Nitrate as Warden of Nitric Oxide Homeostasis in Mammals
Abstract
1. Introduction
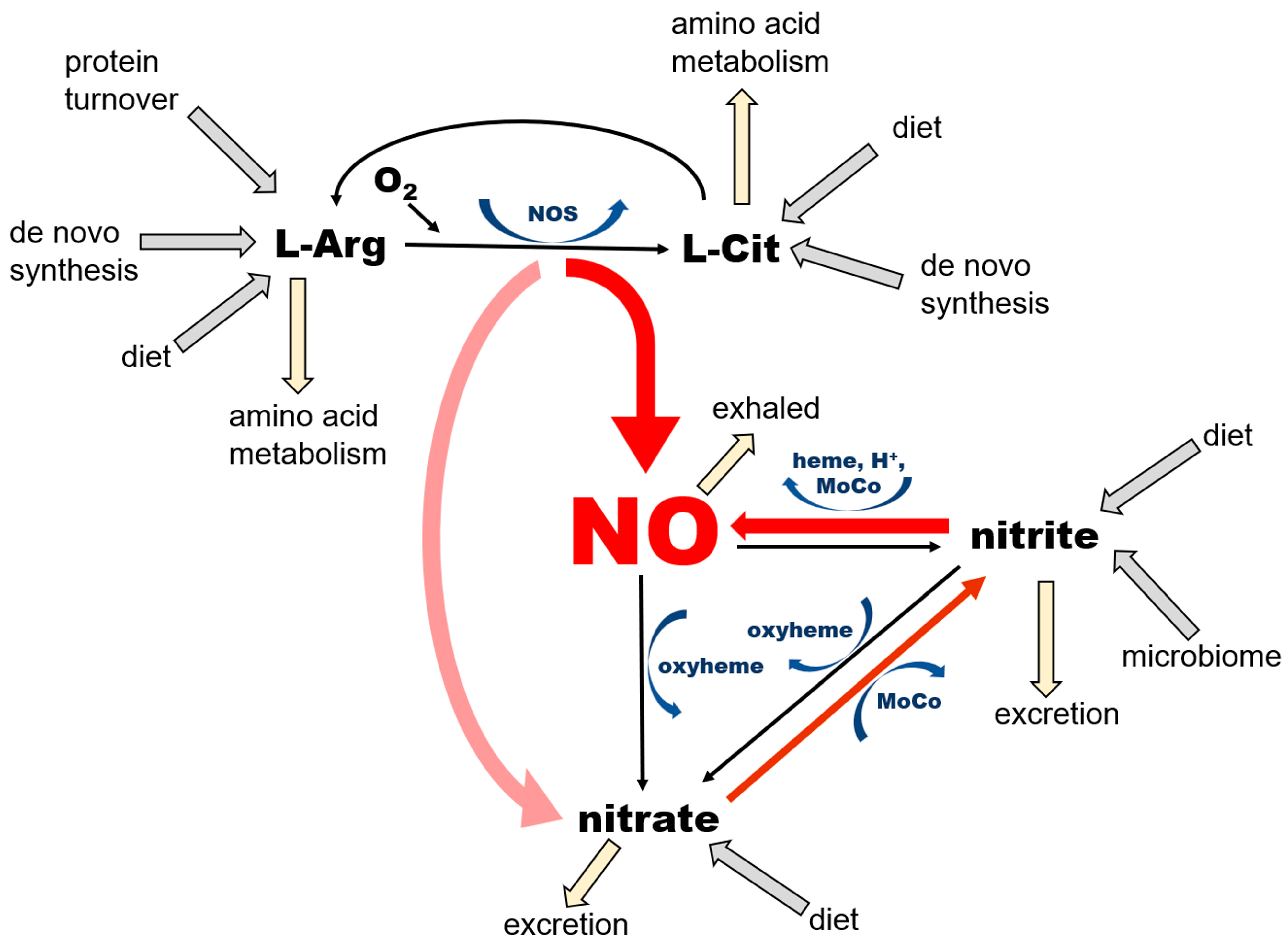
2. Nitric Oxide Cycle
2.1. Short Story of NO Discovery
2.2. Organization of the NO Pathway with Emphasis on Its Cyclicity and Self-Sustaining Nature
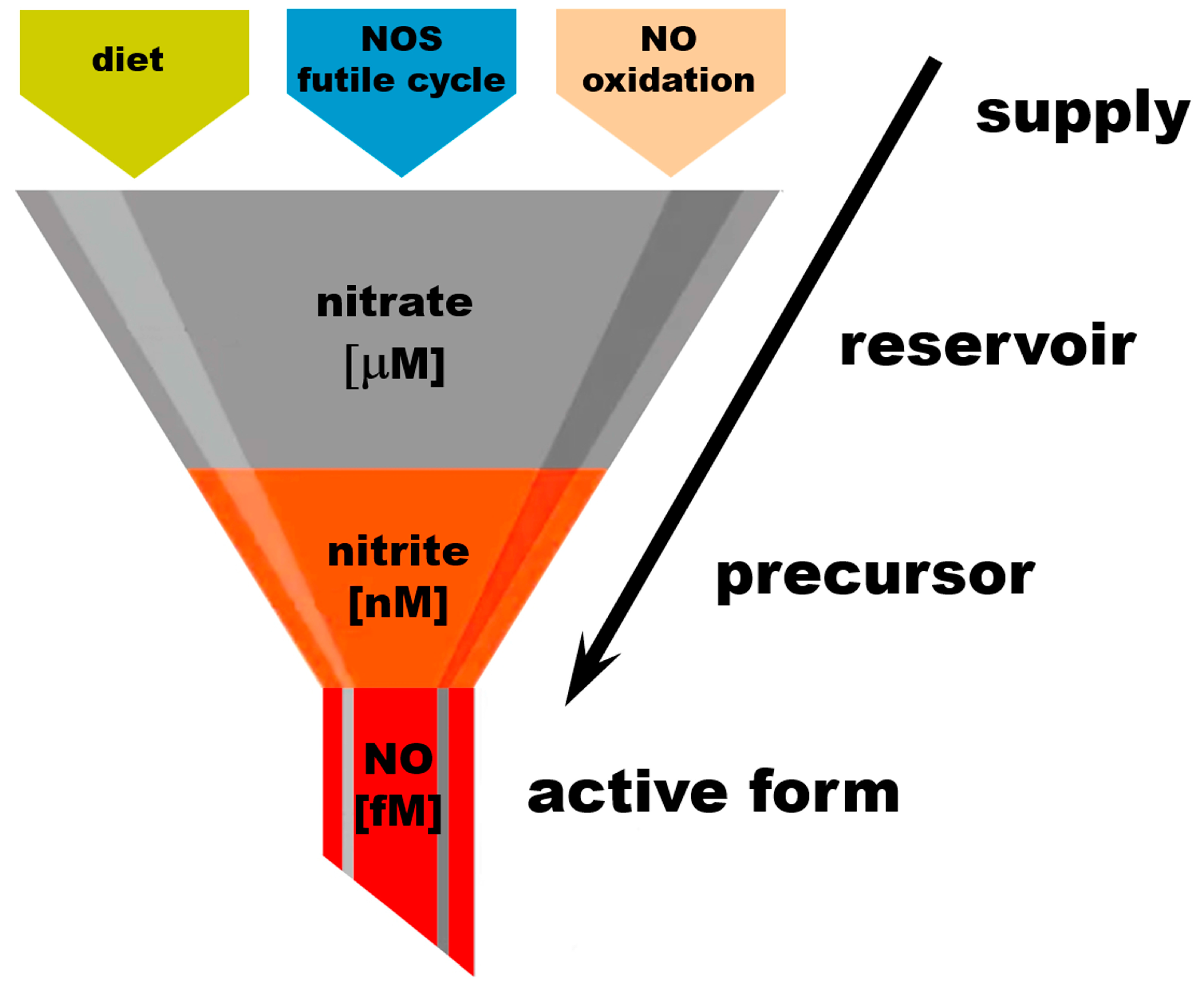
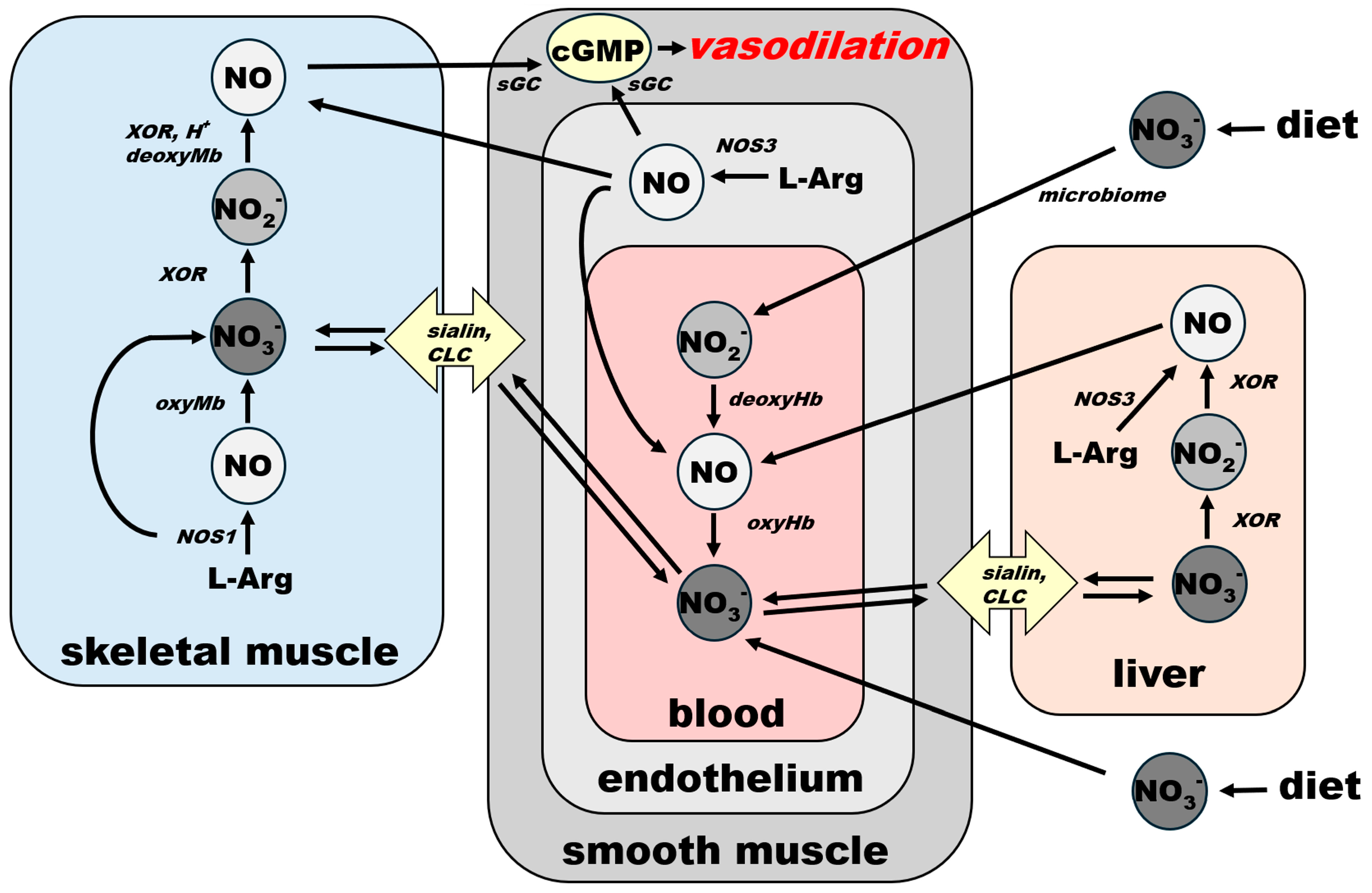
2.3. Nitrate as an Exclusive Storage Molecule of NO Cycle
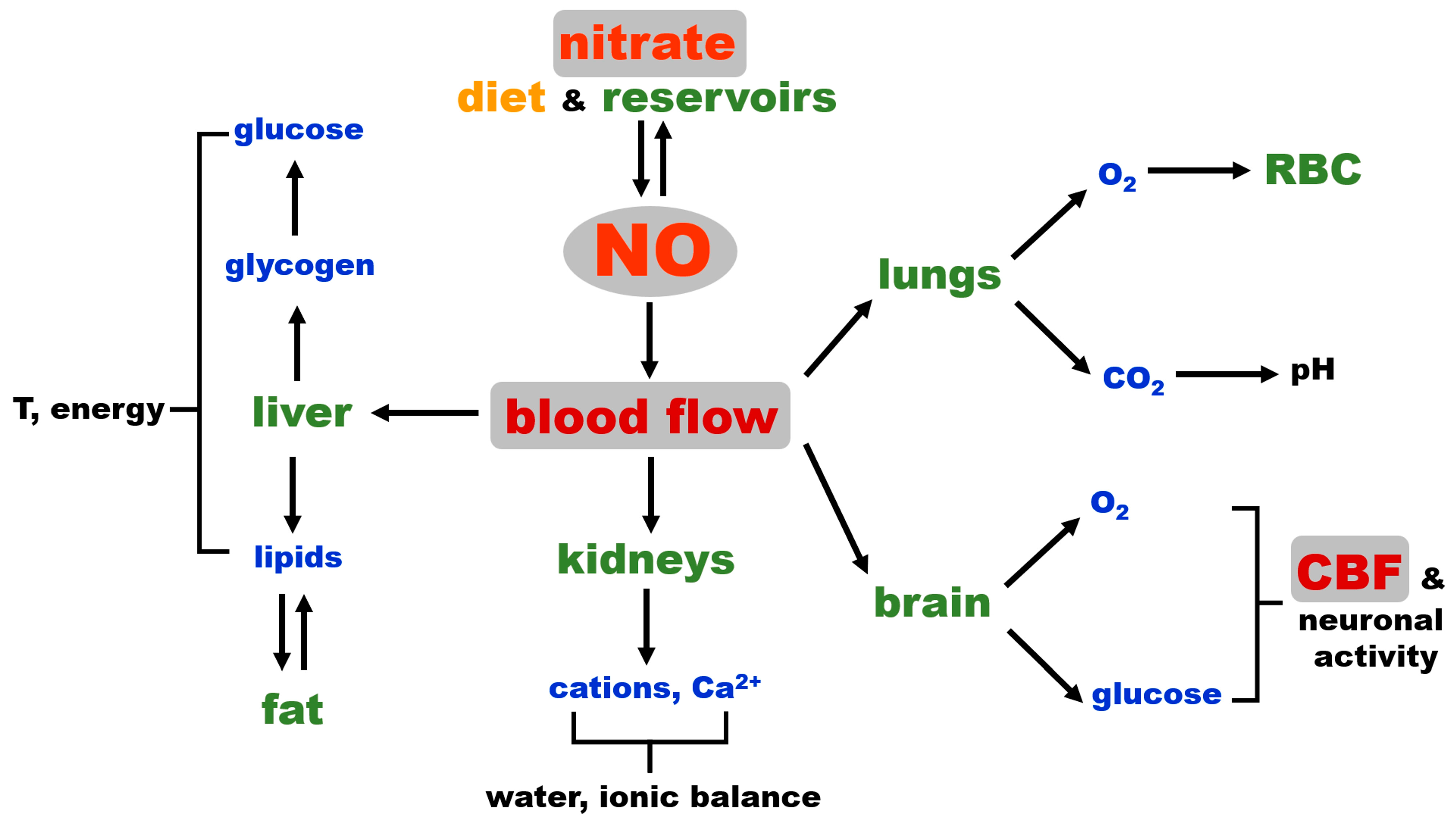
2.4. NO and Blood Flow Control as a Critical Factor for Maintaining General Homeostasis in Mammals
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AO | aldehyde oxidase |
| BH4 | tetrahydrobiopterin |
| BAEC | bovine aortic endothelial cells |
| CBF | cerebral blood flow |
| cGMP | guanosine 3′,5′-cyclic monophosphate |
| CLC | chloride channels and transporters |
| COPD | chronic pulmonary obstructive disease |
| eNOS/NOS3 | endothelial nitric oxide synthase |
| nNOS/NOS1 | neuronal nitric oxide synthase |
| FAD | flavin adenine dinucleotide |
| FMN | flavin mononucleotide |
| IPF | idiopathic pulmonary fibrosis |
| mARC | mitochondrial amidoxime reducing component |
| MoCo proteins | molybdopterin motive containing proteins |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NO | nitric oxide |
| NO2− | nitrite |
| NO3− | nitrate |
| NOS | nitric oxide synthase |
| ONOO | peroxinitrite |
| RBC | red blood cell |
| sGC | soluble guanylate cyclase |
| SO | sulfite oxidase |
| XOR | xanthine oxidoreductase |
References
- Moroz, L.L.; Mukherjee, K.; Romanova, D.Y. Nitric oxide signaling in ctenophores. Front. Neurosci. 2023, 17, 1125433. [Google Scholar] [CrossRef]
- Cristino, L.; Guglielmotti, V.; Cotugno, A.; Musio, C.; Santillo, S. Nitric oxide signaling pathways at neural level in invertebrates: Functional implications in cnidarians. Brain Res. 2008, 1225, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.; Almeida-Souza, T.H.; Silva, R.S.; Santos, H.F.; Santos, E.V.; Gois, A.M.; Leal, P.C.; Santos, J.R. Involvement of nitric oxide in the neurobiology of fear-like behavior. Nitric Oxide 2022, 124, 24–31. [Google Scholar] [CrossRef]
- Donald, J.A.; Forgan, L.G.; Cameron, M.S. The evolution of nitric oxide signalling in vertebrate blood vessels. J. Comp. Physiol. B 2015, 185, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.R., Jr. Historical origins of the discovery of mammalian nitric oxide (nitrogen monoxide) production/physiology/pathophysiology. Biochem. Pharmacol. 2020, 176, 113793. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Lundberg, J.M.; Alving, K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut 1994, 35, 1543–1546. [Google Scholar] [CrossRef]
- Zweier, J.L.; Wang, P.; Samouilov, A.; Kuppusamy, P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995, 1, 804–809. [Google Scholar] [CrossRef]
- Zweier, J.L.; Samouilov, A.; Kuppusamy, P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta 1999, 1411, 250–262. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Schechter, A.N.; Kim-Shapiro, D.B.; Patel, R.P.; Hogg, N.; Shiva, S.; Cannon, R.O., 3rd; Kelm, M.; Wink, D.A.; Espey, M.G.; et al. The emerging biology of the nitrite anion. Nat. Chem. Biol. 2005, 1, 308–314. [Google Scholar] [CrossRef]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O., 3rd; et al. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics, and tolerance formation. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Nitrite in nitric oxide biology: Cause or consequence? A systems-based review. Free Radic. Biol. Med. 2006, 41, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Hendgen-Cotta, U.B.; Luedike, P.; Berenbrink, M.; Klare, J.P.; Steinhoff, H.J.; Semmler, D.; Shiva, S.; Williams, D.; Kipar, A.; et al. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 2012, 126, 325–334. [Google Scholar] [CrossRef] [PubMed]
- van Faassen, E.E.; Babrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.T.; Lundberg, J.O.; Mason, R.; et al. Nitrite as Regulator of Hypoxic Signaling in Mammalian Physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef]
- Pinder, A.G.; Pittaway, E.; Morris, K.; James, P.E. Nitrite directly vasodilates hypoxic vasculature via nitric oxide-dependent and -independent pathways. Br. J. Pharmacol. 2009, 157, 1523–1530. [Google Scholar] [CrossRef]
- Duncan, C.; Li, H.; Dykhuizen, R.; Frazer, R.; Johnston, P.; MacKnight, G.; Smith, L.; Lamza, K.; McKenzie, H.; Batt, L.; et al. Protection against oral and gastrointestinal diseases: Importance of dietary nitrate intake, oral nitrate reduction and enterosalivary nitrate circulation. Comp. Biochem. Physiol. A Physiol. 1997, 118, 939–948. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef]
- Bryan, N.S.; Ahmed, S.; Lefer, D.J.; Hord, N.; von Schwarz, E.R. Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action. Nitric Oxide 2023, 132, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to NO: Potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef]
- Volino-Souza, M.; Oliveira, G.V.; Pinheiro, V.D.S.; Conte-Junior, C.A.; Alvares, T.D.S. The effect of dietary nitrate on macro- and microvascular function: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 64, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Derella, C.C.; Anderson, K.C.; Woessner, M.N.; Paterson, C.; Allen, J.D. Ergogenic Effect of Nitrate Supplementation in Clinical Populations: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3832. [Google Scholar] [CrossRef]
- Tripodi, G.; Lombardo, M.; Kerav, S.; Aiello, G.; Baldelli, S. Nitric Oxide in Parkinson’s Disease: The Potential Role of Dietary Nitrate in Enhancing Cognitive and Motor Health via the Nitrate-Nitrite-Nitric Oxide Pathway. Nutrients 2025, 17, 393. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef]
- Huang, L.; Borniquel, S.; Lundberg, J.O. Enhanced xanthine oxidoreductase expression and tissue nitrate reduction in germ free mice. Nitric Oxide 2010, 22, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 2017, 105, 48–67. [Google Scholar] [CrossRef]
- Archer, D.L. Evidence that ingested nitrate and nitrite are beneficial to health. J. Food Prot. 2002, 65, 872–875. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Tunau-Spencer, K.J.; Jenkins, A.; Hellinga, D.G.; Walter, P.J.; Cai, H.; Schechter, A.N. Skeletal Muscle, Skin, and Bone as Three Major Nitrate Reservoirs in Mammals: Chemiluminescence and (15)N-Tracer Studies in Yorkshire Pigs. Nutrients 2024, 16, 2674. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Santolini, J.; Wang, Z.Q.; Wei, C.C.; Adak, S. Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 2004, 279, 36167–36170. [Google Scholar] [CrossRef]
- Shiva, S.; Wang, X.; Ringwood, L.A.; Xu, X.; Yuditskaya, S.; Annavajjhala, V.; Miyajima, H.; Hogg, N.; Harris, Z.L.; Gladwin, M.T. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2006, 2, 486–493. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Blekkenhorst, L.C.; Bondonno, N.P.; Sim, M.; Woodman, R.J.; Croft, K.D.; Lewis, J.R.; Hodgson, J.M.; Bondonno, C.P. A food composition database for assessing nitrate intake from plant-based foods. Food Chem. 2022, 394, 133411. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Dezhangah, S.; Esmi, F.; Gharavi-nakhjavani, M.S.; Hashempour-Baltork, F.; Mirza Alizadeh, A. A worldwide systematic review, meta-analysis and meta-regression of nitrate and nitrite in vegetables and fruits. Ecotoxicol. Environ. Saf. 2023, 257, 114934. [Google Scholar] [CrossRef] [PubMed]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, X.; Sun, Q.; Fan, Z.; Xia, D.; Ding, G.; Ong, H.L.; Adams, D.; Gahl, W.A.; Zheng, C.; et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 13434–13439. [Google Scholar] [CrossRef]
- Qu, X.M.; Wu, Z.F.; Pang, B.X.; Jin, L.Y.; Qin, L.Z.; Wang, S.L. From Nitrate to Nitric Oxide: The Role of Salivary Glands and Oral Bacteria. J. Dent. Res. 2016, 95, 1452–1456. [Google Scholar] [CrossRef]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef]
- Akhtar, S.; Sagar, K.; Singh, A.; Hote, M.P.; Roy, A.; Sharma, A. Inflammation-induced sialin mediates nitrate efflux in dysfunctional endothelium affecting NO bioavailability. Nitric Oxide 2024, 146, 37–47. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Pusch, M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Lam, K.K.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide—Biol. Chem. 2016, 55–56, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Suschek, C.V.; Feibel, D.; von Kohout, M.; Oplander, C. Enhancement of Nitric Oxide Bioavailability by Modulation of Cutaneous Nitric Oxide Stores. Biomedicines 2022, 10, 2124. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Lüneburg, N.; Xanthakis, V.; Schwedhelm, E.; Sullivan, L.M.; Maas, R.; Anderssohn, M.; Riederer, U.; Glazer, N.L.; Vasan, R.S.; Böger, R.H. Reference Intervals for Plasma L-Arginine and the L-Arginine:Asymmetric Dimethylarginine Ratio in the Framingham Offspring Cohort. J. Nutr. 2011, 141, 2186–2190. [Google Scholar] [CrossRef]
- Maas, R.; Xanthakis, V.; Goen, T.; Muller, J.; Schwedhelm, E.; Boger, R.H.; Vasan, R.S. Plasma Nitrate and Incidence of Cardiovascular Disease and All-Cause Mortality in the Community: The Framingham Offspring Study. J. Am. Heart Assoc. 2017, 6, e006224. [Google Scholar] [CrossRef]
- Boger, R.H. The pharmacodynamics of L-arginine. J. Nutr. 2007, 137, 1650S–1655S. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, A.R.; Emery, P.W.; Pearson, J.D.; Mann, G.E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1990, 173, 940–948. [Google Scholar] [CrossRef]
- Wyatt, A.W.; Steinert, J.R.; Mann, G.E. Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem. Soc. Symp. 2004, 71, 143–156. [Google Scholar] [CrossRef]
- Gornik, H.L.; Creager, M.A. Arginine and endothelial and vascular health. J. Nutr. 2004, 134, 2880S–2887S; discussion 2895S. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef]
- Jonvik, K.L.; Nyakayiru, J.; Pinckaers, P.J.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Nitrate-Rich Vegetables Increase Plasma Nitrate and Nitrite Concentrations and Lower Blood Pressure in Healthy Adults. J. Nutr. 2016, 146, 986–993. [Google Scholar] [CrossRef]
- Gilliard, C.N.; Lam, J.K.; Cassel, K.S.; Park, J.W.; Schechter, A.N.; Piknova, B. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 2018, 75, 1–7. [Google Scholar] [CrossRef]
- Piknova, B.; Schechter, A.N.; Park, J.W.; Vanhatalo, A.; Jones, A.M. Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis. Exerc. Sport Sci. Rev. 2022, 50, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, J.; Poon, E.; Davies, K. The dystrophin-associated protein complex. J. Cell Sci. 2002, 115, 2801–2803. [Google Scholar] [CrossRef]
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Hunt, A.P.; Santolini, J.; Lehnert, N.; Stuehr, D.J. Mechanism and regulation of ferrous heme-nitric oxide (NO) oxidation in NO synthases. J. Biol. Chem. 2019, 294, 7904–7916. [Google Scholar] [CrossRef] [PubMed]
- Garbincius, J.F.; Michele, D.E. Dystrophin-glycoprotein complex regulates muscle nitric oxide production through mechanoregulation of AMPK signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 13663–13668. [Google Scholar] [CrossRef]
- Dombernowsky, N.W.; Olmestig, J.N.E.; Witting, N.; Kruuse, C. Role of neuronal nitric oxide synthase (nNOS) in Duchenne and Becker muscular dystrophies—Still a possible treatment modality? Neuromuscul. Disord. 2018, 28, 914–926. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Barton, E.R. The dystrophin-associated glycoprotein complex: What parts can you do without? Proc. Natl. Acad. Sci. USA 2000, 97, 13464–13466. [Google Scholar] [CrossRef]
- Sander, M.; Chavoshan, B.; Harris, S.A.; Iannaccone, S.T.; Stull, J.T.; Thomas, G.D.; Victor, R.G. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 2000, 97, 13818–13823. [Google Scholar] [CrossRef]
- Nelson, M.D.; Rosenberry, R.; Barresi, R.; Tsimerinov, E.I.; Rader, F.; Tang, X.; Mason, O.; Schwartz, A.; Stabler, T.; Shidban, S.; et al. Sodium nitrate alleviates functional muscle ischaemia in patients with Becker muscular dystrophy. J. Physiol. 2015, 593, 5183–5200. [Google Scholar] [CrossRef]
- Wylie, L.J.; Park, J.W.; Vanhatalo, A.; Kadach, S.; Black, M.I.; Stoyanov, Z.; Schechter, A.N.; Jones, A.M.; Piknova, B. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 2019, 597, 5565–5576. [Google Scholar] [CrossRef] [PubMed]
- Black, M.I.; Wylie, L.J.; Kadach, S.; Piknova, B.; Park, J.W.; Stoyanov, Z.; L’Heureux, J.E.; Schechter, A.N.; Vanhatalo, A.; Jones, A.M. Effects of low and high dietary nitrate intake on human saliva, plasma and skeletal muscle nitrate and nitrite concentrations and their functional consequences. Free Radic. Biol. Med. 2024, 225, 881–893. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Walter, P.J.; Cai, H.; Upanan, S.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Distribution of dietary nitrate and its metabolites in rat tissues after (15)N-labeled nitrate administration. Sci. Rep. 2023, 13, 3499. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Compensatory mechanisms in myoglobin deficient mice preserve NO homeostasis. Nitric Oxide 2019, 90, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kadach, S.; Piknova, B.; Black, M.I.; Park, J.W.; Wylie, L.J.; Stoyanov, Z.; Thomas, S.M.; McMahon, N.F.; Vanhatalo, A.; Schechter, A.N.; et al. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 2022, 121, 1–10. [Google Scholar] [CrossRef]
- Frisbee, J.C. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R307–R316. [Google Scholar] [CrossRef]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef]
- Wang, Y.; Simons, M. Flow-regulated lymphatic vasculature development and signaling. Vasc. Cell 2014, 6, 14. [Google Scholar] [CrossRef]
- Ohhashi, T.; Kawai, Y.; Maejima, D.; Hayashi, M.; Watanabe-Asaka, T. Physiological Roles of Lymph Flow-Mediated Nitric Oxide in Lymphatic System. Lymphat. Res. Biol. 2023, 21, 253–261. [Google Scholar]
- Esplugues, J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002, 135, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Picon-Pages, P.; Garcia-Buendia, J.; Munoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. Brain glucose metabolism: Role of nitric oxide. Biochem. Pharmacol. 2025, 232, 116728. [Google Scholar] [CrossRef] [PubMed]
- Roger, N.; Barbera, J.A.; Roca, J.; Rovira, I.; Gomez, F.P.; Rodriguez-Roisin, R. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 156, 800–806. [Google Scholar] [CrossRef]
- Hasuda, T.; Satoh, T.; Shimouchi, A.; Sakamaki, F.; Kyotani, S.; Matsumoto, T.; Goto, Y.; Nakanishi, N. Improvement in exercise capacity with nitric oxide inhalation in patients with precapillary pulmonary hypertension. Circulation 2000, 101, 2066–2070. [Google Scholar] [CrossRef]
- Blanco, I.; Ribas, J.; Xaubet, A.; Gómez, F.P.; Roca, J.; Rodriguez-Roisin, R.; Barberà, J.A. Effects of inhaled nitric oxide at rest and during exercise in idiopathic pulmonary fibrosis. J. Appl. Physiol. 2011, 110, 638–645. [Google Scholar] [CrossRef]
- Gwyn, D.R.; Lindeman, K.S.; Hirshman, C.A. Inhaled nitric oxide attenuates bronchoconstriction in canine peripheral airways. Am. J. Respir. Crit. Care Med. 1996, 153, 604–609. [Google Scholar] [CrossRef]
- Hunter, C.J.; Dejam, A.; Blood, A.B.; Shields, H.; Kim-Shapiro, D.B.; Machado, R.F.; Tarekegn, S.; Mulla, N.; Hopper, A.O.; Schechter, A.N.; et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. 2004, 10, 1122–1127. [Google Scholar] [CrossRef]
- Casey, D.B.; Badejo, A.M., Jr.; Dhaliwal, J.S.; Murthy, S.N.; Hyman, A.L.; Nossaman, B.D.; Kadowitz, P.J. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H524–H533. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Stewart, G.M.; Obokata, M.; Koepp, K.E.; Borlaug, B.A. Peripheral and pulmonary effects of inorganic nitrite during exercise in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 814–823. [Google Scholar] [CrossRef]
- Pana, R.; Hornby, L.; Shemie, S.D.; Dhanani, S.; Teitelbaum, J. Time to loss of brain function and activity during circulatory arrest. J. Crit. Care 2016, 34, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piknova, B.; Park, J.W.; Schechter, A.N. Nitrate as Warden of Nitric Oxide Homeostasis in Mammals. Nutrients 2025, 17, 1544. https://doi.org/10.3390/nu17091544
Piknova B, Park JW, Schechter AN. Nitrate as Warden of Nitric Oxide Homeostasis in Mammals. Nutrients. 2025; 17(9):1544. https://doi.org/10.3390/nu17091544
Chicago/Turabian StylePiknova, Barbora, Ji Won Park, and Alan N. Schechter. 2025. "Nitrate as Warden of Nitric Oxide Homeostasis in Mammals" Nutrients 17, no. 9: 1544. https://doi.org/10.3390/nu17091544
APA StylePiknova, B., Park, J. W., & Schechter, A. N. (2025). Nitrate as Warden of Nitric Oxide Homeostasis in Mammals. Nutrients, 17(9), 1544. https://doi.org/10.3390/nu17091544




