Nutraceuticals for Gut–Brain Axis Health: A Novel Approach to Combat Malnutrition and Future Personalised Nutraceutical Interventions
Abstract
1. Introduction
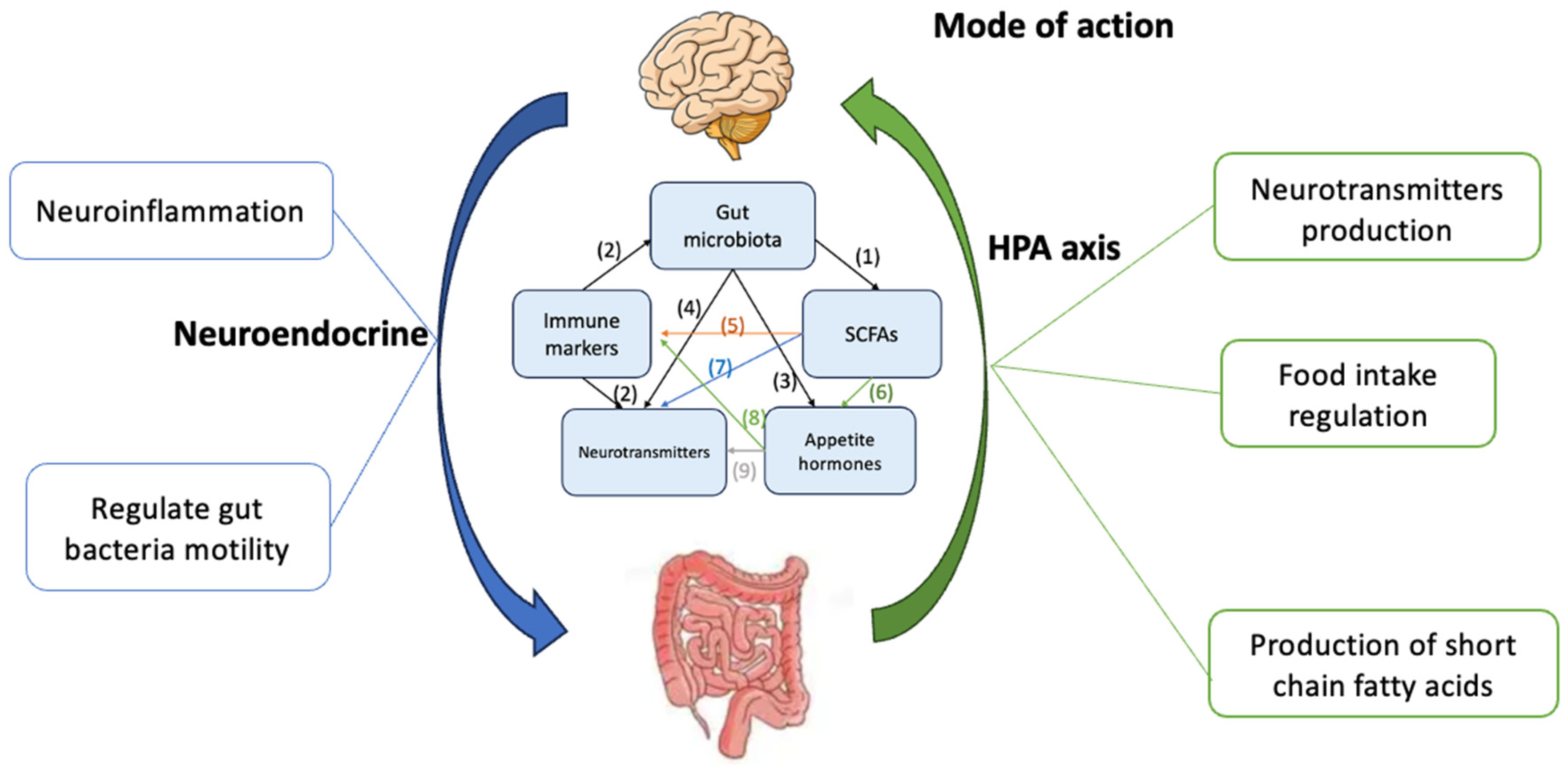
2. Combatting Malnutrition via Various GBA Biomarkers
2.1. Gut Microbiota Effects on SCFAs Regulation
2.2. Immune Markers Effect on Gut Microbiota and NTs Regulation
2.3. Gut Microbiota Effects on Appetite Hormones Regulation
2.4. Gut Microbiota Effects on NTs Regulation
2.5. SCFAs Effect on Immune Markers Regulation
2.6. SCFAs Effect on Appetite Hormones Regulation
2.7. SCFAs Effect on NTs Regulation
2.8. Appetite Hormones Effect on Immune Markers Regulation
2.9. NTs Effect in Appetite Hormone Regulation
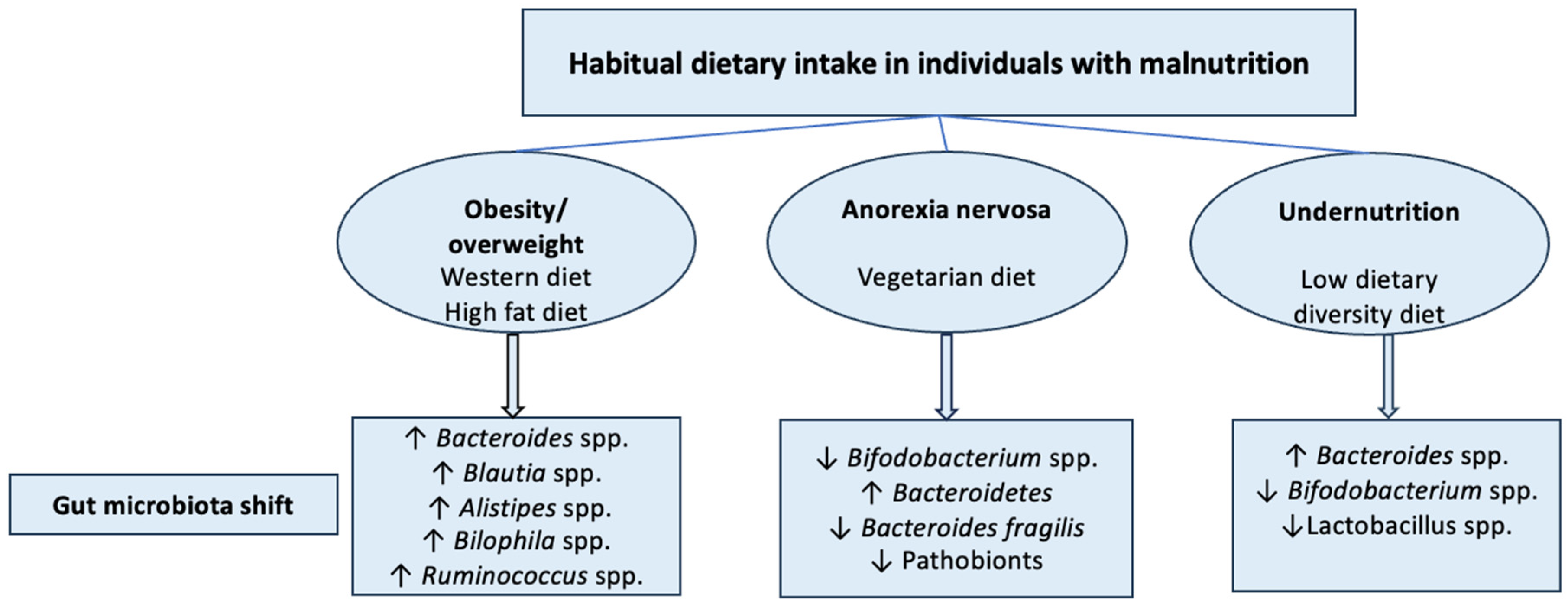
2.10. Malnutrition and GBA Dysregulation
3. Emerging Nutraceuticals for Treating Malnutrition via GBA Targeting
3.1. Probiotics
Effect of Probiotics on Gut Microbiota Composition and NTs Modulation
3.2. Prebiotics
Effect of Prebiotic Type on Neuroactive Metabolites
3.3. Synbiotics
3.4. Postbiotics
Effect of Postbiotics on Appetite Regulation
3.5. Paraprobiotics
Effect of Paraprobiotics on Neuroactive Compounds Regulation
4. Challenges and Future Prospectives
4.1. Approaches in Nutraceutical Interventions for Individuals with Malnutrition
4.1.1. In Vitro Approach
4.1.2. In Vivo Approach
4.2. Vision for the Future: Multi-Omics Technologies Driven Tailored Nutraceuticals in Malnutrition
4.2.1. Genomics of Malnutrition
4.2.2. Harnessing Microbiomics for Nutraceutical Interventions
4.2.3. Metabolomics and Malnutrition—Unveiling the Metabolic Fingerprint
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AN | Anorexia nervosa |
| BMI | Body mass index |
| CNS | Central nervous system |
| CIpB | Caseinolytic protease B |
| CFU | Colony-forming unit |
| CRP | C-reactive protein |
| DA | Dopamine |
| ECs | Enterochromaffin cells |
| F/B | Firmicute/Bacteroidete |
| FTT | Failure to thrive |
| FOS | Fructooligosaccharide |
| GBA | Gut–brain axis |
| GOS | Galactoologosaccharide |
| GLP-1 | Glucagon-like peptide-1 |
| GABA | Gamma aminobutyric acid |
| GIT | Gastrointestinal tract |
| GALT | Gut-associated lymphoid tissue |
| HMOs | Human milk oligosaccharides |
| HFD | High fat diet |
| IL-6 | Interleukin-6 |
| IL-17 | Interleukin-17 |
| IL-1β | Interleukin-1 beta |
| mRNA | Messenger RNA |
| MUAC | Mid-upper arm circumference |
| NE | Norepinephrine |
| NTs | Neurotransmitters |
| NF-κB | Nuclear factor-κB |
| OFS | Oligofructose |
| OTU | Operational taxonomic unit |
| PYY | Peptide YY |
| SCFAs | Short-chain fatty acids |
| TNF-α | Tumour necrosis factor-α |
| 5-HT | Serotonin |
References
- Mitra, S.; Dash, R.; Al Nishan, A.; Habiba, S.U.; Moon, I.S. Brain modulation by the gut microbiota: From disease to therapy. J. Adv. Res. 2023, 53, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Devi, K.; Coutinho, H.D.; Mishra, A.P. Exploration of gut microbiome and inflammation: A review on key signalling pathways. Cell. Signal. 2024, 118, 111140. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Sig. Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.K.; Ramadan, H.K.-A.; Elbeh, K.; Haridy, N.A. Bridging the gap: Associations between gut microbiota and psychiatric disorders. Middle East. Curr. Psychiatry 2024, 31, 2. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Malnutrition. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 25 April 2025).
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E93–E101. [Google Scholar]
- National Institute of Mental Health (NIMH). Anorexia Nervosa. 2023. Available online: https://www.nimh.nih.gov/health/topics/eating-disorders (accessed on 25 April 2025).
- Mancuso, S.G.; Newton, J.R.; Bosanac, P.; Rossell, S.L.; Nesci, J.B.; Castle, D.J. Classification of eating disorders: Comparison of relative prevalence rates using DSM-IV and DSM-5 criteria. Br. J. Psychiatry 2015, 206, 519–520. [Google Scholar] [CrossRef]
- Merino del Portillo, M.; Clemente-Suárez, V.J.; Ruisoto, P.; Jimenez, M.; Ramos-Campo, D.J.; Beltran-Velasco, A.I.; Martínez-Guardado, I.; Rubio-Zarapuz, A.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional Modulation of the Gut–Brain Axis: A Comprehensive Review of Dietary Interventions in Depression and Anxiety Management. Metabolites 2024, 14, 549. [Google Scholar] [CrossRef]
- Elazzazy, A.M.; Baeshen, M.N.; Alasmi, K.M.; Alqurashi, S.I.; Desouky, S.E.; Khattab, S.M.R. Where Biology Meets Engineering: Scaling Up Microbial Nutraceuticals to Bridge Nutrition, Therapeutics, and Global Impact. Microorganisms 2025, 13, 566. [Google Scholar] [CrossRef]
- Jaberi, K.R.; Alamdari-Palangi, V.; Savardashtaki, A.; Vatankhah, P.; Jamialahmadi, T.; Tajbakhsh, A.; Sahebkar, A. Modulatory Effects of Phytochemicals on Gut–Brain Axis: Therapeutic Implication. Curr. Dev. Nutr. 2024, 8, 103785. [Google Scholar] [CrossRef]
- Agusti, A.; Moya-Pérez, A.; Campillo, I.; Montserrat-de la Paz, S.; Cerrudo, V.; Perez-Villalba, A. Bifidobacterium pseudocatenulatum CECT 7765 ameliorates neuroendocrine alterations associated with an exaggerated stress response and anhedonia in obese mice. Mol. Neurobiol. 2018, 55, 5337–5352. [Google Scholar]
- Jang, H.-M.; Han, S.-K.; Kim, J.-K.; Oh, S.-J.; Jang, H.-B.; Kim, D.-H. Lactobacillus sakei alleviates high-Fat-Diet-Induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-κB activation. Mol. Nutr. Food Res. 2019, 63, e1800978. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, S.P.; Meyer, D.; Westerterp, K.R. Effects of oligofructose on appetite profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and energy intake. Br. J. Nutr. 2011, 106, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Bomhof, M.R.; Saha, D.C.; Reid, D.T.; Paul, H.A.; Reimer, R.A. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity 2014, 22, 763–771. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Sig. Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- de Vos, W.M.; de Vos, E.A. Role of the intestinal microbiome in health and disease: From correlation to causation. Nutr. Rev. 2012, 70 (Suppl. 1), S45–S56. [Google Scholar] [CrossRef]
- Dave, M.; Higgins, P.D.; Middha, S.; Rioux, K.P. The human gut microbi-ome: Current knowledge, challenges, and future directions. Transl. Res. 2012, 160, 246–257. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex- Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems 2019, 4, e00261-19. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; Van Der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Mahony, S. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Davey, K.J.; O’mahony, S.M.; Schellekens, H.; O’sullivan, O.; Bienenstock, J.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gender-dependent consequences of chronic olanzapine in the rat: Effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology 2012, 221, 155–169. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016, 63, 217–227. [Google Scholar]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity pathogen-esis: An endocrine society scientific statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar]
- Macfarlane, G.T.; Gibson, G.R.; Cummings, J.H. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 1992, 72, 57–64. [Google Scholar] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M.; Moran, M.A. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljević, S.; Čepić, A.; Bojić, S.; Veljović, K.; Mihajlović, S.; Đedović, N.; Jevtić, B.; Momčilović, M.; Lazarević, M.; Mostarica Stojković, M.; et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Sci. Rep. 2019, 9, 918. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, H.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018, 11, 1386–1397. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef]
- Breton, J.; Legrand, R.; Akkermann, K.; Jarv, A.; Harro, J.; Dechelotte, P.; Fetissov, S.O. Elevated Plasma Concentrations of Bacterial ClpB Protein in Patients with Eating Disorders. Int. J. Eat. Disord. 2016, 49, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Tennoune, N.; Legrand, R.; Ouelaa, W.; Breton, J.; Lucas, N.; Bole-Feysot, C.; do Rego, J.C.; Dechelotte, P.; Fetissov, S.O. Sex-related effects of nutritional supplementation of Escherichia coli: Relevance to eating disorders. Nutrition 2015, 31, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Toro, O.; Varela, P.; López-Vidriero, I.; Morandé, G.; Marcos, A. Effects of a nutritional intervention with yogurt on lymphocyte subsets and cytokine production capacity in anorexia nervosa patients. Eur. J. Nutr. 2006, 45, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 963–975. [Google Scholar] [CrossRef]
- Kishi, T.; Elmquist, J.K. Body weight is regulated by the brain: A link between feeding and emotion. Mol. Psychiatry 2005, 10, 132–146. [Google Scholar]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Dhillo, W.S.; Seal, L.J.; Cohen, M.A.; Batterham, R.L.; Taheri, S.; Stanley, S.A.; Ghatei, M.A.; et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001, 50, 2540–2547. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gaerlan, S.; De Leoz, M.L.; Dimapasoc, L.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677. [Google Scholar] [CrossRef]
- Williams, C.M.; Jackson, K.G. Inulin and oligofructose: Effects on lipid metabolism from human studies. Br. J. Nutr. 2002, 87 (Suppl. 2), S261–S264. [Google Scholar] [CrossRef]
- Overduin, J.; Schoterman, M.H.; Calame, W.; Schonewille, A.J.; Ten Bruggencate, S.J. Dietary galacto-oligosaccharides and calcium: Effects on energy intake, fat-pad weight and satiety-related, gastrointestinal hormones in rats. Br. J. Nutr. 2013, 109, 1338–1348. [Google Scholar] [CrossRef]
- Walls, A.B.; Heimbürger, C.M.; Bouman, S.D.; Schousboe, A.; Waagepetersen, H.S. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience 2009, 158, 284–292. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behaviour and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264, e119. [Google Scholar] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [PubMed]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Sawaki, E.; Koga, Y.; Benno, Y. Cerebral Low-Molecular Metabolites Influenced by Intestinal Microbiota: A Pilot Study. Front. Syst. Neurosci. 2013, 7, 9. [Google Scholar]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.A.M. Gut-brain: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar]
- Sugama, S.; Kakinuma, Y. Loss of dopaminergic neurons occurs in the ventral tegmental area and hypothalamus of rats following chronic stress: Possible pathogenetic loci for depression involved in Parkinson’s disease. Neurosci. Res. 2016, 111, 48–55. [Google Scholar]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef] [PubMed]
- Roubalova, R.; Prochazkova, P.; Dvorak, J.; Hill, M.; Papezova, H.; Kreisinger, J.; Bulant, J.; Lambertova, A.; Holanova, P.; Bilej, M.; et al. Altered Serum Immunological and Biochemical Parameters and Microbiota Composition in Patients With AN During Realimentation. Front. Nutr. 2021, 8, 680870. [Google Scholar] [CrossRef] [PubMed]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-Chain Fatty Acids Suppress Lipopolysaccharide-Induced Production of Nitric Oxide and Proinflammatory Cytokines Through Inhibition of NF-κB Pathway in RAW264.7 Cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Shah, P.; Nankova, B.B.; Parab, S.; La Gamma, E.F. Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res. 2006, 1107, 13–23. [Google Scholar] [CrossRef]
- Wu, R.; Dong, W.; Qiang, X.; Wang, H.; Blau, S.A.; Ravikumar, T.S.; Wang, P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit. Care Med. 2009, 37, 2421–2426. [Google Scholar] [CrossRef]
- Meister, B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behaviour and body weight. Physiol. Behav. 2007, 92, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Meguid, M.M.; Fetissov, S.O.; Varma, M.; Sato, T.; Zhang, L.; Laviano, A.; Rossi-Fanelli, F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 2000, 16, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Wellman, P.J. Norepinephrine and the control of food intake. Nutrition 2000, 16, 837–842. [Google Scholar] [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary patterns associated with obesity outcomes in adults: An umbrella review of systematic reviews. Public. Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Chelmi, M.E.; Liampas, A.; Yfanti, C.M.; Panagouli, E.; Vlachopapadopoulou, E.; Michalacos, S.; Bacopoulou, F.; Psaltopoulou, T.; Tsitsika, A. Vegetarian diets and eating disorders in adolescents and young adults: A systematic review. Children 2020, 8, 12. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Meehan, C.L.; Davies, P.S.W.; Wall, C.; Lovell, A.; Hill, R.J. Dietary intake influences gut microbiota development of healthy Australian children from the age of one to two years. Sci. Rep. 2019, 9, 12476. [Google Scholar]
- Kim, M.S.; Hwang, S.S.; Park, E.J.; Bae, J.W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Profir, M.; Enache, R.M.; Roşu, O.A.; Pavelescu, L.A.; Creţoiu, S.M.; Gaspar, B.S. Malnutrition and Its Influence on Gut sIgA–Microbiota Dynamics. Biomedicines 2025, 13, 179. [Google Scholar] [CrossRef]
- Conde, K.; Fang, S.; Xu, Y. Unraveling the serotonin saga: From discovery to weight regulation and beyond—A comprehensive scientific review. Cell Biosci. 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Alaaraj, N.M.; Rogol, A.D. The link between malnutrition, immunity, infection, inflammation and growth: New pathological mechanisms. World J. Adv. Res. Rev. 2022, 15, 157–167. [Google Scholar] [CrossRef]
- Parmar, R.M.; Can, A.S. Physiology, Appetite and Weight Regulation. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574539/ (accessed on 25 April 2025).
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Egea Rodrigues, C.; Floegel, A.; Ahrens, W. Omics Biomarkers in Obesity: Novel Etiological Insights and Targets for Precision Prevention. Curr. Obes. Rep. 2020, 9, 219–230. [Google Scholar] [CrossRef]
- Labban, R.S.M.; Alfawaz, H.; Almnaizel, A.T.; Hassan, W.M.; Bhat, R.S.; Moubayed, N.M.; El-Ansary, A. High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Transl. Neurosci. 2020, 11, 147–160. [Google Scholar] [CrossRef]
- Aukan, M.I.; Nymo, S.; Ollestad, K.H.; Boyesen, G.A.; DeBenedictis, J.N.; Rehfeld, J.F.; Martins, C. Differences in gastrointestinal hormones and appetite ratings among obesity classes. Appetite 2022, 171, 105940. [Google Scholar] [CrossRef]
- Haines, M.S. Endocrine complications of anorexia nervosa. J. Eat. Disord. 2023, 11, 24. [Google Scholar] [CrossRef]
- Brunelli, D.T.; Boldrini, V.O.; Bonfante, I.L.; Duft, R.G.; Mateus, K.; Costa, L.; Cavaglieri, C.R. Obesity increases gene expression of markers associated with immunosenescence in obese middle-aged individuals. Front. Immunol. 2022, 12, 806400. [Google Scholar] [CrossRef]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Jagtiani, E.; Adsare, S. Microencapsulation: Probiotics, Prebiotics, and Nutraceuticals. J. Nanotechnol. Nanomater. 2022, 3, 34–60. [Google Scholar]
- Larroya-García, A.; Navas-Carrillo, D.; Orenes-Piñero, E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Crit. Rev. Food Sci. Nutr. 2019, 59, 3102–3116. [Google Scholar] [CrossRef] [PubMed]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Webberley, T.S.; Masetti, G.; Bevan, R.J.; Kerry-Smith, J.; Jack, A.A.; Michael, D.R. The impact of probiotic supplementation on cognitive, pathological and metabolic markers in a transgenic mouse model of Alzheimer’s disease. Front. Neurosci. 2022, 16, 843105. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef]
- Agustí, A.; Campillo, I.; Balzano, T.; Benítez-Páez, A.; López-Almela, I.; Romaní-Pérez, M.; Forteza, J.; Felipo, V.; Avena, N.M.; Sanz, Y. Bacteroides uniformis CECT 7771 modulates the brain reward response to reduce binge eating and anxiety-like behavior in rat. Mol. Neurobiol. 2021, 58, 4959–4979. [Google Scholar] [CrossRef]
- Solis, B.; Nova, E.; Gómez, S. The effect of fermented milk on interferon production in malnourished children and in anorexia nervosa patients undergoing nutritional care. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 4), S27–S33. [Google Scholar] [CrossRef]
- Fiolić, M.; Ćuk, M.C.; Tiljak, M.K. The role of L. reuteri DSM17938 in nutritional recovery and treatment of constipation in children and adolescents with anorexia nervosa—A randomized, double blind, placebo controlled study. Clin. Nutr. ESPEN 2021, 46, 47–53. [Google Scholar]
- Trinh, S.; Käver, L.; Schlösser, A.; Simon, A.; Kogel, V.; Voelz, C. Gut-associated lymphatic tissue in food-restricted rats: Influence of refeeding and probiotic supplementation. Microorganisms 2023, 11, 1411. [Google Scholar] [CrossRef]
- Qian, L.; Gao, R.; Huang, J.; Qin, H. Supplementation of triple viable probiotics combined with dietary intervention is associated with gut microbial improvement in humans on a high-fat diet. Exp. Ther. Med. 2019, 18, 2262–2270. [Google Scholar] [CrossRef]
- Hassan, N.E.; El-Masry, S.A.; El Shebini, S.M.; Ahmed, N.H.; Mehanna, N.S.; Abdel Wahed, M.M.; Alian, K. Effect of weight loss program using prebiotics and probiotics on body composition, physique, and metabolic products: Longitudinal intervention study. Sci. Rep. 2024, 14, 10960. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Howard, J.K.; Cani, P.D. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2016, 6, 48–60. [Google Scholar] [CrossRef]
- Liu, L.; Poveda, C.; Jenkins, P.E.; Walton, G.E. An in vitro approach to studying the microbial community and impact of pre-and probiotics under anorexia nervosa-related dietary restrictions. Nutrients 2021, 13, 4447. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Nuzhat, S.; Hasan, S.T.; Palit, P. Effects of probiotic and synbiotic supplementation on ponderal and linear growth in severely malnourished young infants in a randomized clinical trial. Sci. Rep. 2023, 13, 1845. [Google Scholar] [CrossRef]
- Aflatoonian, M.; Taghavi Ardakani, A.; Modarresi, S.Z. The effect of synbiotic supplementation on growth parameters in mild to moderate FTT children aged 2–5 years. Probiotics Antimicrob. Proteins 2020, 12, 119–124. [Google Scholar] [CrossRef]
- Famouri, F.; Khoshdel, A.; Golshani, A.; Kheiri, S.; Saneian, H.; Kelishadi, R. Effects of synbiotics on treatment of children with failure to thrive: A triple blind placebo-controlled trial. J. Res. Med. Sci. 2014, 19, 1046. [Google Scholar]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Damink, S.W.M.O.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar]
- Higashikawa, F.; Noda, M.; Awaya, T.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: Arandomized double-blind placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Ishida, Y.; Aihara, K.; Sawada, D.; Ashida, N.; Sugawara, T.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of fragmented Lactobacillus amylovorus CP1563 on lipid metabolism in overweight and mildly obese individuals: A randomized controlled trial. Microb. Ecol. Health Dis. 2016, 27, 30312. [Google Scholar]
- Tang, C.; Lu, Z. Health promoting activities of probiotics. J. Food Biochem. 2019, 43, e12944. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Frese, S.A.; Hutton, A.A.; Contreras, L.N.; Shaw, C.A.; Palumbo, M.C.; Casaburi, G.; Xu, G.; Davis, J.C.C.; Lebrilla, C.B.; Henrick, B.M. Persistence of Supplemented Bifidobacterium longum subsp. infantis EVC001 in Breastfed Infants. mSphere 2017, 2, e00501-17. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Cowen, P.J.; Fairburn, C.G.; Parry-Billings, M.; Calder, P.C.; Newsholme, E. Plasma concentrations of tryptophan and dieting. BMJ 1990, 300, 1499–1500. [Google Scholar]
- Bahceci, M.; Gokalp, D.; Bahceci, S.; Tuzcu, A.; Atmaca, S.; Arikan, S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity c-reactive protein levels. Is adipocyte size associated with inflammation in adults? J. Endocrinol. Invest. 2007, 30, 210–214. [Google Scholar] [CrossRef]
- Choi, B.S.; Brunelle, L.; Pilon, G.; Cautela, B.G.; Tompkins, T.A.; Drapeau, V. Lacticaseibacillus rhamnosus HA-114 improves eating behaviors and mood-related factors in adults with overweight during weight loss: A randomized controlled trial. Nutr. Neurosci. 2023, 26, 667–679. [Google Scholar] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303. [Google Scholar] [PubMed]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Posovszky, C.; Wabitsch, M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 2: Therapeutic potential of enteroendocrine cells in the treatment of obesity. Horm. Res. Paediatr. 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar]
- Burokas, A.; Arboleya, S.; Moloney, R.D. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef]
- van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate Mediates a Microbiome-Brain-Beta-Cell Axis to Promote Metabolic Syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 14, 491–502. [Google Scholar]
- Rauch, C.E.; Mika, A.S.; McCubbin, A.J.; Huschtscha, Z.; Costa, R.J.S. Effect of prebiotics, probiotics, and synbiotics on gastrointestinal outcomes in healthy adults and active adults at rest and in response to exercise—A systematic literature review. Front. Nutr. 2022, 9, 1003620. [Google Scholar] [CrossRef]
- Hadi, A.; Alizadeh, K.; Hajianfar, H.; Mohammadi, H.; Miraghajani, M. Efficacy of synbiotic supplementation in obesity treatment: A systematic review and meta-analysis of clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 584–596. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eslami, M.; Pakmehr, A.; Pourghazi, F.; Kami, A.; Ejtahed, H.-S.; Mohajeri-Tehrani, M.; Hasani-Ranjbar, S.; Larijani, B. The Anti-obesity Effects of Postbiotics: A Systematic Review of Pre-Clinical and Clinical Studies. Clin. Nutr. ESPEN 2024, 64, 370–389. [Google Scholar] [CrossRef]
- Garcia, N.; Gutierrez, E. Anorexia nervosa and microbiota: Systematic review and critical appraisal. Eat. Weight. Disord. 2023, 28, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, J.; Hashimoto, N.; Yin, T.; Sandagdorj, B.; Arakawa, C.; Inoue, T.; Suzuki, S. Heat-killed lactobacillus brevis KB290 Attenuates Visceral Fat Accumulation Induced by High-fat Diet in Mice. J. Appl. Microbiol. 2021, 131, 1998–2009. [Google Scholar] [CrossRef]
- Mudaliar, S.B.; Poojary, S.S.; Prasad, A.S.B.; Mazumder, N. Probiotics and Paraprobiotics: Effects on Microbiota-Gut-Brain Axis and Their Consequent Potential in Neuropsychiatric Therapy. Probiotics Antimicro. Prot. 2024, 16, 1440–1464. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E. Postbiotics as Metabolites and Their Biotherapeutic Potential. Int. J. Mol. Sciences. 2024, 25, 5441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Xin, S.S.; Ding, L.N.; Ding, W.Y.; Hou, Y.L.; Liu, C.Q.; Zhang, X.D. The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2019, 2019, 3862971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhanasekaran, D.; Venkatesan, M.; Sabarathinam, S. Efficacy of Microbiome-Targeted Interventions in Obesity Management-A comprehensive systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2025, 19, 103208. [Google Scholar] [CrossRef]
- Rahman, Z.; Dandekar, M.P. Implication of Paraprobiotics in Age-Associated Gut Dysbiosis and Neurodegenerative Diseases. Neuromol. Med. 2023, 25, 14–26. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Lara, J.; Mathers, J.C. Personalising nutritional guidance for more effective behaviour change. Proc. Nutr. Soc. 2015, 74, 130–138. [Google Scholar] [CrossRef]
- Gibney, M.; Walsh, M.; Goosens, J. Personalized Nutrition: Paving the Way to Better Population Health, in Good Nutrition: Perspectives for the 21st Century; Eggersdorfer, M., Kraemer, K., Cordaro, J.B., Fanzo, J., Gibney, M., Kennedy, E., Labrique, A., Steffen, J., Eds.; Karger Publishers: Basel, Switzerland, 2016; pp. 235–248. [Google Scholar]
- Gibbons, S.M.; Gurry, T.; Lampe, J.W.; Chakrabarti, A.; Dam, V.; Everard, A.; Goas, A.; Gross, G.; Kleerebezem, M.; Lane, J.; et al. Perspective: Leveraging the gut microbiota to predict personalized responses to dietary, prebiotic, and probiotic interventions. Adv. Nutr. 2022, 13, 1450–1461. [Google Scholar] [CrossRef]
- Gurry, T.; Nguyen, L.T.T.; Yu, X.; Alm, E.J. Functional heterogeneity in the fermentation capabilities of the healthy human gut microbiota. PLoS ONE 2021, 16, e0254004. [Google Scholar] [CrossRef]
- Kim, M.; Huda, M.N.; O’Connor, A.; Albright, J.; Durbin-Johnson, B.; Bennett, B.J. Hepatic transcriptional profile reveals the role of diet and genetic backgrounds on metabolic traits in female progenitor strains of the collaborative cross. Physiol. Genom. 2021, 53, 173–192. [Google Scholar] [CrossRef]
- Lancaster, S.M.; Lee-McMullen, B.; Abbott, C.W.; Quijada, J.V.; Hornburg, D.; Park, H.; Perelman, D.; Peterson, D.J.; Tang, M.; Robinson, A.; et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 2022, 30, 848–862.e7. [Google Scholar] [CrossRef]
- Pham, V.T.; Mohajeri, M.H. The application of in vitro human intestinal models on the screening and development of pre- and probiotics. Benef. Microbes 2018, 9, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Casciano, F.; Gianotti, A. Intestinal fermentation in vitro models to study food-induced gut microbiota shift: An updated review. FEMS Microbiol. Lett. 2020, 367, fnaa097. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Gibson, G.R. Carbohydrate fermentation, energy transduction and gas metabolism in the human large intestine. In Gastrointestinal Microbiology, Mackie, R.I., White, B.A., Eds.; Springer: Boston, MA, USA, 1997; pp. 269–318. [Google Scholar]
- Walton, G.E.; van den Heuvel, E.; Kosters, M.H.W.; Rastall, R.A.; Tuohy, K.M.; Gibson, G.R. Arandomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br. J. Nutr. 2012, 107, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Nelis, H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 2010, 83, 89–105. [Google Scholar] [CrossRef]
- Xiang, Y.; Wen, H.; Yu, Y.; Li, M.; Fu, X.; Huang, S. Gut-on-chip: Recreating human intestine in vitro. J. Tissue Eng. 2020, 11, 204173142096531. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.-W.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.-Z.; et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef]
- Christoforidou, Z.; Ortiz, M.M.; Poveda, C.; Abbas, M.; Walton, G.; Bailey, M.; Lewis, M.C. Sexual dimorphism in immune development and in response to nutritional intervention in neonatal piglets. Front. Immunol. 2019, 10, 2705. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or exaggerating causality for the gut microbiome: Lessons from human microbiota-associated rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Petersen, K.; Barger, K.; E Hansen, K.; Anderson, C.A.M.; Baer, D.J.; Lampe, J.W.; Rasmussen, H.; Matthan, N.R. Perspective: Design and conduct of human nutrition randomized controlled trials. Adv. Nutr. 2021, 12, 4–20. [Google Scholar] [CrossRef]
- Kane, P.B.; Bittlinger, M.; Kimmelman, J. Individualized therapy trials: Navigating patient care, research goals and ethics. Nat. Med. 2021, 27, 1679–1686. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Foxx, A.J.; Meléndez, K.P.F.; Hariharan, J.; Kozik, A.J.; Wattenburger, C.J.; Godoy-Vitorino, F.; Rivers, A.R. Advancing equity and inclusion in microbiome research and training. Msystems 2021, 6, e0115121. [Google Scholar] [CrossRef] [PubMed]
- Abdill, R.J.; Adamowicz, E.M.; Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 2022, 20, e3001536. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Heinken, A.; Basile, A.; Hertel, J.; Thinnes, C.; Thiele, I. Genome-scale metabolic modeling of the human microbiome in the era of personalized medicine. Annu. Rev. Microbiol. 2021, 75, 199–222. [Google Scholar] [CrossRef]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and position of the international society of nutrigenetics/nutrigenomics on personalised nutrition: Part 1-fields of precision nutrition. Lifestyle Genom. 2016, 9, 12–27. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef]
- Palmnäs, M.; Brunius, C.; Shi, L.; Rostgaard-Hansen, A.; Torres, N.E.; González-Domínguez, R.; Zamora-Ros, R.; Ye, Y.L.; Halkjær, J.; Tjønneland, A.; et al. Perspective: Metabotyping—A potential personalized nutrition strategy for precision prevention of cardiometabolic disease. Adv. Nutr. 2020, 11, 524–532. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Trevisano, R.G.; Gregnani, M.F.; de Azevedo, B.C.; de Almeida, S.S. The Effect of Association between Fat Mass and Obesity-associated Gene Polymorphism (rs9939609) on the Body Composition of Older People: A Systematic Review. Curr. Aging Sci. 2022, 15, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, G.; Avsec, Ž.; Gagneur, J.; Theis, F.J. Deep learning: New computational modelling techniques for genomics. Nat. Rev. Genet. 2019, 20, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Lagoumintzis, G.; Patrinos, G.P. Triangulating nutrigenomics, metabolomics and microbiomics toward personalized nutrition and healthy living. Hum. Genomics. 2023, 17, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovas, J.M.; Martínez, J.A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Lubomski, M.; Xu, X.; Holmes, A.J.; Muller, S.; Yang, J.Y.; Davis, R.L.; Sue, C.M. Nutritional intake and gut microbiome composition predict Parkinson’s disease. Front. Aging Neurosci. 2022, 10, 881872. [Google Scholar] [CrossRef]
- Canfora, E.E.; Van Der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; DeJong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- O’Grady, J.; Shanahan, F. Macronutrients, microbiome and precision nutrition. Curr. Opin. Gastroenterol. 2021, 37, 145–151. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vila, A.V.; Study, L.C.; Weersma, R.K.; Medema, M.H. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef]
- Mills, S.; Lane, J.A.; Smith, G.J.; Grimaldi, K.A.; Ross, R.P.; Stanton, C. Precision nutrition and the microbiome part II: Potential opportunities and pathways to commercialisation. Nutrients 2019, 11, 1468. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Jalanka, J.; Staudacher, H.M. Can Gut Microbiota Composition Predict Response to Dietary Treatments? Nutrients 2019, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Rollo, M.E.; Williams, R.L.; Burrows, T.; Kirkpatrick, S.I.; Bucher, T.; Collins, C.E. What Are They Really Eating? A Review on New Approaches to Dietary Intake Assessment and Validation. Curr. Nutr. Rep. 2016, 5, 307–314. [Google Scholar] [CrossRef]
- Tebani, A.; Bekri, S. Paving the way to precision nutrition through metabolomics. Front. Nutr. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Djoumbou-Feunang, Y.; Dorrestein, P.C.; O Dragsted, L.; Draper, J.; et al. Perspective: Dietary biomarkers of intake and exposure—Exploration with omics approaches. Adv. Nutr. 2020, 11, 200–215. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Ramos-Lopez, O.; Perusse, L.; Kato, H.; Ordovas, J.M.; Martínez, J.A. Reprint of: Precision nutrition: A review of current approaches and future endeavors. Trends Food Sci. Technol. 2022, 130, 51–62. [Google Scholar] [CrossRef]
- Garcia-Bailo, B.; El-Sohemy, A. Recent advances and current controversies in genetic testing for personalized nutrition. Curr. Opin. Clin. Nutr. Metab. Care. 2021, 24, 289–295. [Google Scholar] [CrossRef]
- McNamara, A.E.; Brennan, L. Potential of food intake biomarkers in nutrition research. Proc. Nutr. Soc. 2020, 79, 487–497. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes. Nutr. 2018, 13, 14. [Google Scholar] [CrossRef]
- Fu, L.; Song, L.; Zhou, X.; Chen, L.; Zheng, L.; Hu, D.; Zhu, S.; Hu, Y.; Gong, D.; Chen, C.L.; et al. Serum metabolomics analysis of malnutrition in patients with gastric cancer: A cross sectional study. BMC Cancer 2024, 24, 1195. [Google Scholar] [CrossRef]
- Santamarina, A.B.; de Freitas, J.A.; Franco, L.A.; Nehmi-Filho, V.; Fonseca, J.V.; Martins, R.C.; Turri, J.A.; da Silva, B.F.; Fugi, B.E.; da Fonseca, S.S.; et al. Nutraceutical blends predict enhanced health via microbiota reshaping improving cytokines and life quality: A Brazilian double-blind randomized trial. Sci. Rep. 2024, 14, 11127. [Google Scholar] [CrossRef]

| Energy Imbalance | Manifestation | Characteristic | Examples | Ref. | |
|---|---|---|---|---|---|
| Overweight/ obesity | Positive | Excessive nutrients intake, accumulation of excess body fat | BMI ≥ 25 for overweight, ≥30 for obesity | Obesity, metabolic syndrome | [5,6] |
| Anorexia nervosa | Negative | Severe restriction of food intake | BMI < 18.5, severe restriction of food intake, extreme weight loss | Anorexia nervosa | [7,8] |
| Undernutrition | Negative | Insufficient intake or absorption of essential nutrients | BMI < 18.5, low body weight, stunted growth, reduced MUAC, weakened immune system | Kwashiorkor, severe acute malnutrition, failure to thrive | [5] |
| GBA Biomarkers | Obesity | Anorexia Nervosa | Undernutrition | Physiological Relevance | Ref. |
|---|---|---|---|---|---|
| SCFAs | ↓ Total SCFAs | ↓ Butyrate; ↓ propionate | ↓ Butyrate; ↓ propionate | Energy source, gut barrier integrity, anti-inflammatory effects, appetite regulation, gut microbiota metabolism | [86,87] |
| Neurotransmitters | ↓ Serotonin | ↓ Serotonin; ↓ dopamine | ↓ Serotonin; ↓ dopamine | Influence mood, appetite, energy expenditure, and reward pathways | [85,88,89] |
| Appetite hormones | ↓ PYY; ↓ GLP-1; ↑ leptin | ↓ Leptin; ↑ ghrelin; ↑ PYY | ↑ ghrelin; ↓ leptin | Appetite and body weight regulation, energy homeostasis regulation | [90,91] |
| Immune markers | ↑ TNF-α; ↑ IL-6; ↑ CRP; ↓ IL-10 | ↑ TNF-α; ↑ IL-1β | ↑ TNF-α | Metabolic regulation; Increased gut permeability indued by pro-inflammatory markers | [88,92] |
| Nutraceuticals | Study Population | Intervention Time and Daily Dose | Model | Main NDs Related Findings | Ref |
|---|---|---|---|---|---|
| Probiotics studies | |||||
| Probiotic: Bifidobacterium pseudocatenulatum CECT 7765 | Obesity | 14 weeks, 1 × 109 CFU/day | Mice | ↓ Weight, ↑ leptin receptor mRNA, ↓ Leptin, ↓ DA, ↓ NE, ↑ 5-HT concentrations in the hypothalamus | [12] |
| Probiotic 1: Lactobacillus sakei OK67, probiotic 2: Lactobacillus sakei PK16 | Obesity | 4 weeks; 2 × 109 CFU/day | Mice | In both treatments: ↓ Firmicutes, ↓ Proteobacteria, ↑ Verrucomicrobia, ↓ delta-Proteobacteria, ↓ Deferribacteres, ↓ weight; ↓ TNF-α; ↓ NF-κB; ↓ anxiety like behaviours | [13] |
| Probiotic: Lactobacillus paracasei HII01 | Obesity | 12 weeks, 1 × 108 CFU/day | Rats | ↓ Weight; ↓ ratio of F/B; ↓ IL-1 mRNA; ↓ IL-6 mRNA | [96] |
| Probiotic 1: Lactobacillus brevis DPC6108 probiotic 2: Lactobacillus brevis DSM32386 | Obesity | 12 weeks, 1 × 1010 CFU/day | Mice | In both treatments: ↓ weight, ↑ faecal flora diversity, ↑ ratio of F/B, ↑ GABA in the small intestine | [97] |
| Mixed probiotic supplementation: Lactobacillus salivarius CUL61, Lactobacillus paracasei CUL08, Bifidobacterium bifidum CUL20, and Bifidobacterium animalis subsp. lactis CUL34 | Obesity | 12 weeks, 5 × 108 CFU/day | Mice | ↓ Weight, ↓ Lactobacilli; ↓ Enterobacteria, ↓ Coliforms↓ Yeast, ↑ Enterococci, ↑ IL-10 mRNA, ↓ IL-18 mRNA | [98] |
| Probiotic: Lactobacillus reuteri MM4–1A | Obesity | 6 weeks, 5 × 109 CFU/day | Mice | ↓ Ratio of F/B, ↓ weight, ↑ TNF-α, ↓ IL-1b, ↓ IL-6 in the hippocampus | [99] |
| Probiotics: Yoghurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus.thermophilus | Patients with AN | 10 weeks, 375 g yoghurt/day | Human | ↑ Interferon-γ, ↑ CD4+/CD8+ ratio, ↑ T lymphocyte subset | [45] |
| Multi-probiotic supplementation: Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium longum, Lactobacillus rhamnosus, Lactobacillus reuteri | Obesity and food addiction | 12 weeks, each strain is 1.8 × 109 CFU including: Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium longum. The 1 × 109 CFU/capsule including Lactobacillus rhamnosus, Lactobacillus reuteri | Human | ↓ Weight, ↓ leptin, ↓ neuropeptide | [46] |
| Probiotic: Bacteroides uniformis CECT 7771 | Food addiction | Rats that fasted 12 h and received a daily dose of 1 × 108 CFU | Rats | The effects of Bacteroides. uniformis on the brain reward response are mediated by changes in the levels of DA, NE and 5-HT in the nucleus accumbens as well as in the expression of dopamine receptors in the prefrontal cortex and intestine. An increase in the OTUs and the phylogenetic diversity | [100] |
| Probiotic: yoghurt containing Lactobacillus. Bulgaris, Streptococcus. thermophilus | Two different situations: (1) Malnourished children; (2) Patients with AN | 10 weeks, 125 g yoghurt/day | Human | In both groups: ↑ Interferon-γ | [101] |
| Probiotic: Lactobacillus reuteri DSM17938 | Patients with AN | 13 weeks, 2 × 108 CFU/day | Human | ↑ Weight, ↑ body mass index | [102] |
| Mixed probiotics supplementation: Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. bulgaricus | Translational activity-based anorexia | 2 days, 1 × 109 CFU/mL | Rats | An increase formation of GALT provided with probiotics supplementation, possibly related to gut microbiome, also contributes to the imbalanced levels of pro-inflammatory and anti-inflammatory cytokines observed in patients with AN. | [103] |
| Mixed probiotics supplementation: Lactobacillus acidophilus, Bifidobacterium longum, and Enterococcus faecalis | Participants on a high fat diet | 4 months, 2 g probiotic powder/day (1.0 × 107 CFU/g) | Human | ↑ Ruminococcaceae and Lachnospiraceae family, ↓ Bacteroidaceae family | [104] |
| Mixed of probiotics strains in fermented milk: Lactobacillus acidophilus CUL60, Lactobacillus acidophilus CUL21, Lactobacillus acidophilus NCFM, Bifidobacterialactis HNO19, Bifidobacteriaanimalis-supsplactis CUL34, and Bifidobacteriabifidum CUL20 | Obesity | 3 months, 100 g/day (One fermented milk cup contained 10 × 109 CFU) | Human | ↓ Weight, ↓ leptin, ↓ SCFA, ↑ Lactobacillus, ↑ Bifidobacteria, ↑ Bacteroidetes, ↓ Firmicutes, ↓ ratio of F/B | [105] |
| Prebiotics studies | |||||
| Prebiotic-supplemented diet containing OFS | Overweight | 13 days (1) 10 g OFS/day or (2) 16 g OFS/day | Human | In both treatments: ↑ PYY, ↑ GLP-1, ↓ energy intake. PYY and GLP-1 levels were significantly lower with 16 g/d OFS compared with 10 g/d OFS. Energy intake was significantly lower with 16 g/d OFS compared with 10 g/d OFS | [14] |
| Prebiotic-supplemented diet: OFS | Overweight and obese adults | 12 weeks, 21g/day | Human | ↓ Body weight, ↓ fat mass, ↓ energy intake, ↓ ghrelin | [15] |
| Prebiotic: OFS-enriched inulin | Overweight or obesity | 16 weeks, 8 g/day | Human | ↓ Weight; ↓ IL-6; ↑ Bifidobacterium spp; ↓ Bacteroides vulgatus | [106] |
| Prebiotic-supplemented diet: chicory-derived fructan | Healthy non-obese adults | 2 weeks, 16 g chicory-derived fructan/day | Human | ↑ PYY, ↑ GLP-1, ↓ hunger | [107] |
| Prebiotic: Inulin | Wild type mice | 14 weeks, 7.5% inulin/day | Mice | PYY was reduced by 87% | [108] |
| Probiotic: Saccharomyces. Boulardii Prebiotic: FOS | Mimic of AN gut condition based on AN patients’ dietary pattern | 16 days; Saccharomyces. Boulardii: 5 × 108 CFU/day; FOS: 1.67 g/day | In vitro gut model system | In Saccharomyces Boulardii treatment: ↑ GABA and 5-HT in proximal, ↑ total bacteria in transverse colon. In FOS treatment: ↑ acetate, Bifidobacterium spp., Roseburia genus and total bacteria in proximal, transverse and distal colon; ↑ butyrate in proximal and distal colon; ↑ propionate, EPI and DA in proximal colon. | [109] |
| Prebiotic treatment: OFS Probiotics treatment: Bifidobacterium animalis subsp. lactis, synbiotic treatment: probiotic (Bifidobacterium animalis subsp. lactis) with prebiotic (OFS) | Rats with high fat diet-induced obese | 8 weeks, prebiotic: 10% (wt/wt) OFS/day, probiotic: 1 × 1010 CFU/day, symbiotic: 10% (wt/wt) OFS with Bifidobacterium animalis subsp. lactis of 1 × 1010 CFU/day | Rats | In OFS treatment: ↑ GLP-1, ↑ PYY, ↓ leptin, ↑ Bacteroides spp., ↑ Lactobacillus spp., ↑ Bifidobacterium spp., ↑ Bifidobacterium. animalis, ↓ C. coccoides, ↓ C. leptum, ↓ Clostridium Cluster XI and I, ↓ Enterobacteriaceae, ↓ the ratio of F/B. In Bifidobacterium animalis subsp. lactis treatment: ↑ GLP-2, ↑ Bifidobacterium.animalis | [16] |
| Synbiotics studies | |||||
| Synbiotic treatment: probiotic (Bifidobacterium animalis subsp. lactis) with prebiotic (polydextrose), probiotic treatment: Bifidobacterium animalis subsp. lactis | Overweight and obese | 6 months, synbiotics: 12 g/day of polydextrose and 1010 CFU of Bifidobacterium animalis subsp. lactis plus, probiotic: 1010 CFU/day | Human | Synbiotics treatment: ↓ weight, ↑ Akkermansia, ↑ Christensenellaceae, ↑ Methanobrevibacter, ↓ Paraprevotella Probiotic treatment: ↑ Lactobacillus, ↑ Akkermansia | [110] |
| Synbiotic: probiotic (Lactobacillus rhamnosus CGMCC1.3724) with prebiotic (OFS and inulin) | Obese | 24 weeks, 1.6 × 108 CFU of Lactobacillus rhamnosus CGMCC1.3724 and 300 mg of a mix of OFS and inulin/day | Human | ↓ Weight; ↓ leptin; ↑ Lachnospiraceae | [17] |
| Synbiotic: mixed probiotic (Lactobacillus acidophilus, Bifidobacterium lactis, Bifidobacterium longum, Bifidobacterium bifidum) with prebiotic (galactooligosaccharide) | Overweight | 3 months, 15 × 109 CFU of mixed strains (Lactobacillus acidophilus DDS-1, Bifidobacterium lactis UABla-12, Bifidobacterium longum UABl-14, and Bifidobacterium bifidum UABb-10) and 5.5 g galactooligosaccharide/day | Human | ↑ Bifidobacterium; ↑ Lactobacillus; ↑ Ruminococcus; ↑ Verrucomicrobiae | [18] |
| Probiotic: Bifidobacterium. infantis EVC001), Synbiotic treatment: probiotic (Bifidobacterium. infantis EVC001) with prebiotic (Lacto-N-neotetraose [LNnT]) | Children with severe acute malnutrition | 4 weeks, probiotic: 8 × 109 CFU/day; Synbiotic: probiotic (8 × 109 CFU) plus 1.6 g prebiotic/day | Human | ↑ Rate of weight gain in probiotic group compared to synbiotic group | [111] |
| Synbiotic: mixed probiotic (Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, Bifidobacterium infantis, Bifidobacterium breve, and Streptococcus thermophilus) with prebiotic (FOS) | Children with FTT | 30 days, synbiotic: probiotic (1 × 109 CFU) plus 1.0 g prebiotic/day | Human | ↑ Weight | [112] |
| Synbiotic: probiotic (Bacillus coagulans) with prebiotic (FOS) | Children with FTT | 6 months, 100 mg FOS and 150 million spore Bacillus coagulans/day | Human | ↑ Weight, ↑ BMI | [113] |
| Postbiotics studies | |||||
| Inulin-propionate ester | Overweight (cultured human colonic cell model) | 24 weeks, 10 g/day | Human | ↓ Weight, ↑ PYY, ↑ GLP-1 | [114] |
| Acetate sodium | Overweight/obese men | 3 days, distal and proximal colon: (100 or 180 mmol/L dissolved in saline 120 mL) | Human | Distal colon: ↑ PYY, ↓ TNF-α; Proximal colon: no significant difference. | [115] |
| Paraprobiotics studies | |||||
| Heat-killed LP28 | Overweight | 12 weeks, 7.5 mL (1011 cells) | Human | ↓Body fat mass, ↓ BMI, ↓ waist circumference, ↓ body fat percentages | [116] |
| Fragmented CP1563 | Overweight and mildly obese | 12 weeks, 200 mg paraprobiotics in a 500 mL beverage | Human | ↓ Body fat percentage, ↓ whole body fat, ↓ visceral fat | [117] |
| Approach | Main Points | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| In vitro | Studies conducted outside a living organism (e.g., cell cultures, gut organoids, or microbiome and colon simulations). Test bioavailability, absorption, and metabolism of nutraceuticals. Study direct effects on gut microbiota and epithelial cells. | No ethical concerns High-throughput screening | Limited relevance to whole organism physiology Cannot fully replicate gut brain axis interactions. | [109,152] |
| In vivo | Studies conducted within a living organism in animal models and human clinical trials. Administer nutraceuticals orally or through diet. Study systemic effects on gut microbiota and gut brain axis biomarkers. | Captures systemic and physiological effects on GBA interactions. More aligned with human biology and directly relevant to human outcomes | Ethical concerns Long term study is expensive and challenging | [153,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Qi, W.; Zhang, N.; Zhang, J.; Liu, S.; Wang, H.; Jiang, L.; Sun, Y. Nutraceuticals for Gut–Brain Axis Health: A Novel Approach to Combat Malnutrition and Future Personalised Nutraceutical Interventions. Nutrients 2025, 17, 1551. https://doi.org/10.3390/nu17091551
Liu L, Qi W, Zhang N, Zhang J, Liu S, Wang H, Jiang L, Sun Y. Nutraceuticals for Gut–Brain Axis Health: A Novel Approach to Combat Malnutrition and Future Personalised Nutraceutical Interventions. Nutrients. 2025; 17(9):1551. https://doi.org/10.3390/nu17091551
Chicago/Turabian StyleLiu, Litai, Wen Qi, Na Zhang, Jinhao Zhang, Shen Liu, Huan Wang, Lianzhou Jiang, and Ying Sun. 2025. "Nutraceuticals for Gut–Brain Axis Health: A Novel Approach to Combat Malnutrition and Future Personalised Nutraceutical Interventions" Nutrients 17, no. 9: 1551. https://doi.org/10.3390/nu17091551
APA StyleLiu, L., Qi, W., Zhang, N., Zhang, J., Liu, S., Wang, H., Jiang, L., & Sun, Y. (2025). Nutraceuticals for Gut–Brain Axis Health: A Novel Approach to Combat Malnutrition and Future Personalised Nutraceutical Interventions. Nutrients, 17(9), 1551. https://doi.org/10.3390/nu17091551






