Clinical Trial: A Mediterranean Low-FODMAP Diet Alleviates Symptoms of Non-Constipation IBS—Randomized Controlled Study and Volatomics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Ethics
2.3. Randomization and Masking
2.4. Intervention Diets
2.5. Exploratory Analysis
SCFA and BCFA Analysis
2.6. Study Outcomes
2.7. Statistical Analyses
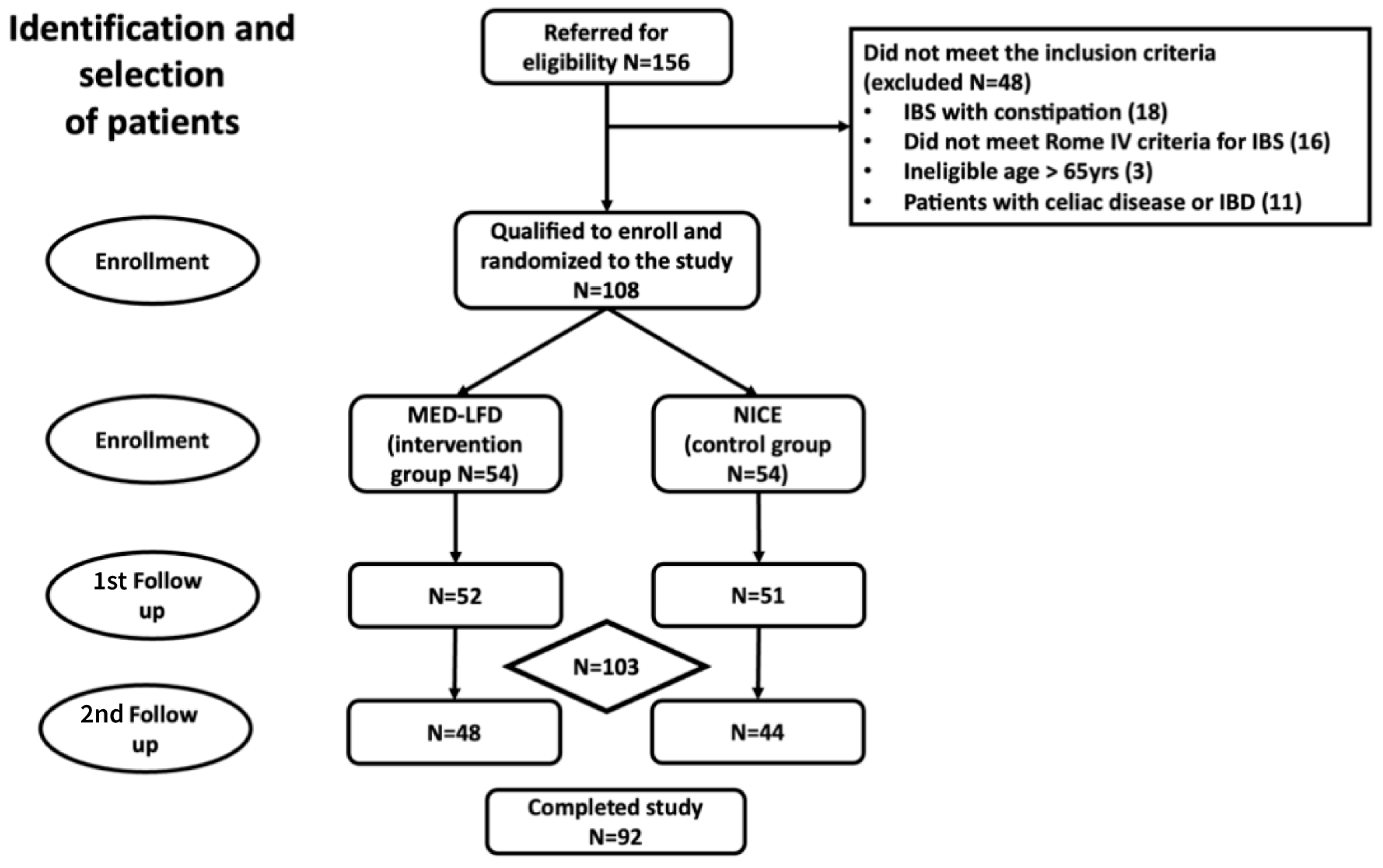
3. Results
3.1. Participants
3.2. Overall and Individual Symptom Relief
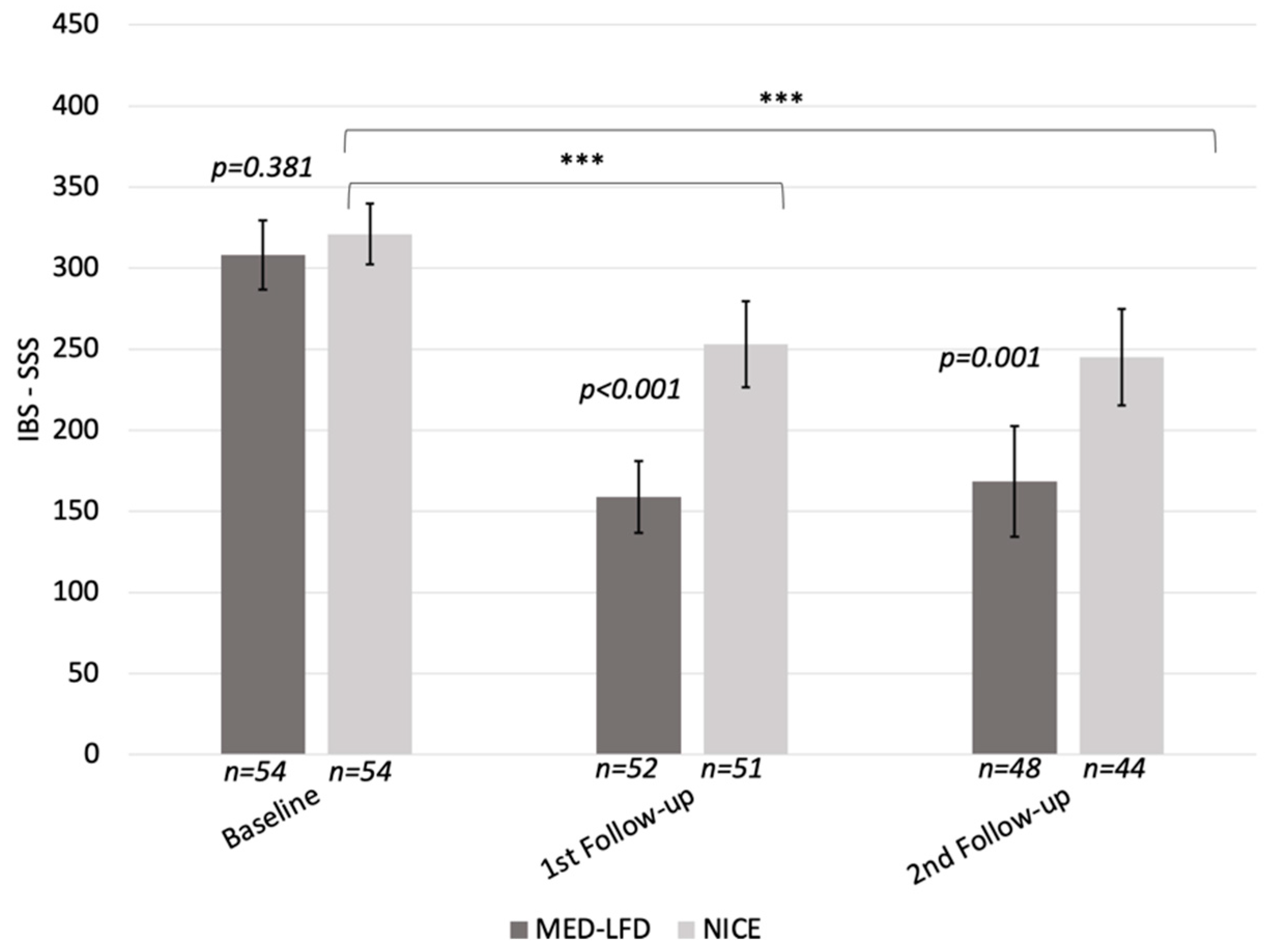
3.2.1. IBS-SSS Change in the PP Population
3.2.2. IBS-SSS Response Rate in the ITT and PP Populations
3.2.3. GSRS-IBS, IBS-QoL, SF-12, HADS Changes in the PP Population
3.2.4. BSFS Changes in the PP Analysis
3.2.5. Adequate Symptom Relief in the ITT and PP Populations
3.3. Adherence to Intervention
3.4. Predictors of Dietary Intervention Response
3.5. Safety
3.6. Exploratory Analysis Results
Stool SCFAs and BCFAs Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| BCFA | Branched Chain Fatty Acids |
| BSFS | Bristol Stool Form Scale |
| FODMAP | Fermentable oligo-, di-, monosaccharides and polyols |
| GC/MS | Gas Chromatography/Mass Spectrometry |
| GSRS-IBS | Gastrointestinal Symptom Rating Scale-IBS version |
| HADS | Hospital Anxiety and Depression Scale |
| HADS-D | The HADS questionnaire measures depression |
| HADS-A | The HADS questionnaire measures anxiety |
| IBS | Irritable Bowel Syndrome |
| IBS-SSS | IBS severity scoring system |
| ITT | Intention-to-treat population |
| QoL | Quality of Life |
| LFD | Low-FODMAP Diet |
| MED | Mediterranean Diet |
| MED–LFD | Mediterranean Diet–Low-FODMAP Diet |
| MCS | Mental Composite Score |
| NICE | National Institute for Health and Care Excellence |
| PCS | Physical Composite Score |
| PP | Per Protocol |
| SAE | Serious Adverse Event |
| SF12 | 12-Item Short Form Survey |
| SCFAs | Short-chain Fatty Acids |
References
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.-S. The Role of Inflammation in Irritable Bowel Syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Mahurkar-Joshi, S.; Liu, C.; Jaffe, N.; Labus, J.S.; Dong, T.S.; Gupta, A.; Patel, S.; Mayer, E.A.; Chang, L. The Association Between a Mediterranean Diet and Symptoms of Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2024, 22, 164–172.e6. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Shimbori, C.; Reed, D.E.; Yu, Y.; Rabbia, V.; Lu, J.; Jimenez-Vargas, N.; Sessenwein, J.; Lopez-Lopez, C.; Pigrau, M.; et al. Histamine Production by the Gut Microbiota Induces Visceral Hyperalgesia through Histamine 4 Receptor Signaling in Mice. Sci. Transl. Med. 2022, 14, eabj1895. [Google Scholar] [CrossRef] [PubMed]
- Monash University Understanding the Traffic Lights in the Monash FODMAP Diet App. Available online: http://www.monashfodmap.com/blog/traffic-light-system/ (accessed on 13 December 2024).
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol. Res. 2017, 38, 163–171. [Google Scholar]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated with More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Schnabel, L.; Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Monteiro, C.A.; Hercberg, S.; Benamouzig, R.; et al. Association Between Ultra-Processed Food Consumption and Functional Gastrointestinal Disorders: Results from the French NutriNet-Santé Cohort. Off. J. Am. Coll. Gastroenterol. ACG 2018, 113, 1217–1228. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and Histamine Intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota Regulates Visceral Pain in the Mouse. eLife 2017, 6, e25887. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, J.; Zhu, S.; Xin, L.; Yu, C.; Shen, Z. The Role of Short Chain Fatty Acids in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Nikolaki, M.D.; Kasti, A.N.; Katsas, K.; Petsis, K.; Lambrinou, S.; Patsalidou, V.; Stamatopoulou, S.; Karlatira, K.; Kapolos, J.; Papadimitriou, K.; et al. The Low-FODMAP Diet, IBS, and BCFAs: Exploring the Positive, Negative, and Less Desirable Aspects—A Literature Review. Microorganisms 2023, 11, 2387. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.G.; Gibson, P.R. The Low FODMAP Diet for Treatment of Irritable Bowel Syndrome and Other Gastrointestinal Disorders. Gastroenterol. Hepatol. 2013, 9, 450–452. [Google Scholar]
- Tuck, C.J.; Reed, D.E.; Muir, J.G.; Vanner, S.J. Implementation of the Low FODMAP Diet in Functional Gastrointestinal Symptoms: A Real-World Experience. Neurogastroenterol. Motil. 2020, 32, e13730. [Google Scholar] [CrossRef]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a Low FODMAP Diet in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef]
- O’Brien, L.; Kasti, A.; Halmos, E.P.; Tuck, C.; Varney, J. Evolution, Adaptation, and New Applications of the FODMAP Diet. JGH Open 2024, 8, e13066. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. The Effect of a Mediterranean Diet on the Symptoms of Depression in Young Males (the “AMMEND: A Mediterranean Diet in MEN with Depression” Study): A Randomized Controlled Trial. Am. J. Clin. Nutr. 2022, 116, 572–580. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Zito, F.P.; Polese, B.; Vozzella, L.; Gala, A.; Genovese, D.; Verlezza, V.; Medugno, F.; Santini, A.; Barrea, L.; Cargiolli, M.; et al. Good Adherence to Mediterranean Diet Can Prevent Gastrointestinal Symptoms: A Survey from Southern Italy. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 564–571. [Google Scholar] [CrossRef]
- Altomare, A.; Del Chierico, F.; Rocchi, G.; Emerenziani, S.; Nuglio, C.; Putignani, L.; Angeletti, S.; Lo Presti, A.; Ciccozzi, M.; Russo, A.; et al. Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study. Nutrients 2021, 13, 1479. [Google Scholar] [CrossRef]
- Li, L.; Ran, Y.; Zhuang, Y.; Xu, Y.; Wang, L.; Chen, L.; Sun, Y.; Ye, F.; Mei, L.; Dai, F. Proinflammatory Diet Increases the Risk of Irritable Bowel Syndrome: A Prospective Study of 129,408 UK Biobank Participants and Mendelian Randomization Analysis. Dig. Dis. Sci. 2024, 69, 4140–4151. [Google Scholar] [CrossRef] [PubMed]
- Kasti, A.; Petsis, K.; Lambrinou, S.; Katsas, K.; Nikolaki, M.; Papanikolaou, I.S.; Hatziagelaki, E.; Triantafyllou, K. A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut! Microorganisms 2022, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- NICE Irritable Bowel Syndrome in Adults: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/cg61/chapter/Recommendations#dietary-and-lifestyle-advice (accessed on 26 September 2024).
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The Irritable Bowel Severity Scoring System: A Simple Method of Monitoring Irritable Bowel Syndrome and Its Progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Kasti, A.N.; Katsas, K.; Petsis, K.; Lambrinou, S.; Synodinou, K.D.; Kapetani, A.; Smart, K.L.; Nikolaki, M.D.; Halvatsiotis, P.; Triantafyllou, K.; et al. Is the Mediterranean Low Fodmap Diet Effective in Managing Irritable Bowel Syndrome Symptoms and Gut Microbiota? An Innovative Research Protocol. Nutrients 2024, 16, 1592. [Google Scholar] [CrossRef]
- Schindler, D.; Uschner, D.; Razsolkov, D.; Mihaylov, D.; Rueckbeil, M.V.; Manolov, M.; Pham, T.M.; Martini, M.; Hilgers, R.-D.; Heussen, N.; et al. randomizeR: Randomization for Clinical Trials; CRAN: Vienna, Austria, 2023. [Google Scholar]
- Zanzer, Y.C.; Theis, S. Systematic Review and Meta-Analysis of Habitual Intake of Fermentable Oligo-, Di-, Mono- Saccharides and Polyols in the General Population and Revisiting the Low FODMAP Diet Concept. J. Funct. Foods 2024, 112, 105914. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets That Differ in Their FODMAP Content Alter the Colonic Luminal Microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 53–61. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Sultan, N.; Varney, J.E.; Halmos, E.P.; Biesiekierski, J.R.; Yao, C.K.; Muir, J.G.; Gibson, P.R.; Tuck, C.J. How to Implement the 3-Phase FODMAP Diet into Gastroenterological Practice. J. Neurogastroenterol. Motil. 2022, 28, 343–356. [Google Scholar] [CrossRef]
- Monash University Online FODMAP and IBS Training for Dietitians. Available online: https://www.monashfodmap.com/online-training/dietitian-course/ (accessed on 13 December 2024).
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Maagaard, L.; Ankersen, D.V.; Végh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of Patients with Functional Bowel Symptoms Treated with a Low FODMAP Diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Jiang, Z.; Ma, J.; Yang, D. Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1727. [Google Scholar] [CrossRef]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding Nutritional Epidemiology and Its Role in Policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile Organic Compounds in Feces Associate with Response to Dietary Intervention in Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391.e1. [Google Scholar] [CrossRef]
- Monash University Fodmap Calculator. Available online: https://www.monashfodmapcalculator.com.au/ (accessed on 13 December 2024).
- Zhang, S.; Wang, H.; Zhu, M.-J. A Sensitive GC/MS Detection Method for Analyzing Microbial Metabolites Short Chain Fatty Acids in Fecal and Serum Samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Dimzas, N.; Argyriou, K.; Zachou, M.; Kasti, A.; Petsis, K.; Lambrinou, S.; Tsolaki, A.; Potamianos, P.S.; Kapsoritakis, A. Translation, Validation, and First Application of the Greek Version of an Irritable Bowel Syndrome Severity Scoring System. Ann. Gastroenterol. 2024, 37, 182–190. [Google Scholar] [CrossRef]
- Wiklund, I.K.; Fullerton, S.; Hawkey, C.J.; Jones, R.H.; Longstreth, G.F.; Mayer, E.A.; Peacock, R.A.; Wilson, I.K.; Naesdal, J. An Irritable Bowel Syndrome-Specific Symptom Questionnaire: Development and Validation. Scand. J. Gastroenterol. 2003, 38, 947–954. [Google Scholar] [CrossRef]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; DiCesare, J.; Puder, K.L. Quality of Life in Persons with Irritable Bowel Syndrome: Development and Validation of a New Measure. Dig. Dis. Sci. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Michopoulos, I.; Douzenis, A.; Kalkavoura, C.; Christodoulou, C.; Michalopoulou, P.; Kalemi, G.; Fineti, K.; Patapis, P.; Protopapas, K.; Lykouras, L. Hospital Anxiety and Depression Scale (HADS): Validation in a Greek General Hospital Sample. Ann. Gen. Psychiatry 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Mangel, A.W.; Hahn, B.A.; Heath, A.T.; Northcutt, A.R.; Kong, S.; Dukes, G.E.; McSorley, D. Adequate Relief as an Endpoint in Clinical Trials in Irritable Bowel Syndrome. J. Int. Med. Res. 1998, 26, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.C.F.; Lembo, A.J.; Conboy, L.A.; Kaptchuk, T.J.; Kelly, J.M.; Quilty, M.T.; Kerr, C.E.; Jacobson, E.E.; Hu, R.; Friedlander, E.; et al. Adequate Relief in a Treatment Trial with IBS Patients: A Prospective Assessment. Am. J. Gastroenterol. 2009, 104, 912–919. [Google Scholar] [CrossRef]
- Varjú, P.; Farkas, N.; Hegyi, P.; Garami, A.; Szabó, I.; Illés, A.; Solymár, M.; Vincze, Á.; Balaskó, M.; Pár, G.; et al. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols (FODMAP) Diet Improves Symptoms in Adults Suffering from Irritable Bowel Syndrome (IBS) Compared to Standard IBS Diet: A Meta-Analysis of Clinical Studies. PLoS ONE 2017, 12, e0182942. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Mumolo, M.G.; Bronzini, F.; Pancetti, A.; Bertani, L.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Marchi, S.; et al. Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome? Nutrients 2020, 12, 3368. [Google Scholar] [CrossRef]
- Staudacher, H.M. Nutritional, Microbiological and Psychosocial Implications of the Low FODMAP Diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 16–19. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Mahoney, S.; Canale, K.; Opie, R.S.; Loughman, A.; So, D.; Beswick, L.; Hair, C.; Jacka, F.N. Clinical Trial: A Mediterranean Diet Is Feasible and Improves Gastrointestinal and Psychological Symptoms in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2024, 59, 492–503. [Google Scholar] [CrossRef]
- Paduano, D.; Cingolani, A.; Tanda, E.; Usai, P. Effect of Three Diets (Low-FODMAP, Gluten-Free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life. Nutrients 2019, 11, 1566. [Google Scholar] [CrossRef]
- Khalighi Sikaroudi, M.; Soltani, S.; Ghoreishy, S.M.; Ebrahimi, Z.; Shidfar, F.; Dehnad, A. Effects of a Low FODMAP Diet on the Symptom Management of Patients with Irritable Bowel Syndrome: A Systematic Umbrella Review with the Meta-Analysis of Clinical Trials. Food Funct. 2024, 15, 5195–5208. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Ranjbar, M.; Mohammadi, H.; Adibi, P. Nutritional Interventions in Adult Patients with Irritable Bowel Syndrome: An Umbrella Review of Systematic Reviews and Meta-Analyses of Randomized Clinical Trials. Nutr. Rev. 2024, 83, e1343–e1354. [Google Scholar] [CrossRef]
- Rej, A.; Sanders, D.S.; Shaw, C.C.; Buckle, R.; Trott, N.; Agrawal, A.; Aziz, I. Efficacy and Acceptability of Dietary Therapies in Non-Constipated Irritable Bowel Syndrome: A Randomized Trial of Traditional Dietary Advice, the Low FODMAP Diet, and the Gluten-Free Diet. Clin. Gastroenterol. Hepatol. 2022, 20, 2876–2887.e15. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Irving, P.M.; Lomer, M.C.; Whelan, K. β-Galactooligosaccharide in Conjunction with Low FODMAP Diet Improves Irritable Bowel Syndrome Symptoms but Reduces Fecal Bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Goyal, O.; Batta, S.; Nohria, S.; Kishore, H.; Goyal, P.; Sehgal, R.; Sood, A. Low Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Prospective, Randomized Trial. J. Gastroenterol. Hepatol. 2021, 36, 2107–2115. [Google Scholar] [CrossRef]
- Zahedi, M.J.; Behrouz, V.; Azimi, M. Low Fermentable Oligo-Di-Mono-Saccharides and Polyols Diet versus General Dietary Advice in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Trial. J. Gastroenterol. Hepatol. 2018, 33, 1192–1199. [Google Scholar] [CrossRef]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-Term Irritable Bowel Syndrome Symptom Control with Reintroduction of Selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef]
- Natalia Pedersen, N.N.A.; Gh, L.J. Ehealth: Low FODMAP Diet vs Lactobacillus Rhamnosus GG in Irritable Bowel Syndrome. World J. Gastroenterol. 2014, 20, 16215–16226. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Bertin, L.; Zanconato, M.; Crepaldi, M.; Marasco, G.; Cremon, C.; Barbara, G.; Barberio, B.; Zingone, F.; Savarino, E.V. The Role of the FODMAP Diet in IBS. Nutrients 2024, 16, 370. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic Review with Meta-Analysis: The Prevalence of Anxiety and Depression in Patients with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef]
- van Lanen, A.-S.; de Bree, A.; Greyling, A. Efficacy of a Low-FODMAP Diet in Adult Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 3505–3522. [Google Scholar] [CrossRef]
- Stalder, E. The Effects of a Low FODMAP Diet on People with Irritable Bowel Syndrome: A Literature Review. The Eleanor Mann School of Nursing Undergraduate Honors Theses. Bachelor’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2022. [Google Scholar]
- Kortlever, T.L.; Ten Bokkel Huinink, S.; Offereins, M.; Hebblethwaite, C.; O’Brien, L.; Leeper, J.; Mulder, C.J.J.; Barrett, J.S.; Gearry, R.B. Low-FODMAP Diet Is Associated with Improved Quality of Life in IBS Patients-A Prospective Observational Study. Nutr. Clin. Pract. 2019, 34, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.; Schantz Laursen, B.; Drewes, A.M.; Krarup, A.L. The Incidence of Sexual Dysfunction in Patients with Irritable Bowel Syndrome. Sex. Med. 2019, 7, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.; Morris, C.B.; Hu, Y.; Toner, B.B.; Diamant, N.; Whitehead, W.E.; Dalton, C.B.; Leserman, J.; Patrick, D.L.; Bangdiwala, S.I. Characterization of Health Related Quality of Life (HRQOL) for Patients with Functional Bowel Disorder (FBD) and Its Response to Treatment. Am. J. Gastroenterol. 2007, 102, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef]
- Kim, D.; Konyn, P.; Cholankeril, G.; Ahmed, A. Physical Activity Is Associated with Nonalcoholic Fatty Liver Disease and Significant Fibrosis Measured by FibroScan. Clin. Gastroenterol. Hepatol. 2022, 20, e1438–e1455. [Google Scholar] [CrossRef]
- So, D.; Gibson, P.R.; Muir, J.G.; Yao, C.K. Dietary Fibres and IBS: Translating Functional Characteristics to Clinical Value in the Era of Personalised Medicine. Gut 2021, 70, 2383–2394. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-Style Dietary Intervention Supplemented with Fish Oil Improves Diet Quality and Mental Health in People with Depression: A Randomized Controlled Trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Bizzozero-Peroni, B.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Jiménez-López, E.; Núñez de Arenas-Arroyo, S.; Saz-Lara, A.; Díaz-Goñi, V.; Mesas, A.E. The Impact of the Mediterranean Diet on Alleviating Depressive Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2024, 83, 29–39. [Google Scholar] [CrossRef]
- Bouayed, J. Polyphenols: A Potential New Strategy for the Prevention and Treatment of Anxiety and Depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Salvatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and Its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef]
- Altun, A.; Brown, H.; Szoeke, C.; Goodwill, A. The Mediterranean Dietary Pattern and Depression Risk: A Systematic Review. Neurol. Psychiatry Brain Res. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.H.; Zarate-Lopez, N.; Martin, L. Group Education on the Low FODMAP Diet Improves Gastrointestinal Symptoms but Neither Anxiety or Depression in Irritable Bowel Syndrome. J. Hum. Nutr. Diet. 2022, 35, 425–434. [Google Scholar] [CrossRef]
- Nájera-Maldonado, J.M.; Salazar, R.; Alvarez-Fitz, P.; Acevedo-Quiroz, M.; Flores-Alfaro, E.; Hernández-Sotelo, D.; Espinoza-Rojo, M.; Ramírez, M. Phenolic Compounds of Therapeutic Interest in Neuroprotection. J. Xenobiot. 2024, 14, 227–246. [Google Scholar] [CrossRef]
- Conley, T.E.; Slater, R.; Moss, S.; Bulmer, D.C.; de la Negro, J.R.; Ijaz, U.Z.; Pritchard, D.M.; Parkes, M.; Probert, C. Microbiome-Driven IBS Metabotypes Influence Response to the Low FODMAP Diet: Insights from the Faecal Volatome. eBioMedicine 2024, 107, 105282. [Google Scholar] [CrossRef]
- Zielińska, M.; Łuszczki, E.; Michońska, I.; Dereń, K. The Mediterranean Diet and the Western Diet in Adolescent Depression-Current Reports. Nutrients 2022, 14, 4390. [Google Scholar] [CrossRef]
- Zinkow, A.; Grodzicki, W.; Czerwińska, M.; Dziendzikowska, K. Molecular Mechanisms Linking Omega-3 Fatty Acids and the Gut–Brain Axis. Molecules 2025, 30, 71. [Google Scholar] [CrossRef]
- Clevers, E.; Tran, M.; Van Oudenhove, L.; Störsrud, S.; Böhn, L.; Törnblom, H.; Simrén, M. Adherence to Diet Low in Fermentable Carbohydrates and Traditional Diet for Irritable Bowel Syndrome. Nutrition 2020, 73, 110719. [Google Scholar] [CrossRef]
- Rej, A.; Avery, A.; Aziz, I.; Black, C.J.; Bowyer, R.K.; Buckle, R.L.; Seamark, L.; Shaw, C.C.; Thompson, J.; Trott, N.; et al. Diet and Irritable Bowel Syndrome: An Update from a UK Consensus Meeting. BMC Med. 2022, 20, 287. [Google Scholar] [CrossRef]
- Porcari, S.; Mullish, B.H.; Asnicar, F.; Ng, S.C.; Zhao, L.; Hansen, R.; O’Toole, P.W.; Raes, J.; Hold, G.; Putignani, L.; et al. International Consensus Statement on Microbiome Testing in Clinical Practice. Lancet Gastroenterol. Hepatol. 2024, 10, 154–167. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, L.; Wang, X.; Fox, M.; Luo, L.; Du, L.; Chen, B.; Chen, X.; He, H.; Zhu, S.; et al. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet Compared with Traditional Dietary Advice for Diarrhea-Predominant Irritable Bowel Syndrome: A Parallel-Group, Randomized Controlled Trial with Analysis of Clinical and Microbiological Factors Associated with Patient Outcomes. Am. J. Clin. Nutr. 2021, 113, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- De La Cuesta-Zuluaga, J.; Mueller, N.; Álvarez-Quintero, R.; Velásquez-Mejía, E.; Sierra, J.; Corrales-Agudelo, V.; Carmona, J.; Abad, J.; Escobar, J. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The Challenges of Control Groups, Placebos and Blinding in Clinical Trials of Dietary Interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Serra, J.; Fernandez-Bañares, F.; Mearin, F. The Low-FODMAP Diet for Irritable Bowel Syndrome: Lights and Shadows. Gastroenterol. Hepatol. 2016, 39, 55–65. [Google Scholar] [CrossRef]
- Krauss, A. Assessing the Overall Validity of Randomised Controlled Trials. Int. Stud. Philos. Sci. 2021, 34, 159–182. [Google Scholar] [CrossRef]
- Bardacke, J.A.; Yarrow, L.; Rosenkranz, S.K. The Long-Term Effects of a Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet for Irritable Bowel Syndrome Management. Curr. Dev. Nutr. 2023, 7, 101997. [Google Scholar] [CrossRef]
- ΝΙH. 24-Hour Dietary Recall (24HR) At a Glance | Dietary Assessment Primer. 2024. Available online: https://dietassessmentprimer.cancer.gov/profiles/recall/ (accessed on 24 December 2024).
- Hoving, L.R.; Heijink, M.; van Harmelen, V.; van Dijk, K.W.; Giera, M. GC-MS Analysis of Short-Chain Fatty Acids in Feces, Cecum Content, and Blood Samples. Methods Mol. Biol. 2018, 1730, 247–256. [Google Scholar] [CrossRef]
- Clench, M.R.; Tetler, L.W. CHROMATOGRAPHY: GAS | Detectors: Mass Spectrometry. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 448–455. [Google Scholar] [CrossRef]
- Shimadzu. LabSolutions Series. 2024. Available online: https://www.shimadzu.com/an/products/software-informatics/labsolutions-series/index.html (accessed on 24 December 2024).
- SIS. NIST/EPA/NIH MS/MS Mass Spectral Library 2014. 2014. Available online: https://www.sisweb.com/software/nist-msms-2014.htm (accessed on 24 December 2024).
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; E MacDonald, P.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Rawlings, J.O.; Pantula, S.G.; Dickey, D.A. (Eds.) Polynomial Regression. In Applied Regression Analysis: A Research Tool; Springer: Berlin/Heidelberg, Germany, 1998; pp. 235–268. [Google Scholar] [CrossRef]

| Mediterranean Pyramid [32] | Servings | Med–LFD | Servings | |
|---|---|---|---|---|
| Consumption in each main meal | Bread, pasta, rice, other cereals | 1–2 | Bread, pasta, rice, other cereals | Not in every meal (see daily consumption below) |
| Fruits | 1–2 | Fruits | ||
| Vegetables | ≥2 | Vegetables | ||
| Daily consumption | Olive oil | The major source of fat | Olive oil | 6 |
| Milk and Dairy products | 2 | Milk and Dairy products | 3 | |
| Nuts/seeds | 1–2 | Nuts | 3 | |
| Herbs, spices | Non defined | Herbs, spices | Moderate consumption | |
| Garlic, onions | Non defined | - | - | |
| Bread, pasta, rice, other cereals | 4–8 | Bread, pasta, rice, other cereals | 8 * | |
| Fruits | 4–8 | Fruits | 4 * | |
| Vegetables | ≥8 | Vegetables | 4 * | |
| Weekly consumption | Meat | 2 | Meat | 2 |
| Eggs | 2 | Eggs | 2 | |
| Fish | 2 | Fish | 4 | |
| Poultry | 2 | Poultry | 2 | |
| Wine | Moderate consumption | Wine | Moderate consumption ** | |
| Legumes | 2–3 | - | - | |
| Potatoes | ≤3 | Potatoes | 8 | |
| Sweets | ≤2 | - | - |
| Total | MED–LFD (n = 54) | NICE (n = 54) | p | |
|---|---|---|---|---|
| Age (years), Mean (SD) | 40 (11) | 39 (11) | 41 (12) | 0.358 |
| Females, n (%) | 84 (77.8) | 43 (79.6) | 41 (75.9) | 0.643 |
| Educational level, n (%) | ||||
| Secondary | 31 (28.7) | 11 (20.4) | 20 (37) | 0.056 |
| Tertiary | 77 (71.3) | 43 (79.6) | 34 (63) | |
| Marital status, n (%) | ||||
| Married | 47 (43.5) | 18 (33.3) | 29 (53.7) | 0.066 |
| Unmarried | 48 (44.4) | 26 (48.2) | 22 (40.7) | |
| Divorced | 11 (10.2) | 8 (14.8) | 3 (5.6) | |
| Widowed | 2.1 (1.9) | 2 (3.7) | 0 (0) | |
| Income status, n (%) | ||||
| <500 € | 37 (34.3) | 18 (33.4) | 19 (35.2) | |
| 500–1000 € | 40 (37) | 20 (37) | 20 (37) | 0.971 |
| >1000 € | 31 (28.7) | 16 (29.6) | 15 (27.8) | |
| Smoking habits, n (%) † | ||||
| Current smoker | 31 (32.0) | 12 (24.5) | 19 (39.6) | |
| Former smoker | 23 (23.7) | 11 (22.5) | 12 (25.0) | 0.174 |
| Non-smoker | 43 (44.3) | 26 (53.0) | 17 (35.4) | |
| Body Mass Index, n (%) | ||||
| Underweight | 4 (3.7) | 1 (1.9) | 3 (5.6) | 0.535 |
| Normal | 46 (42.6) | 21 (38.9) | 25 (46.3) | |
| Overweight | 36 (33.3) | 19 (35.2) | 17 (31.5) | |
| Obese | 22 (20.4) | 13 (24.1) | 9 (16.7) | |
| IBS-SSS, Mean (SD) | 314 (74) | 308 (79) | 321 (70) | 0.381 |
| Baseline Score | Difference from Baseline at First Follow-Up | Difference from Baseline at Second Follow-Up) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MED–LFD, n = 54 | NICE, n = 54 | p | MED–LFD, n = 52 | NICE, n = 51 | p | MED–LFD, n = 48 | NICE, n = 44 | p | |
| GSRS-IBS total score, median (IQR) | 4.02 (0.94) | 3.95 (0.82) | 0.683 | −1.67 (0.99) ** | −0.64 (0.95) ** | <0.001 | −1.53 (1.32) ** | −0.5 (1.22) * | <0.001 |
| Pain subscale | 4.25 [3.50, 5.50] | 4.00 [3.50, 6.00] | 0.737 | −2.17 (1.35) ** | −0.96 (1.60) ** | <0.001 | −1.82 (1.64) ** | −0.63 (1.76) * | 0.001 |
| Bloating subscale | 5.17 [3.67, 6.00] | 4.67 [4.00, 6.00] | 0.763 | −2.01 (1.39) ** | −0.85 (1.45) ** | <0.001 | −1.82 (1.77) ** | −0.63 (1.56) * | 0.001 |
| Constipation subscale | 2.50 [1.00, 4.00] | 2.00 [1.00, 3.50] | 0.422 | −0.48 (1.56) * | −0.16 (1.85) | 0.338 | −0.65 (1.66) * | −0.65 (1.76) * | 0.996 |
| Diarrhoea subscale | 4.38 [3.00, 5.25] | 4.50 [2.75, 5.25] | 0.812 | −1.97 (1.62) ** | −0.82 (1.58) ** | <0.001 | −1.76 (1.96) ** | −0.47 (1.77) | 0.001 |
| Satiety subscale | 3.75 [3.00, 5.00] | 3.50 [2.50, 4.50] | 0.356 | −1.23 (1.51) ** | −0.28 (1.58) | 0.003 | −1.21 (1.85) ** | −0.06 (1.49) | 0.002 |
| IBS-QoL total score, median (IQR) | 47.5 (20.6) | 49.4 (21.5) | 0.649 | +24.2 (20.8) ** | +8.9 (12.9) ** | <0.001 | +26.5 (21.1) ** | +11.6 (17.4) ** | <0.001 |
| Dysphoria subscale | 40.6 [21.9, 60.9] | 42.2 [18.8, 62.5] | 0.981 | +30.0 (24.8) ** | +13.9 (20.0) ** | <0.001 | +32.4 (26.4) ** | +16.9 (23) ** | 0.004 |
| Interference with Activity subscale | 35.7 [25, 71.4] | 44.6 [28.6, 60.7] | 0.629 | +27.3 (25.0) ** | +10.1 (15.7) ** | <0.001 | +29.4 (25.6) ** | +10.9 (21) * | <0.001 |
| Body Image subscale | 56.3 [37.5, 68.8] | 62.5 [43.8, 81.3] | 0.102 | +22.6 (25.8) ** | +5.0 (14.5) * | <0.001 | +21.4 (28.1) ** | +7.4 (20.2) * | 0.009 |
| Health Worry subscale | 54.2 [33.3, 66.7] | 58.3 [41.7, 66.7] | 0.721 | +24.5 (23.4) ** | +8.5 (17.8) * | <0.001 | +27.8 (23.9) ** | +10.1 (20.9) * | <0.001 |
| Food Avoidance subscale | 25 [8.3, 41.7] | 25 [16.7, 41.7] | 0.769 | +10.9 (31.1) * | +5.7 (16.7) * | 0.295 | +14.4 (29.7) * | +4.7 (24.5) | 0.093 |
| Social Reaction subscale | 56.3 [34.4, 71.9] | 56.3 [43.8, 75] | 0.391 | +24.4 (23.7) ** | +6.3 (17.5) * | <0.001 | +29 (22.7) ** | +13.4 (20.7) ** | <0.001 |
| Sexual subscale | 50 [25, 75] | 62.5 [37.5, 75] | 0.769 | +20.9 (30.2) ** | +8.5 (20.3) ** | 0.017 | +20.8 (27.2) ** | +15.4 (29.1) * | 0.361 |
| Relationships subscale | 62.5 [41.7, 79.2] | 66.7 [41.7, 83.3] | 0.773 | +18.4 (23.8) ** | +5.5 (16.8) * | 0.002 | +21.7 (24.5) ** | +8.5 (25.1) * | 0.013 |
| HADS, mean (SD) | |||||||||
| Depression scale | 8.10 (4.68) | 8.59 (3.82) | 0.565 | −2.88 (4.71) ** | −1.18 (3.01) * | 0.035 | −3 (4.29) ** | −0.64 (3.61) | 0.007 |
| Anxiety scale | 8.94 (4.64) | 9.45 (4.67) | 0.592 | −2.18 (4.64) * | −0.83 (2.93) | 0.092 | −3.08 (4.78) ** | −0.98 (4.38) | 0.034 |
| SF-12 total score, mean (SD) | 40.2 (7.9) | 39.3 (7.0) | 0.559 | +6.39 (7.97) ** | +4.01 (5.78) ** | 0.089 | +8.66 (8.23) ** | +3.3 (6.61) * | 0.001 |
| Physical component | 43.4 (8.6) | 41.6 (9.3) | 0.319 | +5.54 (8.29) ** | +4.73 (8.04) ** | 0.619 | +6.65 (6.4) ** | +1.34 (8.37) | 0.001 |
| Mental component | 37.0 (12.0) | 37.0 (9.9) | 0.990 | +7.24 (11.8) ** | +3.29 (10.5) * | 0.077 | +10.67 (13.64) ** | +5.26 (11.71) * | 0.047 |
| Baseline | First Follow-Up | Second Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MED–LFD | NICE | p | MED–LFD | NICE | p | MED–LFD | NICE | p | |
| Patients, n | 54 | 54 | - | 52 | 51 | - | 48 | 44 | - |
| BSFS type, % | |||||||||
| 1–2 | 0.0 | 0.0 | 5.77 | 11.76 | 8.33 | 4.55 | |||
| 3–5 | 59.26 | 47.17 | 0.210 | 84.62 | 60.78 | 0.023 | 81.25 | 61.36 | 0.021 |
| 6–7 | 40.74 | 52.83 | 9.62 | 27.45 | 10.42 | 34.09 | |||
| First Follow-Up | Second Follow-Up | |||
|---|---|---|---|---|
| Variables | Univariate OR (95% CI) | Multivariate OR (95% CI) | Univariate OR (95% CI) | Multivariate OR (95% CI) |
| Intervention (MED–LFD) | 3.27 (1.36, 7.82) * | 6.66 (1.46, 30.4) * | 3.17 (1.44, 6.98) * | 4.85 (1.31, 17.96) * |
| Age (years) | 0.98 (0.95, 1.02) | 0.96 (0.89, 1.05) | 1.00 (0.96, 1.03) | 1.03 (0.95, 1.11) |
| Gender (female) | 1.89 (0.74, 4.86) | 1.38 (0.28, 6.76) | 0.52 (0.20, 1.36) | 0.46 (0.09, 2.30) |
| Smoking | ||||
| Smoker | Reference | Reference | Reference | Reference |
| Ex-smoker | 0.38 (0.12, 1.23) | 0.29 (0.04, 2.04) | 0.60 (0.20, 1.80) | 0.44 (0.08, 2.37) |
| Never smoked | 0.67 (0.23, 1.95) | 0.38 (0.06, 2.50) | 1.58 (0.62, 4.04) | 0.87 (0.19, 3.96) |
| Education level (University) | 1.12 (0.46, 2.74) | 1.82 (0.33, 9.97) | 1.42 (0.62, 3.28) | 1.08 (0.21, 5.65) |
| Monthly income | ||||
| <500 €/month | Reference | Reference | Reference | Reference |
| 500–1000 €/month | 0.86 (0.32, 2.33) | 3.89 (0.64, 23.67) | 0.50 (0.20, 1.24) | 0.54 (0.11, 2.63) |
| >1000 €/month | 0.67 (0.24, 1.89) | 2.0 (0.23, 17.13) | 0.84 (0.32, 2.23) | 1.48 (0.19, 11.44) |
| Marital status (non-married) | 1.90 (0.83, 4.36) | 1.14 (0.26, 5.06) | 1.29 (0.60, 2.77) | 1.40 (0.34, 5.80) |
| BMI (kg/m2) | 1.0 (0.93, 1.06) | 0.97 (0.86, 1.08) | 1.03 (0.97, 1.10) | 1.07 (0.97, 1.17) |
| Adherence to intervention | 1.24 (0.89, 1.72) | 1.44 (0.83, 2.50) | 2.29 (1.56, 3.35) ** | 2.71 (1.57, 4.66) ** |
| Baseline IBS-SSS | 1.01 (1.00, 1.02) * | 1.03 (1.01, 1.05) ** | 1.0 (0.99, 1.01) | 1.02 (1.01, 1.04) * |
| Baseline IBS-QoL | 0.97 (0.95, 0.99) * | 0.96 (0.91, 1.02) | 1.0 (0.98, 1.02) | 0.99 (0.94, 1.03) |
| Baseline SF-12 | 0.92 (0.87, 0.98) * | 1.0 (0.84, 1.2) | 1.04 (0.98, 1.10) | 1.1 (0.93, 1.3) |
| Baseline GSRS-IBS | 1.04 (0.63, 1.70) | 0.12 (0.03, 0.46) * | 1.15 (0.73, 1.80) | 0.46 (0.16, 1.31) |
| Baseline HADS Anxiety | 1.02 (0.93, 1.13) | 0.96 (0.77, 1.19) | 0.94 (0.86, 1.03) | 0.92 (0.77, 1.11) |
| Baseline HADS Depression | 1.07 (0.96, 1.19) | 0.91 (0.68, 1.21) | 0.91 (0.82, 0.99) * | 0.95 (0.74, 1.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasti, A.N.; Katsas, K.; Pavlidis, D.E.; Stylianakis, E.; Petsis, K.I.; Lambrinou, S.; Nikolaki, M.D.; Papanikolaou, I.S.; Hatziagelaki, E.; Papadimitriou, K.; et al. Clinical Trial: A Mediterranean Low-FODMAP Diet Alleviates Symptoms of Non-Constipation IBS—Randomized Controlled Study and Volatomics Analysis. Nutrients 2025, 17, 1545. https://doi.org/10.3390/nu17091545
Kasti AN, Katsas K, Pavlidis DE, Stylianakis E, Petsis KI, Lambrinou S, Nikolaki MD, Papanikolaou IS, Hatziagelaki E, Papadimitriou K, et al. Clinical Trial: A Mediterranean Low-FODMAP Diet Alleviates Symptoms of Non-Constipation IBS—Randomized Controlled Study and Volatomics Analysis. Nutrients. 2025; 17(9):1545. https://doi.org/10.3390/nu17091545
Chicago/Turabian StyleKasti, Arezina N., Konstantinos Katsas, Dimitrios E. Pavlidis, Emmanouil Stylianakis, Konstantinos I. Petsis, Sophia Lambrinou, Maroulla D. Nikolaki, Ioannis S. Papanikolaou, Erifili Hatziagelaki, Konstantinos Papadimitriou, and et al. 2025. "Clinical Trial: A Mediterranean Low-FODMAP Diet Alleviates Symptoms of Non-Constipation IBS—Randomized Controlled Study and Volatomics Analysis" Nutrients 17, no. 9: 1545. https://doi.org/10.3390/nu17091545
APA StyleKasti, A. N., Katsas, K., Pavlidis, D. E., Stylianakis, E., Petsis, K. I., Lambrinou, S., Nikolaki, M. D., Papanikolaou, I. S., Hatziagelaki, E., Papadimitriou, K., Kapolos, J., Muir, J. G., & Triantafyllou, K. (2025). Clinical Trial: A Mediterranean Low-FODMAP Diet Alleviates Symptoms of Non-Constipation IBS—Randomized Controlled Study and Volatomics Analysis. Nutrients, 17(9), 1545. https://doi.org/10.3390/nu17091545














