Impact of Phosphate Variability in Patients Undergoing Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Population
2.2. Study Variables
2.3. Statistical Analyses
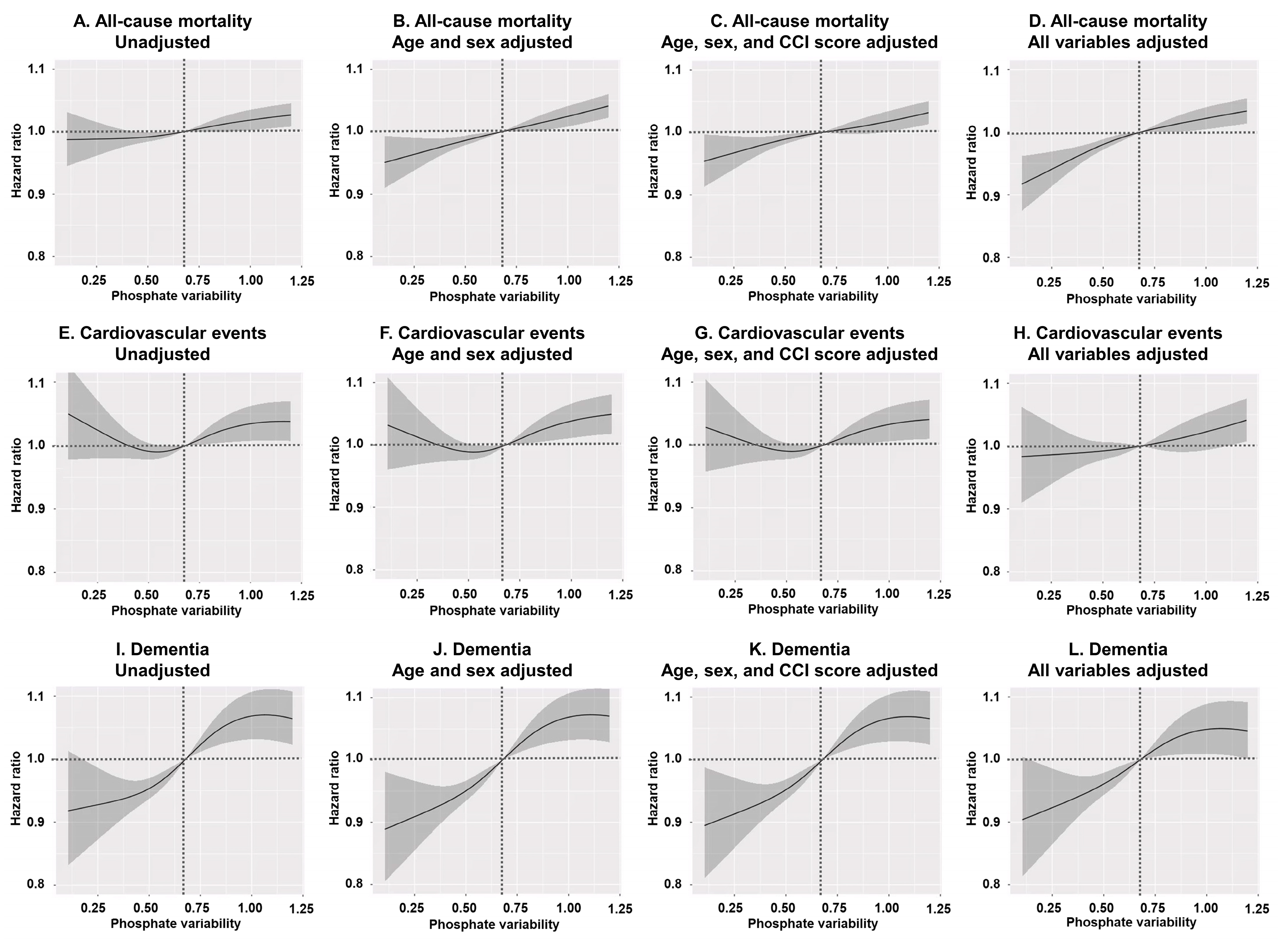
3. Results
3.1. Baseline Characteristics
3.2. Patient Status as Stratified by Phosphate Variability
3.3. Group-Specific Survival Analyses
3.4. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Htay, H.; Cho, Y.; Jha, V.; See, E.; Arruebo, S.; Caskey, F.J.; Damster, S.; Donner, J.A.; Levin, A.; Nangaku, M.; et al. Global structures, practices, and tools for provision of hemodialysis. Nephrol. Dial. Transplant. 2024, 39 (Suppl. S2), ii11–ii17. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Polkinghorne, K.R.; McDonald, S.P.; Ierino, F.L. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am. J. Kidney Dis. 2011, 58, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar]
- Fouque, D.; Horne, R.; Cozzolino, M.; Kalantar-Zadeh, K. Balancing nutrition and serum phosphate in maintenance dialysis. Am. J. Kidney Dis. 2014, 64, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.L.; Wolf, M. Calcium and Phosphate Disorders: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 241–256. [Google Scholar] [CrossRef]
- Cozzolino, M.; Dusso, A.S.; Slatopolsky, E. Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J. Am. Soc. Nephrol. 2001, 12, 2511–2516. [Google Scholar] [CrossRef]
- Yang, W.; Israni, R.K.; Brunelli, S.M.; Joffe, M.M.; Fishbane, S.; Feldman, H.I. Hemoglobin variability and mortality in ESRD. J. Am. Soc. Nephrol. 2007, 18, 3164–3170. [Google Scholar] [CrossRef]
- Di Iorio, B.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Guastaferro, P.; Chiuchiolo, L.; Nigro, F.; De Blasio, A.; Romano, P.; Pota, A.; et al. Variability of blood pressure in dialysis patients: A new marker of cardiovascular risk. J. Nephrol. 2013, 26, 173–182. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, N.; Nie, L.; Lu, C.; Chen, H.; He, W.; Li, M.; Wang, Y.; Zhao, J.; Xiong, J. Visit-to-visit ultrafiltration volume variability predicts all-cause mortality in patients receiving hemodialysis. Ren. Fail. 2023, 45, 2194439. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; You, L.; Yu, X.; Zhang, M.; Zhu, R.; Hao, C.; Zhang, Z.; Chen, J. Association of serum phosphorus variability with coronary artery calcification among hemodialysis patients. PLoS ONE 2014, 9, e93360. [Google Scholar]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [PubMed]
- Kang, S.H.; Kim, B.Y.; Son, E.J.; Kim, G.O.; Do, J.Y. Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients. J. Clin. Med. 2023, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 15 March 2025).
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1039–1130. [Google Scholar]
- Kang, S.H.; Kim, B.Y.; Son, E.J.; Kim, G.O.; Do, J.Y. Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis. Biomedicines 2023, 11, 2838. [Google Scholar] [CrossRef]
- Levitt, H.; Smith, K.G.; Rosner, M.H. Variability in calcium, phosphorus, and parathyroid hormone in patients on hemodialysis. Hemodial. Int. 2009, 13, 518–525. [Google Scholar] [CrossRef]
- Ter Meulen, K.J.; Ye, X.; Wang, Y.; Usvyat, L.A.; van der Sande, F.M.; Konings, C.J.; Kotanko, P.; Kooman, J.P.; Maddux, F.W. Variability of Serum Phosphate in Incident Hemodialysis Patients: Association with All-Cause Mortality. Kidney360 2023, 4, 374–380. [Google Scholar]
- Zhu, M.; Dou, L.; Zhu, M.; Liu, S.; Zhan, Y.; Lu, J.; Ni, Z.; Qian, J.; Cai, H.; Zhang, W. Variability of serum phosphorus and its association with mortality among hemodialysis patients. Clin. Nephrol. 2018, 90, 79–86. [Google Scholar] [CrossRef]
- Mathur, A.; Ahn, J.B.; Sutton, W.; Chu, N.M.; Gross, A.L.; Segev, D.L.; McAdams-DeMarco, M. Secondary hyperparathyroidism (CKD-MBD) treatment and the risk of dementia. Nephrol. Dial. Transplant. 2022, 37, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Fan, L.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. FGF-23 and cognitive performance in hemodialysis patients. Hemodial. Int. 2014, 18, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y. Visit-to-Visit Blood Pressure Variability-What is the current challenge? Am. J. Hypertens. 2017, 30, 112–114. [Google Scholar] [CrossRef] [PubMed]

| Interval of Residual SD of Phosphate Levels | Unweighted Cohort | Weighted Cohort | |||
|---|---|---|---|---|---|
| Mean Phosphate Levels | Residual SDs of Phosphate Levels | Mean Phosphate Levels | Residual SDs of Phosphate Levels | ||
| Q1 | ≤0.47 | 4.55 ± 1.07 | 0.34 ± 0.09 | 4.94 ± 0.01 | 0.35 ± 0.00 |
| Q2 | 0.47 < SD ≤ 0.68 | 4.78 ± 1.05 a | 0.57 ± 0.06 a | 4.95 ± 0.01 | 0.57 ± 0.00 a |
| Q3 | 0.68 < SD ≤ 0.94 | 5.08 ± 1.07 a,b | 0.80 ± 0.07 a,b | 4.99 ± 0.01 a,b | 0.79 ± 0.00 a,b |
| Q4 | >0.94 | 5.47 ± 1.09 a,b,c | 1.25 ± 0.29 a,b,c | 5.03 ± 0.01 a,b,c | 1.22 ± 0.00 a,b,c |
| Q1 (n = 48,817) | Q2 (n = 49,310) | Q3 (n = 49,158) | Q4 (n = 47,730) | p | |
|---|---|---|---|---|---|
| Age (years) | 61.8 ± 0.1 | 61.9 ± 0.1 | 61.8 ± 0.1 | 61.7 ± 0.1 | 0.612 |
| Sex (male, %) | 29,993 (61.4%) | 29,987 (60.8%) | 29,525 (60.1%) | 28,322 (59.3%) | 0.011 |
| HD (months) | 67 ± 1 | 67 ± 1 | 66 ± 1 | 67 ± 1 | 0.385 |
| Body mass index (kg/m2) | 22.8 ± 0.0 | 22.8 ± 0.0 | 22.8 ± 0.0 | 22.7 ± 0. | 0.305 |
| Diabetes (%) | 21,481 (44.0%) | 21,803 (44.2%) | 21,701 (44.1%) | 20,353 (42.6%) | 0.066 |
| CCI score | 8.8 ± 0.0 | 8.8 ± 0.0 | 8.8 ± 0.0 | 8.8 ± 0.0 | 0.079 |
| Arteriovenous fistula (%) | 42,385 (86.8%) | 42,665 (86.5%) | 42,450 (86.4%) | 41,046 (86.0%) | 0.353 |
| Kt/Vurea | 1.57 ± 0.00 | 1.57 ± 0.00 | 1.57 ± 0.00 | 1.58 ± 0.00 | 0.016 |
| UFV (L/session) | 2.31 ± 0.01 | 2.30 ± 0.01 | 2.32 ± 0.01 | 2.34 ± 0.01 | 0.001 |
| Hemoglobin (g/dL) | 10.7 ± 0.0 | 10.7 ± 0.0 | 10.7 ± 0.0 | 10.7 ± 0.0 | 0.765 |
| Serum albumin (g/dL) | 4.01 ± 0.0 | 4.01 ± 0.0 | 4.01 ± 0.0 | 4.01 ± 0.0 | 0.865 |
| Serum calcium (mg/dL) | 8.9 ± 0.0 | 8.9 ± 0.0 | 8.9 ± 0.0 | 8.9 ± 0.0 | 0.020 |
| Serum creatinine (mg/dL) | 9.5 ± 0.0 | 9.5 ± 0.0 | 9.6 ± 0.0 | 9.7 ± 0.0 | <0.001 |
| Use of RASB (%) | 32,055 (65.7%) | 32,912 (66.7%) | 32,757 (66.6%) | 31,874 (66.8%) | 0.249 |
| Use of aspirin (%) | 23,643 (48.4%) | 24,326 (49.3%) | 23,954 (48.7%) | 23,301 (48.8%) | 0.606 |
| Use of clopidogrel (%) | 12,639 (25.9%) | 12,752 (25.9%) | 12,561 (25.6%) | 12,596 (26.4%) | 0.570 |
| Use of statins (%) | 23,950 (49.1%) | 24,398 (49.5%) | 24,720 (50.3%) | 23,965 (50.2%) | 0.211 |
| Use of anti-HTN drugs (%) | 41,440 (84.9%) | 41,977 (85.1%) | 41,787 (85.0%) | 40,634 (85.1%) | 0.951 |
| MI or CHF (%) | 28,416 (58.2%) | 28,571 (57.9%) | 28,634 (58.2%) | 27,978 (58.6%) | 0.795 |
| Atrial fibrillation (%) | 5922 (12.1%) | 6120 (12.4%) | 6154 (12.5%) | 5630 (11.8%) | 0.375 |
| PPB (%) | <0.001 | ||||
| Calcium-based PPB | 30,438 (62.4%) | 30,536 (61.9%) | 30,717 (62.5%) | 29,383 (61.6%) | |
| Non-calcium-based PPB | 13,154 (26.9%) | 13,535 (27.4%) | 13,706 (27.9%) | 14,313 (30.0%) | |
| No PPB | 5225 (10.7%) | 5239 (10.6%) | 4735 (9.6%) | 4034 (8.5%) | |
| Use of vitamin D agents (%) | 26,113 (53.5%) | 26,017 (52.8%) | 26,198 (53.3%) | 25,155 (52.7%) | 0.575 |
| Use of cinacalcet (%) | 5898 (12.1%) | 5950 (12.1%) | 5747 (11.7%) | 5592 (11.7%) | 0.688 |
| Use of CaS (%) | 1362 (2.8%) | 1499 (3.0%) | 1557 (3.2%) | 1528 (3.2%) | 0.270 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| All–cause mortality | ||||
| Q2 | 1.03 (1.00–1.05) | 0.022 | 1.03 (1.00–1.05) | 0.033 |
| Q3 | 1.04 (1.01–1.06) | 0.002 | 1.08 (1.05–1.10) | <0.001 |
| Q4 | 1.06 (1.04–1.09) | <0.001 | 1.08 (1.06–1.11) | <0.001 |
| Cardiovascular events | ||||
| Q2 | 0.97 (0.93–1.01) | 0.111 | 0.96 (0.93–1.00) | 0.085 |
| Q3 | 1.01 (0.97–1.04) | 0.752 | 1.01 (0.97–1.05) | 0.644 |
| Q4 | 1.01 (0.97–1.05) | 0.612 | 1.02 (0.98–1.07) | 0.243 |
| Dementia | ||||
| Q2 | 1.02 (0.97–1.07) | 0.526 | 0.99 (0.94–1.05) | 0.782 |
| Q3 | 1.06 (1.01–1.11) | 0.022 | 1.05 (0.99–1.11) | 0.079 |
| Q4 | 1.12 (1.06–1.18) | <0.001 | 1.08 (1.02–1.14) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Park, S.-Y.; Lim, Y.-J.; Kim, B.-Y.; Choi, J.-Y.; Do, J.-Y.; Kim, A.-Y. Impact of Phosphate Variability in Patients Undergoing Hemodialysis. Nutrients 2025, 17, 1528. https://doi.org/10.3390/nu17091528
Kang S-H, Park S-Y, Lim Y-J, Kim B-Y, Choi J-Y, Do J-Y, Kim A-Y. Impact of Phosphate Variability in Patients Undergoing Hemodialysis. Nutrients. 2025; 17(9):1528. https://doi.org/10.3390/nu17091528
Chicago/Turabian StyleKang, Seok-Hui, So-Young Park, Yu-Jeong Lim, Bo-Yeon Kim, Ji-Young Choi, Jun-Young Do, and A-Young Kim. 2025. "Impact of Phosphate Variability in Patients Undergoing Hemodialysis" Nutrients 17, no. 9: 1528. https://doi.org/10.3390/nu17091528
APA StyleKang, S.-H., Park, S.-Y., Lim, Y.-J., Kim, B.-Y., Choi, J.-Y., Do, J.-Y., & Kim, A.-Y. (2025). Impact of Phosphate Variability in Patients Undergoing Hemodialysis. Nutrients, 17(9), 1528. https://doi.org/10.3390/nu17091528







