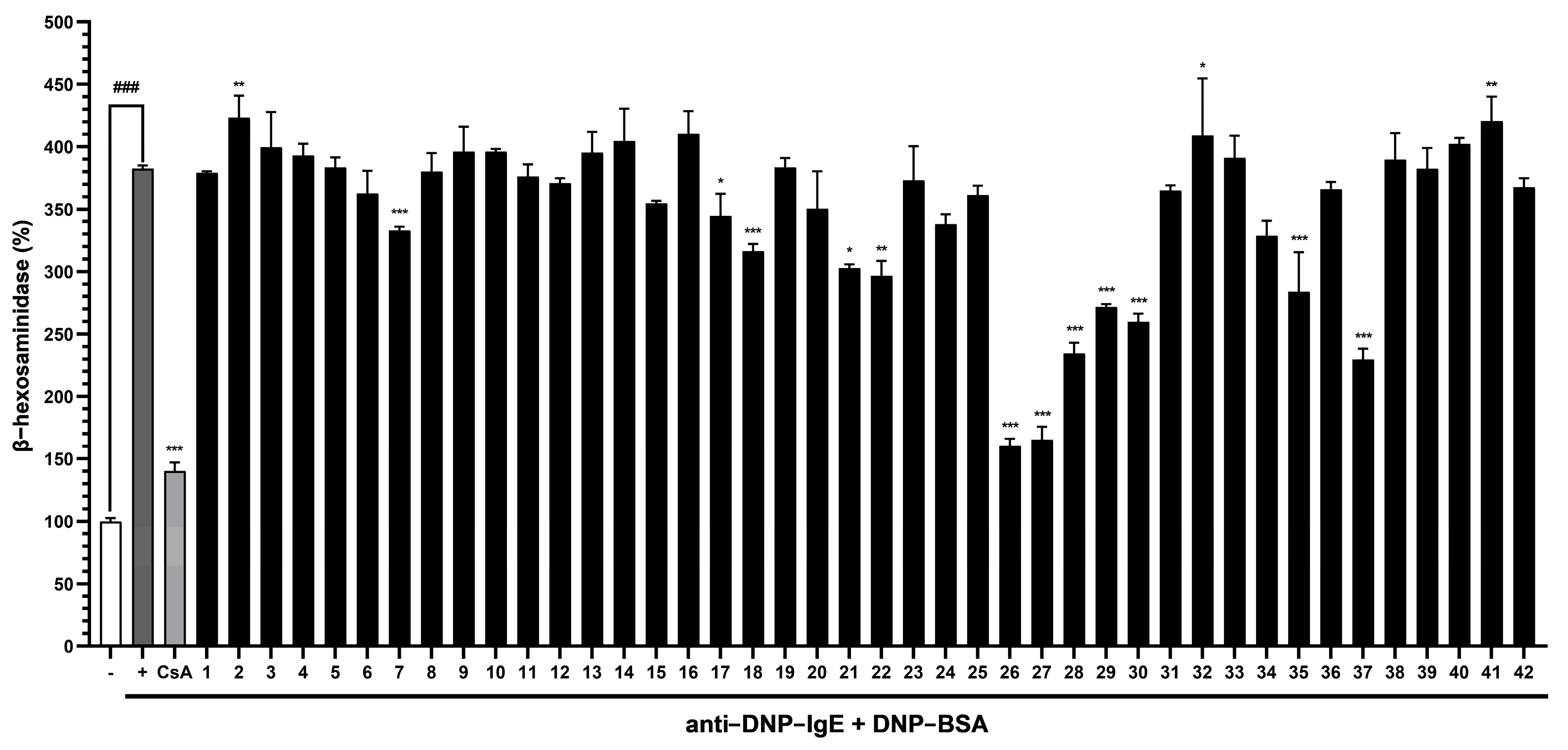
3.3. Evaluation of the Abilities of Compounds (1–42) to Suppress β-Hexosaminidase Release by RBL-2H3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards-Salmon, S.E.; Padmanabhan, S.L.; Kuruvilla, M.; Levy, J.M. Increasing Prevalence of Allergic Disease and Its Impact on Current Practice. Curr. Otorhinolaryngol. Rep. 2022, 10, 278–284. [Google Scholar] [CrossRef]
- Bellanti, J.A. IgE and Non-IgE Food Allergy: A Review of Immunological Mechanisms. J. Food Allergy 2024, 6, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ronchese, F.; Webb, G.R.; Ochiai, S.; Lamiable, O.; Brewerton, M. How Type-2 Dendritic Cells Induce Th2 Differentiation: Instruction, Repression, or Fostering T cell-T Cell Communication? Allergy 2024, 80, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.S.; Maurer, M.; Metcalfe, D.D.; Pejler, G.; Sagi-Eisenberg, R.; Nilsson, G. The Ingenious Mast Cell: Contemporary Insights into Mast Cell Behavior and Function. Allergy 2022, 77, 83–99. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast Cells and Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lee, S.Y.; Guo, J.-Q.; Jin, J.-H.; Fan, Q.; Liao, W.-B. Wikstroemia fragrans (Thymelaeaceae, Daphneae), a New Species from Mount Danxia, China Based on Morphological and Molecular Evidence. PhytoKeys 2022, 213, 67–78. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, M.; Chang, H.; Han, N.; Yin, J. Guaiane Type of Sesquiterpene with NO Inhibitory Activity from the Root of Wikstroemia Indica. Bioorg. Chem. 2020, 99, 103785. [Google Scholar] [CrossRef]
- Lu, C.-L.; Zhu, L.; Piao, J.-H.; Jiang, J.-G. Chemical Compositions Extracted from Wikstroemia indica and Their Multiple Activities. Pharm. Biol. 2012, 50, 225–231. [Google Scholar] [CrossRef]
- Wu, M.; Su, X.; Wu, Y.; Luo, Y.; Guo, Y.; Xue, Y. Glycosylated Coumarins, Flavonoids, Lignans and Phenylpropanoids from Wikstroemia nutans and Their Biological Activities. Beilstein J. Org. Chem. 2022, 18, 200–207. [Google Scholar] [CrossRef]
- Fan, Q.; Jiang, Y.-P.; Zhu, D.-Q.; Xu, W.; Huang, W.-Q.; Huang, X.-J.; Shao, M. Phenols from the Rhizome of Wikstroemia indica. Biochem. Syst. Ecol. 2018, 78, 59–62. [Google Scholar] [CrossRef]
- Huang, W.-H.; Zhou, G.-X.; Wang, G.-C.; Chung, H.-Y.; Ye, W.-C.; Li, Y.-L. A New Biflavonoid with Antiviral Activity from the Roots of Wikstroemia indica. J. Asian Nat. Prod. Res. 2012, 14, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Liu, Y.-P.; Wang, X.-P.; Qiao, Z.-H.; Yu, X.-M.; Zhu, Y.-Z.; Xie, L.; Qiang, L.; Fu, Y.-H. Bioactive Daphnane Diterpenes from Wikstroemia chuii with Their Potential Anti-Inflammatory Effects and Anti-HIV Activities. Bioorg. Chem. 2020, 105, 104388. [Google Scholar] [CrossRef] [PubMed]
- Ingert, N.; Bombarda, I.; Herbette, G.; Faure, R.; Moretti, C.; Raharivelomanana, P. Oleodaphnoic Acid and Coriaceol, Two New Natural Products from the Stem Bark of Wikstroemia coriacea. Molecules 2013, 18, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.-P.; Yuan, J.-J.; Pi, S.-H.; Wang, R.; Tan, R.; Ma, C.-Y.; Zhang, T.; Jiang, H.-Z. Flavones and Lignans from the Stems of Wikstroemia scytophylla Diels. Pharmacogn. Mag. 2017, 13, 488–491. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yang, Y.; Bakri, M.; Gu, D.; Aisa, H.A. Separation of (S)-Dehydrovomifoliol from Leaves of Nitraria sibirica pall. by High-Speed Counter-Current Chromatography. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 573–582. [Google Scholar] [CrossRef]
- Owen, R.W.; Haubner, R.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Isolation, Structure Elucidation and Antioxidant Potential of the Major Phenolic and Flavonoid Compounds in Brined Olive Drupes. Food Chem. Toxicol. 2003, 41, 703–717. [Google Scholar] [CrossRef]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon Nanotubes Supported Tyrosinase in the Synthesis of Lipophilic Hydroxytyrosol and Dihydrocaffeoyl Catechols with Antiviral Activity against DNA and RNA Viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. 1H-NMR Simultaneous Identification of Health-Relevant Compounds in Propolis Extracts. Phytochem. Anal. 2012, 23, 260–266. [Google Scholar] [CrossRef]
- Tang, K.S.C.; Konczak, I.; Zhao, J. Phenolic Compounds of the Australian Native Herb Prostanthera rotundifolia and Their Biological Activities. Food Chem. 2017, 233, 530–539. [Google Scholar] [CrossRef]
- Yang, E.-J.; Kim, S.-I.; Ku, H.-Y.; Lee, D.-S.; Lee, J.-W.; Kim, Y.-S.; Seong, Y.-H.; Song, K.-S. Syringin from Stem Bark of Fraxinus rhynchophylla Protects Aβ(25–35)-Induced Toxicity in Neuronal Cells. Arch. Pharm. Res. 2010, 33, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Li, H.; Zhang, Q.; Zheng, H.; Qin, L. New Thiazinediones and Other Components from Xanthium strumarium. Chem. Nat. Compd. 2006, 42, 567–570. [Google Scholar] [CrossRef]
- Chan, E.W.; Wong, S.; Lim, Y.; Ling, S. Caffeoylquinic Acids in Leaves of Selected Apocynaceae Species: Their Isolation and Content. Phcog. Res. 2014, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Forino, M.; Tenore, G.C.; Tartaglione, L.; Carmela, D.; Novellino, E.; Ciminiello, P. (1S,3R,4S,5R)5-O-Caffeoylquinic Acid: Isolation, Stereo-Structure Characterization and Biological Activity. Food Chem. 2015, 178, 306–310. [Google Scholar] [CrossRef]
- Chi, H.; Qi, X.; Wang, X.; Wang, Y.; Han, X.; Wang, J.; Wang, H. Preparative Separation and Purification of Loliolide and Epiloliolide from Ascophyllum nodosum Using Amine-Based Microporous Organic Polymer for Solid Phase Extraction Coupled with Macroporous Resin and Prep-HPLC. Anal. Methods 2021, 13, 1939–1944. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Moon, J.-H.; Seong, K.-Y.; Park, K.-H. Antimicrobial Activity of 4-Hydroxybenzoic Acid and Trans 4-Hydroxycinnamic Acid Isolated and Identified from Rice Hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J.; Lu, F.; Ralph, S.A.; Boudet, A.-M.; MacKay, J.J.; Sederoff, R.R.; Ito, T.; Kawai, S.; Ohashi, H.; et al. NMR Analysis of Lignins in CAD-Deficient Plants. Part 1. Incorporation of Hydroxycinnamaldehydes and Hydroxybenzaldehydes into Lignins. Org. Biomol. Chem. 2003, 1, 268–281. [Google Scholar] [CrossRef]
- Plouvier, V.; Martin, M.-T.; Brouard, J.-P. Two Pyran Type Glycosides from Tunica prolifera. Phytochemistry 1993, 6, 1618–1620. [Google Scholar] [CrossRef]
- Shao, J.-H.; Chen, J.; Zhao, C.-C.; Shen, J.; Liu, W.-Y.; Gu, W.-Y.; Li, K.-H. Insecticidal and α-Glucosidase Inhibitory Activities of Chemical Constituents from Viburnum Fordiae Hance. Nat. Prod. Res. 2019, 33, 2662–2667. [Google Scholar] [CrossRef]
- Sefkow, M. Enantioselective Synthesis of (−)-Wikstromol Using a New Approach via Malic Acid. J. Org. Chem. 2001, 66, 2343–2349. [Google Scholar] [CrossRef]
- Brenes, M.; Hidalgo, F.J.; García, A.; Rios, J.J.; García, P.; Zamora, R.; Garrido, A. Pinoresinol and 1-acetoxypinoresinol, Two New Phenolic Compounds Identified in Olive Oil. J. Americ. Oil Chem. Soc. 2000, 77, 715–720. [Google Scholar] [CrossRef]
- Ouyang, M.-A.; Wein, Y.-S.; Zhang, Z.-K.; Kuo, Y.-H. Inhibitory Activity against Tobacco Mosaic Virus (TMV) Replication of Pinoresinol and Syringaresinol Lignans and Their Glycosides from the Root of Rhus javanica Var. roxburghiana. J. Agric. Food Chem. 2007, 55, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Jung, Y.-J.; Jung, J.-W.; Shrestha, S.; Lim, D.W.; Han, D.; Baek, N.-I. A New Flavonoid Glycoside from the Root Bark of Morus alba L. Nat. Prod. Res. 2014, 28, 1859–1863. [Google Scholar] [CrossRef]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Umbelliferone—An Antioxidant Isolated from Acacia nilotica (L.) Willd. Ex. Del. Food Chem. 2010, 120, 825–830. [Google Scholar] [CrossRef]
- Kicel, A.; Wolbis, M. Coumarins from the Flowers of Trifolium Repens. Chem. Nat. Compd. 2012, 48, 130–132. [Google Scholar] [CrossRef]
- Li, X.-N.; Tong, S.-Q.; Cheng, D.-P.; Li, Q.-Y.; Yan, J.-Z. Coumarins from Edgeworthia chrysantha. Molecules 2014, 19, 2042–2048. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Tian, X.-Y.; Fang, W.-S. Bioactive Coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007, 9, 59–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.; Wang, Y.; Huang, K. Target-Guided Isolation and Purification of Antioxidants from Selaginella sinensis by Offline Coupling of DPPH-HPLC and HSCCC Experiments. J. Chromatogr. B 2011, 879, 191–196. [Google Scholar] [CrossRef]
- Park, Y.; Moon, B.; Yang, H.; Lee, Y.; Lee, E.; Lim, Y. Complete Assignments of NMR Data of 13 Hydroxymethoxyflavones. Magn. Reson. Chem. 2007, 45, 1072–1075. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Ramadan, M.A.; Khalifa, A.A. Acetophenones, a Chalcone, a Chromone and Flavonoids from Pancratium maritimum. Phytochemistry 1998, 49, 2579–2583. [Google Scholar] [CrossRef]
- Ko, J.-H.; Nam, Y.H.; Joo, S.-W.; Kim, H.-G.; Lee, Y.-G.; Kang, T.H.; Baek, N.-I. Flavonoid 8-O-Glucuronides from the Aerial Parts of Malva Verticillata and Their Recovery Effects on Alloxan-Induced Pancreatic Islets in Zebrafish. Molecules 2018, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sun, A.; Liu, R.; Zhang, Y.; Cui, J. An Efficient Preparative Procedure for Main Flavonoids from the Peel of Trichosanthes kirilowii Maxim. Using Polyamide Resin Followed by Semi-Preparative High Performance Liquid Chromatography. J. Chromatogr. B 2014, 965, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chang, E.J.; Kim, H.J.; Park, J.H.; Choi, S.W. Antioxidative Flavonoids from Leaves of Carthamus tinctorius. Arch. Pharm. Res. 2002, 25, 313–319. [Google Scholar] [CrossRef]
- Ringl, A.; Prinz, S.; Huefner, A.; Kurzmann, M.; Kopp, B. Chemosystematic Value of Flavonoids from Crataegus x Macrocarpa (Rosaceae) with Special Emphasis on (R)- and (S)-Eriodictyol-7-O-glucuronide and Luteolin-7-O-glucuronide. Chem. Biodivers. 2007, 4, 154–162. [Google Scholar] [CrossRef]
- Feng, J.H.; Lee, H.J.; Kim, S.B.; Jung, J.S.; Lim, S.S.; Suh, H.W. Antinociceptive Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract. Sci. Pharm. 2019, 87, 18. [Google Scholar] [CrossRef]
- Huh, J.; Ha, T.K.Q.; Kang, K.B.; Kim, K.H.; Oh, W.K.; Kim, J.; Sung, S.H. C -Methylated Flavonoid Glycosides from Pentarhizidium orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J. Nat. Prod. 2017, 80, 2818–2824. [Google Scholar] [CrossRef]
- Lai, Y.; Zeng, H.; He, M.; Qian, H.; Wu, Z.; Luo, Z.; Xue, Y.; Yao, G.; Zhang, Y. 6,8-Di-C-Methyl-Flavonoids with Neuroprotective Activities from Rhododendron fortunei. Fitoterapia 2016, 112, 237–243. [Google Scholar] [CrossRef]
- Devkota, H.P.; Watanabe, M.; Watanabe, T.; Yahara, S. Diplomorphanins A and B: New C-Methyl Flavonoids from Diplomorpha canescens. Chem. Pharm. Bull. 2013, 61, 242–244. [Google Scholar] [CrossRef]
- Gimenez, V.M.M.; e Silva, M.L.A.; Cunha, W.R.; Januario, A.H.; Costa, E.J.X.; Pauletti, P.M. Influence of Environmental, Geographic, and Seasonal Variations in the Chemical Composition of Miconia Species from Cerrado. Biochem. Syst. Ecol. 2020, 91, 104049. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.-J.; Chen, J.-P.; Shi, C.-Y.; Niu, L.-T.; Zhang, X.; Yao, X.-S. C-Methylated Flavanones from the Rhizomes of Matteuccia intermedia and Their α-Glucosidase Inhibitory Activity. Fitoterapia 2019, 136, 104147. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S. Reproduction and Interpretation of the UV–Vis Spectra of Some Flavonoids. Chem. Pap. 2017, 71, 543–552. [Google Scholar] [CrossRef]
- Slade, D.; Ferreira, D.; Marais, J.P.J. Circular Dichroism, a Powerful Tool for the Assessment of Absolute Configuration of Flavonoids. Phytochemistry 2005, 66, 2177–2215. [Google Scholar] [CrossRef] [PubMed]
- Lecce, M.; Molfetta, R.; Milito, N.D.; Santoni, A.; Paolini, R. FcεRI Signaling in the Modulation of Allergic Response: Role of Mast Cell-Derived Exosomes. Int. J. Mol. Sci. 2020, 21, 5464. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kalogeromitros, D. The Critical Role of Mast Cells in Allergy and Inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–99. [Google Scholar] [CrossRef]
- Zou, F.; Du, Q.; Zhang, Y.; Zuo, L.; Sun, Z. Pseudo-Allergic Reactions Induced by Chinese Medicine Injections: A Review. Chin. Med. 2023, 18, 149. [Google Scholar] [CrossRef]
- Keem, M.-J.; Jo, B.-G.; Lee, S.H.; Kim, T.-Y.; Jung, Y.S.; Jeong, E.-J.; Kim, K.H.; Kim, S.-N.; Yang, M.H. Ameliorative Effects of Wikstroemia trichotoma 95% EtOH Extract on a Mouse Model of DNCB-Induced Atopic Dermatitis. J. Ethnopharmacol. 2024, 333, 118398. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. O-Substituted Quercetin Derivatives: Structural Classification, Drug Design, Development, and Biological Activities, a Review. J. Mol. Struct. 2022, 1254, 132392. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Ortiz-Celiseo, A.; Peniche-Cardeña, A.; Bravo-Ruiz, O.; López-Fentanes, F.C.; Valerio-Alfaro, G.; Fernández, J.J. Studies on the Bioactive Flavonoids Isolated from Azadirachta indica. Nat. Prod. Res. 2020, 34, 3483–3491. [Google Scholar] [CrossRef]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the Way towards Effective Plant-Based Inhibitors of Hyaluronidase and Tyrosinase: A Critical Review on a Structure–Activity Relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; Van Gameren, Y.; Cnossen, E.P.J.; De Vries, J.H.M.; Katan, M.B. The Sugar Moiety Is a Major Determinant of the Absorption of Dietary Flavonoid Glycosides in Man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Keegan, A.D.; Leonard, W.J.; Zhu, J. Recent Advances in Understanding the Role of IL-4 Signaling. Fac. Rev. 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-T.; Lee, Y.; Gullen, E.A.; Cheng, Y.-C. Impacts of Baicalein Analogs with Modification of the 6th Position of A Ring on the Activity toward NF-κB, AP-1 or CREB Mediated Transcription. Bioorg. Med. Chem. Lett. 2008, 18, 5046–5049. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of Dietary Flavonoids towards Improved Bioactivity: An Update on Structure–Activity Relationship. Crit. Rev. Food Sci. Nutr. 2018, 58, 513–527. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Martínez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar] [CrossRef]
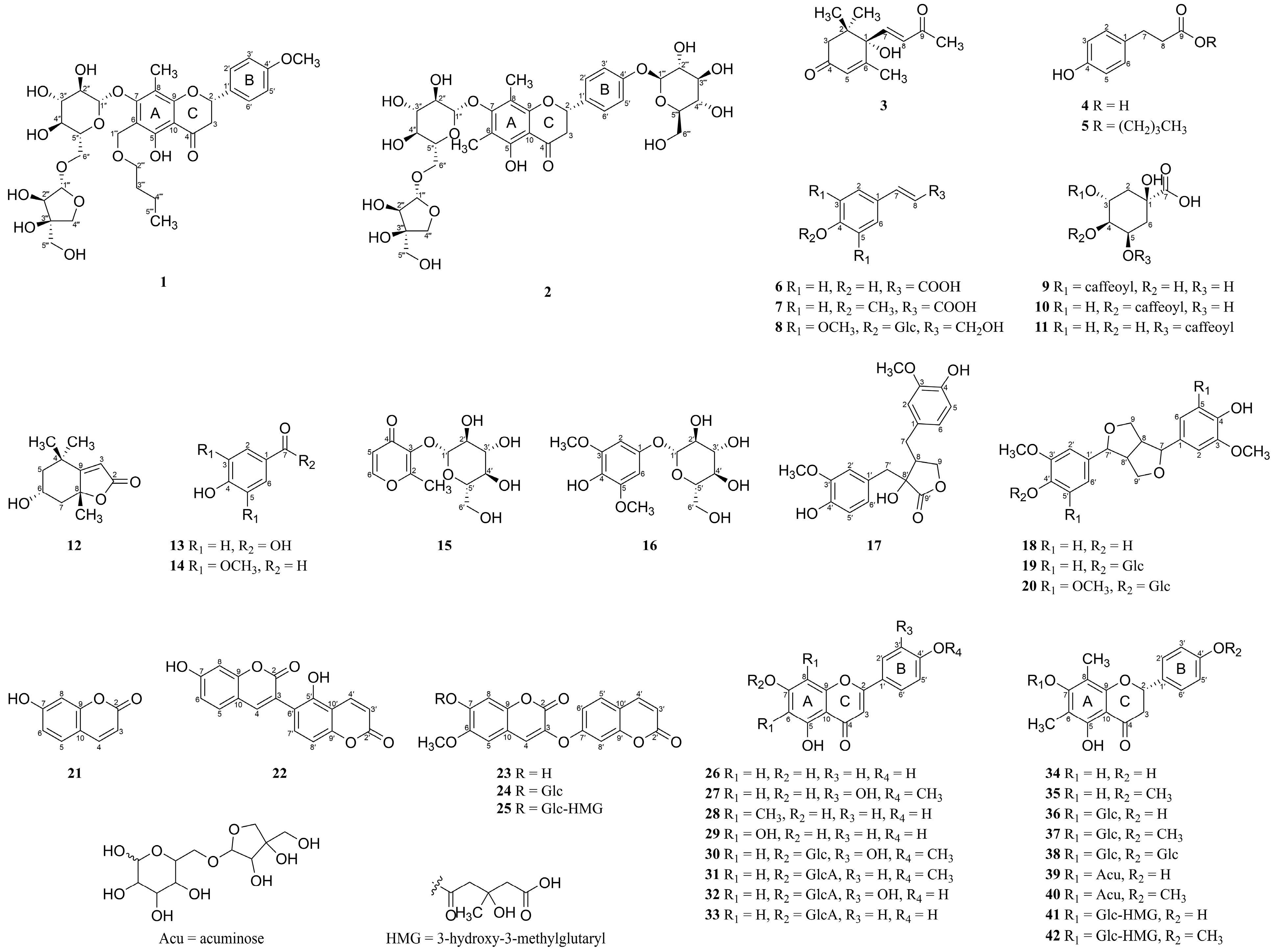
 ) and HMBC (→) correlations of compounds 1 and 2.
) and HMBC (→) correlations of compounds 1 and 2.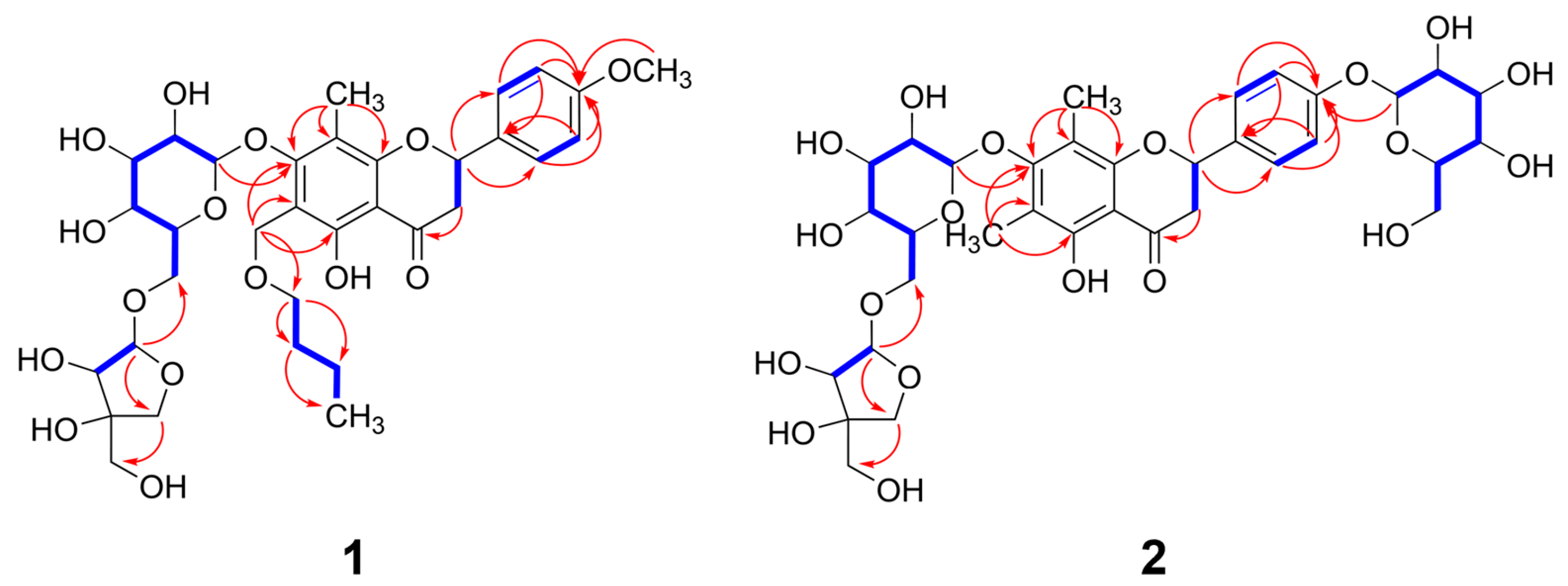

| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 77.9 d | 5.59 dd (13.0/3.0) | 77.8 d | 5.56 dd (12.5/3.0) |
| 3a | 42.1 t | 3.35 dd (17.0/13.0) | 42.3 t | 3.32 m |
| 3b | 2.84 dd (17.0/3.0) | 2.84 dd (17.0/3.0) | ||
| 4 | 198.3 s | 198.4 s | ||
| 5 | 159.3 s | 157.5 s | ||
| 6 | 111.3 s | 111.1 s | ||
| 7 | 162.5 s | 161.3 s | ||
| 8 | 110.1 s | 110.0 s | ||
| 9 | 159.3 s | 157.3 s | ||
| 10 | 104.8 s | 104.8 s | ||
| 1′ | 130.6 s | 132.1 s | ||
| 2′ | 127.9 d | 6.99 d (8.5) | 127.8 d | 7.46 d (9.0) |
| 3′ | 113.9 d | 7.47 d (8.5) | 116.2 d | 7.08 d (9.0) |
| 4′ | 159.4 s | 157.9 s | ||
| 5′ | 113.9 d | 7.47 d (8.5) | 116.2 d | 7.08 d (9.0) |
| 6′ | 127.9 d | 6.99 d (8.5) | 127.8 d | 7.46 d (9.0) |
| 6-CH3 | 8.6 q | 2.08 s | ||
| 8-CH3 | 9.1 q | 2.04 s | 9.2 q | 2.06 s |
| 4′-OCH3 | 55.1 q | 3.78 s | ||
| 1″ | 104.6 d | 4.84 d (7.5) | 104.1 d | 4.59 d (7.5) |
| 2″ | 73.9 d | 3.30 m | 75.3 d | 3.33–3.19 |
| 3″ | 75.6 d | 3.15 m | 77.0 d | 3.33–3.19 |
| 4″ | 69.1 d | 3.15 m | 69.7 d | 3.16 m |
| 5″ | 75.8 d | 3.68 m | 76.6 d | 3.33–3.19 |
| 6″a | 67.2 t | 3.75 m | 67.2 t | 3.70 m |
| 6″b | 3.45 m | 3.43 m | ||
| 1‴ | 109.2 d | 4.77 d (2.5) 1H | 109.2 d | 4.76 d (2.5) |
| 2‴ | 76.3 d | 3.18 m | 73.7 d | 3.33–3.19 |
| 3‴ | 78.7 s | 78.8 s | ||
| 4‴a | 73.2 t | 3.73 d (9.0) | 73.7 t | 3.72 d (9.0) |
| 4‴b | 3.55 d (9.0) | 3.54 d (9.0) | ||
| 5‴ | 63.4 t | 3.27 s | 63.4 t | 3.28 s |
| 1⁗a/1⁗ | 60.1 t | 4.59 d (10.0) | 100.3 d | 4.89 d (7.0) |
| 1⁗b | 4.40 d (10.0) | |||
| 2⁗ | 69.7 t | 3.45 d (6.0) | 73.2 d | 3.33–3.19 |
| 3⁗ | 31.4 t | 1.50 m | 76.2 d | 3.33–3.19 |
| 4⁗ | 19.0 t | 1.36 m | 69.7 d | 3.16 m |
| 5⁗ | 13.8 q | 0.87 t (7.5) | 75.8 d | 3.33–3.19 |
| 6⁗a | 60.7 t | 3.68 m | ||
| 6⁗b | 3.46 m | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keem, M.-J.; Kim, T.-Y.; Park, N.-J.; Choi, S.; Paik, J.-H.; Jo, B.-G.; Kwon, T.-H.; Kim, S.-N.; Lee, S.R.; Yang, M.H. Isolation, Characterization, and Anti-Allergic Evaluation of Phytochemicals from Wikstroemia trichotoma. Nutrients 2025, 17, 1552. https://doi.org/10.3390/nu17091552
Keem M-J, Kim T-Y, Park N-J, Choi S, Paik J-H, Jo B-G, Kwon T-H, Kim S-N, Lee SR, Yang MH. Isolation, Characterization, and Anti-Allergic Evaluation of Phytochemicals from Wikstroemia trichotoma. Nutrients. 2025; 17(9):1552. https://doi.org/10.3390/nu17091552
Chicago/Turabian StyleKeem, Min-Ji, Tae-Young Kim, No-June Park, Sangho Choi, Jin-Hyub Paik, Beom-Geun Jo, Taek-Hwan Kwon, Su-Nam Kim, Seoung Rak Lee, and Min Hye Yang. 2025. "Isolation, Characterization, and Anti-Allergic Evaluation of Phytochemicals from Wikstroemia trichotoma" Nutrients 17, no. 9: 1552. https://doi.org/10.3390/nu17091552
APA StyleKeem, M.-J., Kim, T.-Y., Park, N.-J., Choi, S., Paik, J.-H., Jo, B.-G., Kwon, T.-H., Kim, S.-N., Lee, S. R., & Yang, M. H. (2025). Isolation, Characterization, and Anti-Allergic Evaluation of Phytochemicals from Wikstroemia trichotoma. Nutrients, 17(9), 1552. https://doi.org/10.3390/nu17091552







