Dietary Fiber Intake Improves Osteoporosis Caused by Chronic Lead Exposure by Restoring the Gut–Bone Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of NHANES Database
2.2. Animal Experiment Design
2.3. Tissue Harvesting and Processing
2.4. Lead Quantification
2.5. Micro-CT Analysis
2.6. ELISA
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. RNA Extraction and qRT-PCR Analysis
2.9. Extraction of Bone Tissue Protein
2.10. Western Blot Analysis
2.11. Flow Cytometry for Treg Cell Analysis
2.12. Quantification of Short-Chain Fatty Acids
2.13. 16S rRNA Gene Sequencing
2.14. Statistical Analysis
3. Results
3.1. Dietary Fiber Intake Is Negatively Correlated Osteoporosis in Lead Exposure Population with NHANES
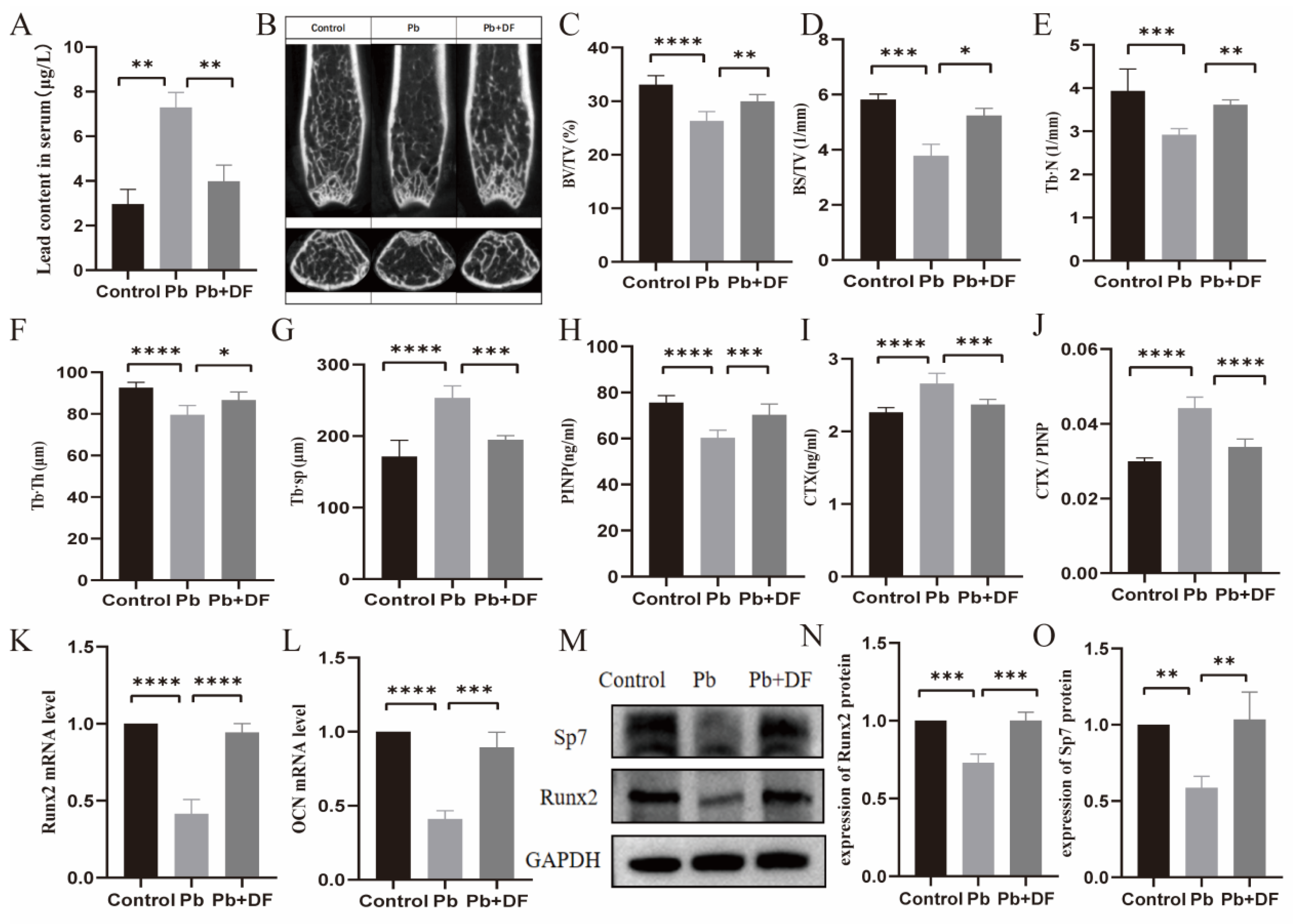
3.2. DF Improved Bone Microstructure Damage in Pb-Exposed Mice
3.3. DF Repaired the Intestinal Barrier Damaged by Pb
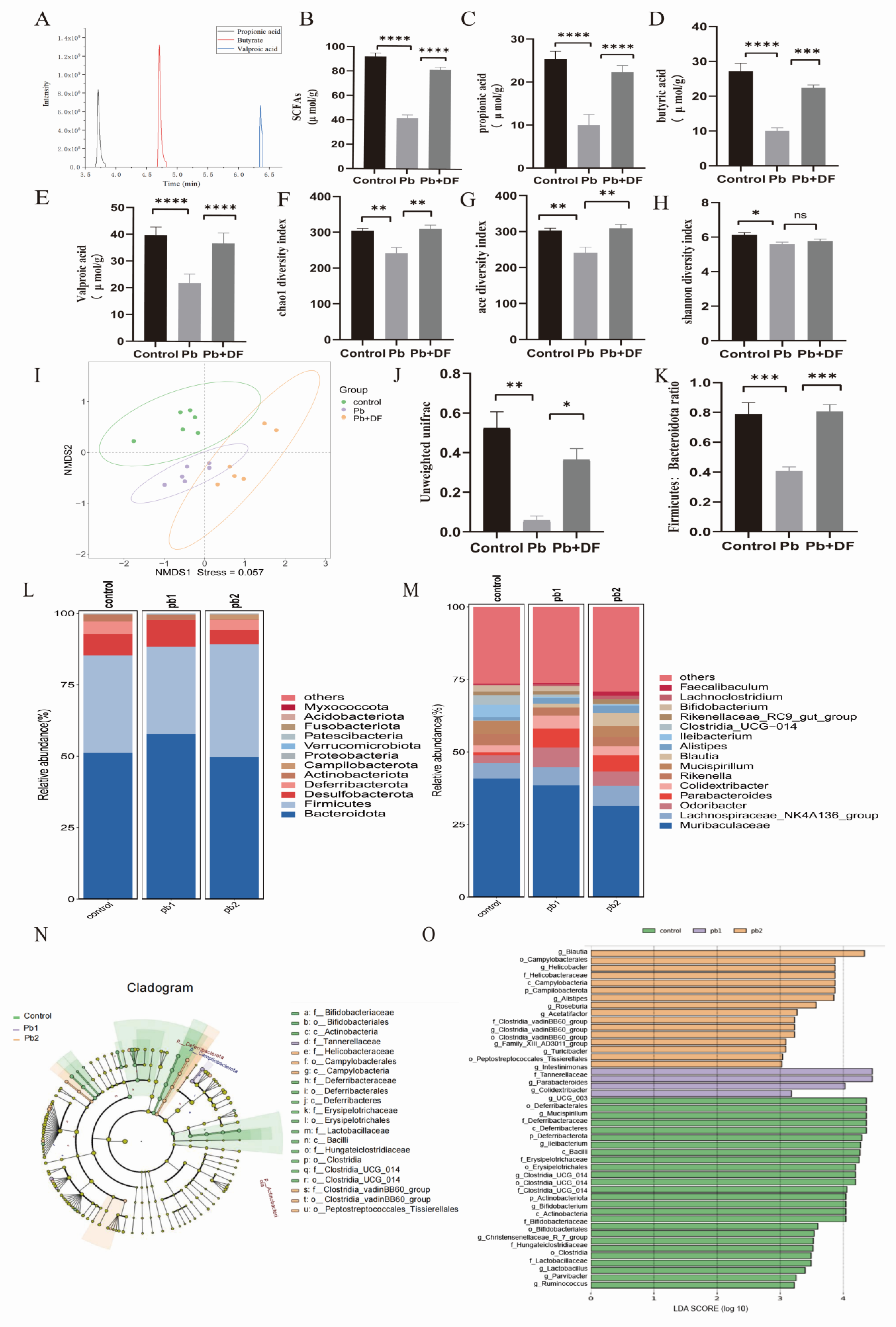
3.4. DF Regulated Changes in Gut Microbiota Structure in Mice Exposed to Pb
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimai, H.P.; Muschitz, C.; Amrein, K.; Bauer, R.; Cejka, D.; Gasser, R.W.; Gruber, R.; Haschka, J.; Hasenöhrl, T.; Kainberger, F.; et al. Osteoporosis-Definition, risk assessment, diagnosis, prevention and treatment (update 2024): Guidelines of the Austrian Society for Bone and Mineral Research. Wien Klin. Wochenschr. 2024, 136 (Suppl. 16), 599–668. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- McClung, M.R.; Pinkerton, J.; Blake, J.; Cosman, F.; Lewiecki, E.; Shapiro, M. Management of Osteoporosis in Postmenopausal Women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar]
- Battistini, B.; Greggi, C.; Visconti, V.V.; Albanese, M.; Messina, A.; De Filippis, P.; Gasperini, B.; Falvino, A.; Piscitelli, P.; Palombi, L.; et al. Metals accumulation affects bone and muscle in osteoporotic patients: A pilot study. Environ. Res. 2024, 250, 118514. [Google Scholar] [CrossRef]
- Elonheimo, H.; Lange, R.; Tolonen, H.; Kolossa-Gehring, M. Environmental Substances Associated with Osteoporosis—A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 738. [Google Scholar] [CrossRef]
- Wu, C.; Xiao, Y.; Jiang, Y. Associations of blood trace elements with bone mineral density: A population-based study in US adults. J. Orthop. Surg. Res. 2023, 18, 827. [Google Scholar] [CrossRef] [PubMed]
- Visconti, V.V.; Gasperini, B.; Greggi, C.; Battistini, B.; Messina, A.; Renzi, M.; Bakhtafrouz, K.; Iundusi, R.; Botta, A.; Palombi, L.; et al. Plasma heavy metal levels correlate with deregulated gene expression of detoxifying enzymes in osteoporotic patients. Sci. Rep. 2023, 13, 10641. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Li, X.; Wang, Z.; Zhu, G.; Jin, T. Effects of lead and cadmium co-exposure on hemoglobin in a Chinese population. Environ. Toxicol. Pharmacol. 2015, 39, 758–763. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Mei, S.; Zheng, Q.Y.; Wang, J.; Yin, Y.R.; Zhang, J.J.; Wang, X.Z. Melatonin or vitamin C attenuates lead acetate-induced testicular oxidative and inflammatory damage in mice by inhibiting oxidative stress mediated NF-κB signaling. Ecotoxicol. Environ. Saf. 2023, 264, 115481. [Google Scholar] [CrossRef]
- Mijošek, T.; Šariri, S.; Kljaković-Gašpić, Z.; Fiket, Ž.; Filipović Marijić, V. Interrelation between environmental conditions, acanthocephalan infection and metal(loid) accumulation in fish intestine: An in-depth study. Environ. Pollut. 2024, 356, 124358. [Google Scholar] [CrossRef]
- Cheng, S.; Qi, X.; Ma, M.; Zhang, L.; Cheng, B.; Liang, C.; Liu, L.; Li, P.; Kafle, O.P.; Wen, Y.; et al. Assessing the Relationship Between Gut Microbiota and Bone Mineral Density. Front. Genet. 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yang, Y.; Zhang, Z.; Nan, Y.; Xiao, M. The toxic effect of lead exposure on the physiological homeostasis of grouper: Insight from gut-liver axis. Mar. Pollut. Bull. 2024, 207, 116926. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, T.; Fan, F.; Jiang, X.; Li, P.; Ding, J.; Sun, X.; Li, Z.; Fang, Y. Potentials of dietary fiber and polyphenols in whole grain wheat flour to release the liver function and intestinal tract injury in lead-induced mice. Int. J. Biol. Macromol. 2024, 278 Pt 2, 134180. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Wang, H. Gut dysbiosis of Rana zhenhaiensis tadpoles after lead (Pb) exposure based on integrated analysis of microbiota and gut transcriptome. Ecotoxicol. Environ. Saf. 2024, 284, 116922. [Google Scholar] [CrossRef]
- Wang, N.; Huo, Y.; Gao, X.; Li, Y.; Cheng, F.; Zhang, Z. Lead exposure exacerbates liver injury in high-fat diet-fed mice by disrupting the gut microbiota and related metabolites. Food Funct. 2024, 15, 3060–3075. [Google Scholar] [CrossRef]
- Wang, N.; Li, C.; Gao, X.; Huo, Y.; Li, Y.; Cheng, F.; Jiang, F.; Zhang, Z. Co-exposure to lead and high-fat diet aggravates systemic inflammation in mice by altering gut microbiota and the LPS/TLR4 pathway. Metallomics 2024, 16, mfae022. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T. Toxic and essential metals: Metabolic interactions with the gut microbiota and health implications. Front. Nutr. 2024, 11, 1448388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Z.; Fu, Z.; Fan, A.; Song, N.; Wang, Q.; Fan, S.; Xu, J.; Xiang, J.; Liu, X. Oral Propolis Nanoemulsions Modulate Gut Microbiota to Balance Bone Remodeling for Enhanced Osteoporosis Therapy. ACS Nano 2024, 18, 26153–26167. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, W.; Zhou, G.; Qi, Y.; Mao, H.R.; Chen, J.; Lu, Z.; Wu, W.; Zou, X.; Deng, D.; et al. Epimedii Folium decoction ameliorates osteoporosis in mice through NLRP3/caspase-1/IL-1β signalling pathway and gut-bone axis. Int. Immunopharmacol. 2024, 137, 112472. [Google Scholar] [CrossRef]
- Feng, R.; Wang, Q.; Yu, T.; Hu, H.; Wu, G.; Duan, X.; Jiang, R.; Xu, Y.; Huang, Y. Quercetin ameliorates bone loss in OVX rats by modulating the intestinal flora-SCFAs-inflammatory signaling axis. Int. Immunopharmacol. 2024, 136, 112341. [Google Scholar] [CrossRef]
- Kondo, T.; Chiba, T.; Tousen, Y. Short-chain fatty acids, acetate and propionate, directly upregulate osteoblastic differentiation. Int. J. Food Sci. Nutr. 2022, 73, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Triggiani, D.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Piazzolla, G.; Jirillo, E.; Triggiani, V. Endocrine, Metabolic, and Immune Pathogenesis of Postmenopausal Osteoporosis. Is there a Therapeutic Role in Natural Products? Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Miao, J.; Fan, X.; Wang, Q.; Sun, M. Effects of valproic acid on bone mineral density and bone metabolism: A meta-analysis. Seizure 2019, 73, 56–63. [Google Scholar] [CrossRef]
- Qi, P.; Chen, X.; Tian, J.; Zhong, K.; Qi, Z.; Li, M.; Xie, X. The gut homeostasis-immune system axis: Novel insights into rheumatoid arthritis pathogenesis and treatment. Front. Immunol. 2024, 15, 1482214. [Google Scholar] [CrossRef]
- Feng, B.; Lu, J.; Han, Y.; Han, Y.; Qiu, X.; Zeng, Z. The role of short-chain fatty acids in the regulation of osteoporosis: New perspectives from gut microbiota to bone health: A review. Medicine 2024, 103, e39471. [Google Scholar] [CrossRef]
- Yang, K.L.; Mullins, B.J.; Lejeune, A.; Ivanova, E.; Shin, J.; Bajwa, S.; Possemato, R.; Cadwell, K.; Scher, J.U.; Koralov, S.B. Mitigation of Osteoclast-Mediated Arthritic Bone Remodeling by Short Chain Fatty Acids. Arthritis Rheumatol. 2024, 76, 647–659. [Google Scholar] [CrossRef]
- Ge, Y.; Jia, Z.; Zhao, S.; Zhang, W.; Shi, X.; Xie, R.; Gong, Y.; Sheng, J.; van ‘t Hof, R.J.; Yang, J.; et al. Mitigating lead-induced osteoporosis: The role of butyrate in gut-bone axis restoration. Ecotoxicol. Environ. Saf. 2024, 283, 116943. [Google Scholar] [CrossRef]
- Guo, M.; Liu, H.; Yu, Y.; Zhu, X.; Xie, H.; Wei, C.; Mei, C.; Shi, Y.; Zhou, N.; Qin, K.; et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes 2023, 15, 2190304. [Google Scholar] [CrossRef]
- Moriya, T.; Fukatsu, K.; Noguchi, M.; Nishikawa, M.; Miyazaki, H.; Saitoh, D.; Ueno, H.; Yamamoto, J. Effects of semielemental diet containing whey peptides on Peyer’s patch lymphocyte number, immunoglobulin A levels, and intestinal morphology in mice. J. Surg. Res. 2018, 222, 153–159. [Google Scholar] [CrossRef]
- Zhao, N.; Yin, X.; Chen, L.; Tang, S.; Lin, H.; Cui, L.; Jin, X.; Xie, Z.; Jiang, N.; Cui, L.; et al. Associations of different dietary patterns, bone mineral density, and fracture risk among elderly women: The China Osteoporosis Prevalence Study. Front. Endocrinol. 2024, 15, 1378158. [Google Scholar] [CrossRef] [PubMed]
- Runting, H.; Qingyue, L.; Yining, Y.; Huiyu, S.; Shu, Y.; Xixi, F. Is bone mineral density in middle-aged and elderly individuals associated with their dietary patterns? A study based on NHANES. Front. Nutr. 2024, 11, 1396007. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, X.; Huo, Y.; Li, Y.; Cheng, F.; Zhang, Z. Lead exposure aggravates glucose metabolism disorders through gut microbiota dysbiosis and intestinal barrier damage in high-fat diet-fed mice. J. Sci. Food Agric. 2024, 104, 3057–3068. [Google Scholar] [CrossRef]
- Li, Y.; Peng, J.; Cheng, Z.; Zhang, K.; Gu, H.; Feng, J.; Liu, Y. Excessive heavy metal enrichment disturbs liver functions through the gut microbe in the great Himalayan leaf-nosed bat (Hipposideros armiger). Ecotoxicol. Environ. Saf. 2024, 282, 116758. [Google Scholar] [CrossRef]
- Yu, C.; Xu, N.; Tao, X.; Liu, G. Chronic lead poisoning-induced budgerigar liver damage, gut microbiota dysbiosis, and metabolic disorder. Ecotoxicol. Environ. Saf. 2024, 278, 116388. [Google Scholar] [CrossRef]
- Li, N.; Wang, H.; Pei, H.; Wu, Y.; Li, L.; Ren, Y.; Wang, S.; Ma, Y.; Luo, M.; Yuan, J.; et al. Genus_Ruminococcus and order_Burkholderiales affect osteoporosis by regulating the microbiota-gut-bone axis. Front. Microbiol. 2024, 15, 1373013. [Google Scholar] [CrossRef]
- Carmouche, J.J.; Puzas, J.E.; Zhang, X.; Tiyapatanaputi, P.; Cory-Slechta, D.A.; Gelein, R.; Zuscik, M.; Rosier, R.N.; Boyce, B.F.; O’Keefe, R.J.; et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ. Health Perspect. 2005, 113, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zu, Q.; Zhang, J.; Liu, S.; Zhang, G.; Chang, X.; Li, X. Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice. Foods 2024, 13, 2220. [Google Scholar] [CrossRef]
- Rizzoli, R.; Chevalley, T. Nutrition and Osteoporosis Prevention. Curr. Osteoporos. Rep. 2024, 22, 515–522. [Google Scholar] [CrossRef]
- Liu, Z.H.; Ai, S.; Xia, Y.; Wang, H.L. Intestinal toxicity of Pb: Structural and functional damages, effects on distal organs and preventive strategies. Sci. Total Environ. 2024, 931, 172781. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, H.; Zheng, S.; Xu, S.; Massey, I.Y.; Zhang, C.; Wang, X.; Yang, F. Pb Toxicity on Gut Physiology and Microbiota. Front. Physiol. 2021, 12, 574913. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Huang, D.; Chen, L.; Ji, Q.; Xiang, Y.; Zhou, Q.; Chen, K.; Zhang, X.; Zou, F.; Zhang, X.; Zhao, Z.; et al. Lead aggravates Alzheimer’s disease pathology via mitochondrial copper accumulation regulated by COX17. Redox Biol. 2024, 69, 102990. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Christudas, S.; Zheng, X.; Xu, B. Dietary fiber konjac glucomannan exerts an antidiabetic effect via inhibiting lipid absorption and regulation of PPAR-γ and gut microbiome. Food Chem. 2023, 403, 134336. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Wang, S.J.; Zhang, X.Y.; Xu, Q.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Yano, H.; Uddin, J.; Emanuel, E.R.; Hu, E.; Zhang, W.; Li, T.T.; Jin, W.B.; Grier, A.; et al. Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J. Exp. Med. 2024, 221, e20232148. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Kim, G.; Kim, S.; Jung, H.; Kang, S.; Park, G.; Shin, H. The Impact of Makgeolli Consumption on Gut Microbiota: An Enterotype-Based Preliminary Study. J. Microbiol. 2024, 62, 965–972. [Google Scholar] [CrossRef]
- Xue, X.; Zhou, H.; Gao, J.; Li, X.; Wang, J.; Bai, W.; Bai, Y.; Fan, L.; Chang, H.; Shi, S. The impact of traditional Chinese medicine and dietary compounds on modulating gut microbiota in hepatic fibrosis: A review. Heliyon 2024, 10, e38339. [Google Scholar] [CrossRef]
- Petakh, P.; Oksenych, V.; Kamyshnyi, A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomed. Pharmacother. 2023, 163, 114892. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Ohno, T.; Iwahashi, H.; Umemura, M.; Murotomi, K. Associations between intestinal lactic acid bacteria species and feeding habits of zoo animals. Microbiome Res. Rep. 2024, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Gong, L.; Liu, Y.; Zhang, X.; Liu, W.; Han, M.; Zhou, D.; Shi, S. Associations between gut microbiota and osteoporosis or osteopenia in a cohort of Chinese Han youth. Sci. Rep. 2024, 14, 20948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jiang, G.; Wang, Y.; Yan, E.; He, L.; Guo, J.; Yin, J.; Zhang, X. Maternal consumption of L-malic acid enriched diets improves antioxidant capacity and glucose metabolism in offspring by regulating the gut microbiota. Redox Biol. 2023, 67, 102889. [Google Scholar] [CrossRef]
- Li, B.; Zhang, F.; Jiang, H.; Wang, C.; Zhao, Q.; Yang, W.; Hu, A. Adequate Intake of Dietary Fiber May Relieve the Detrimental Impact of Blood Lead on Dyslipidemia among US Adults: A Study of Data from the National Health and Nutrition Examination Survey Database. Nutrients 2023, 15, 4434. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, J.; Cen, S.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Modulation of the gut microbiota by a galactooligosaccharide protects against heavy metal lead accumulation in mice. Food Funct. 2019, 10, 3768–3781. [Google Scholar] [CrossRef]


| Forward (5′−3′) | Reverse (5′−3′) | |
|---|---|---|
| ZO-1 | GGGGAAACCCGAAACTGATGC | GTGGAGAGAAGAGTTGGACAGAGGC |
| Occludin | TACTGGTCTCTACGTGGATCAAT | TTCTTCGGGTTTTCACAGCAA |
| Runx2 | GCTTCTCCAACCCACGAATG | GAACTGATAGGACGCTGACGA |
| OCN | CTGACCTCACAGATCCCAAGC | TGGTCTGATAGCTCGTCACAAG |
| GAPDH | CGTATCGGACGCCTGGTT | AGGTCAATGAAGGGGTCGTT |
| Variables, n (%) | Osteoporosis | Non-Osteoporosis | p Value |
|---|---|---|---|
| Blood lead (mean ± SD) | 1.81 ± 1.61 | 1.69 ± 1.66 | <0.001 |
| Dietary fiber intake | 16.16 ± 8.39 | 16.87 ± 8.94 | 0.0051 |
| Variables, n (%) | Osteoporosis | Non-Osteoporosis | OR (95%CI) | p Value |
|---|---|---|---|---|
| Blood lead | 720 (44.64%) | 8014 (50.73%) | 1.28 (1.15–1.42) | <0.001 |
| 893 (55.36%) | 7783 (49.27%) | |||
| Dietary fiber | 845 (52.39%) | 7867 (49.80%) | 0.90 (0.81–1.00) | 0.048 |
| 768 (47.61%) | 7930 (50.20%) |
| Group | Osteoporosis | Non-Osteoporosis | OR (95%CI) | p Value |
|---|---|---|---|---|
| HL dietary fiber and LL Pb | 364 (22.57%) | 4130 (26.14%) | 1.00 (reference) | |
| LL dietary fiber and LL Pb | 356 (22.07%) | 3884 (24.59%) | 1.04 | 0.62 |
| HL dietary fiber and HL Pb | 404 (25.05%) | 3800 (24.06%) | 1.21 | 0.013 |
| LL dietary fiber and HL Pb | 489 (30.32%) | 3983 (25.21%) | 1.39 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Shen, J.; Han, C.; Shi, X.; Gong, Y.; Hu, X.; Jia, Z.; Wang, M.; Wu, Y. Dietary Fiber Intake Improves Osteoporosis Caused by Chronic Lead Exposure by Restoring the Gut–Bone Axis. Nutrients 2025, 17, 1513. https://doi.org/10.3390/nu17091513
Wang R, Shen J, Han C, Shi X, Gong Y, Hu X, Jia Z, Wang M, Wu Y. Dietary Fiber Intake Improves Osteoporosis Caused by Chronic Lead Exposure by Restoring the Gut–Bone Axis. Nutrients. 2025; 17(9):1513. https://doi.org/10.3390/nu17091513
Chicago/Turabian StyleWang, Ruijian, Jin Shen, Chunqing Han, Xiaodong Shi, Yan Gong, Xiping Hu, Zhongtang Jia, Miaomiao Wang, and Yu Wu. 2025. "Dietary Fiber Intake Improves Osteoporosis Caused by Chronic Lead Exposure by Restoring the Gut–Bone Axis" Nutrients 17, no. 9: 1513. https://doi.org/10.3390/nu17091513
APA StyleWang, R., Shen, J., Han, C., Shi, X., Gong, Y., Hu, X., Jia, Z., Wang, M., & Wu, Y. (2025). Dietary Fiber Intake Improves Osteoporosis Caused by Chronic Lead Exposure by Restoring the Gut–Bone Axis. Nutrients, 17(9), 1513. https://doi.org/10.3390/nu17091513






