Dietary Rutin Ameliorates Nanoparticle Zinc Oxide-Induced Toxicity in Mice by Potentiating Antioxidant Defense Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment, Experimental Design, and Sample Collection
- (1)
- Control group: Fed a basal diet;
- (2)
- HZn group: Fed a basal diet supplemented with 5000 mg/kg nano-ZnO;
- (3)
- HZn + R1 group: Fed a basal diet supplemented with 5000 mg/kg nano-ZnO and 300 mg/kg rutin;
- (4)
- HZn + R2 group: Fed a basal diet supplemented with 5000 mg/kg nano-ZnO and 600 mg/kg rutin;
- (5)
- HZn + R3 group: Fed a basal diet supplemented with 5000 mg/kg nano-ZnO and 1200 mg/kg rutin.
2.2. Analysis of Nano-ZnO Charaterization
2.3. Analysis of Serum and Jejunal Parameters
2.4. Histological Analysis
2.5. Determination of Zinc Concentration
2.6. Analysis of mRNA Expression
2.7. Statistical Analysis
3. Results
3.1. The Characterization of Nano-ZnO
3.2. Body Weight
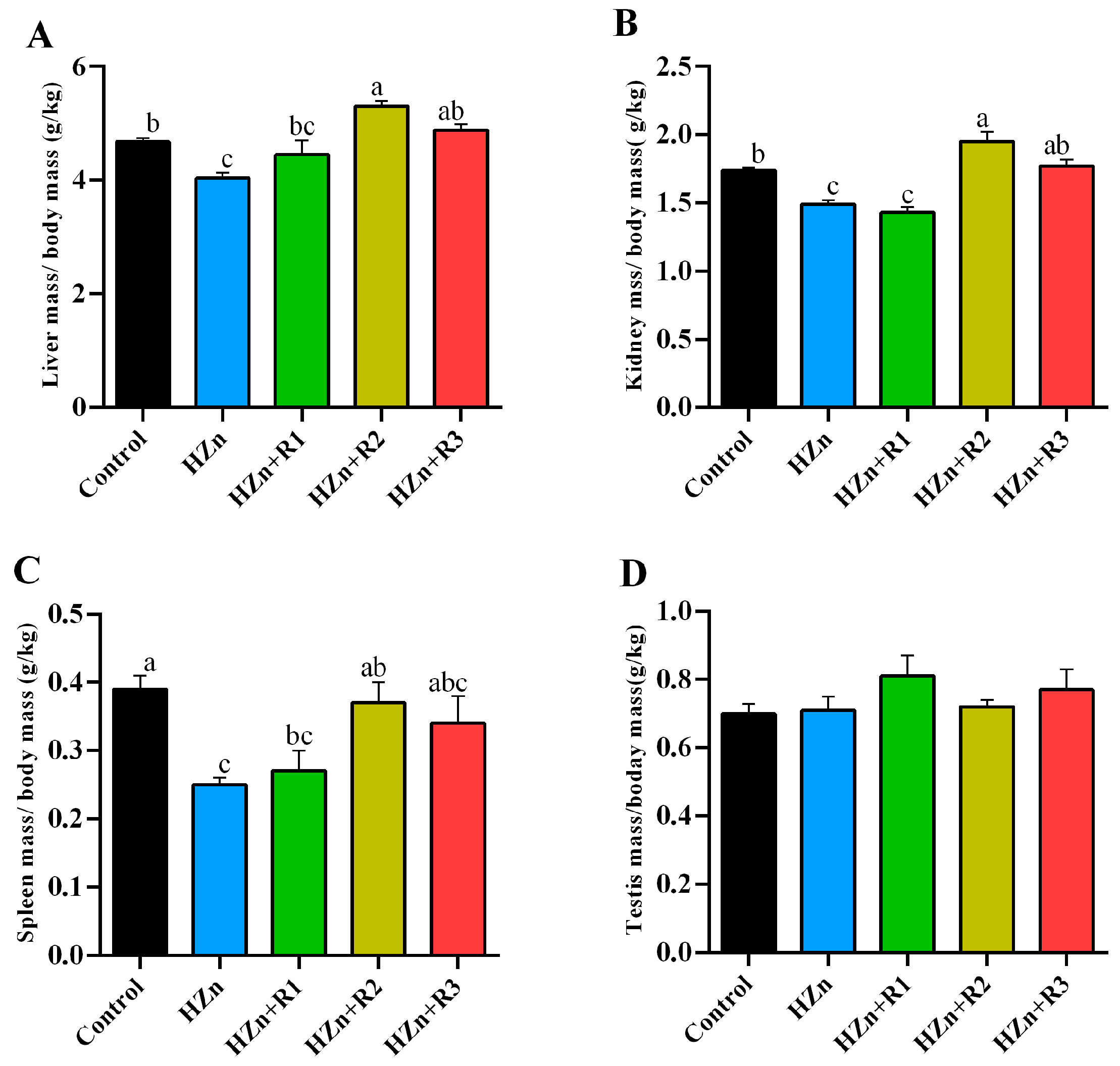
3.3. The Relative Organ Indexes
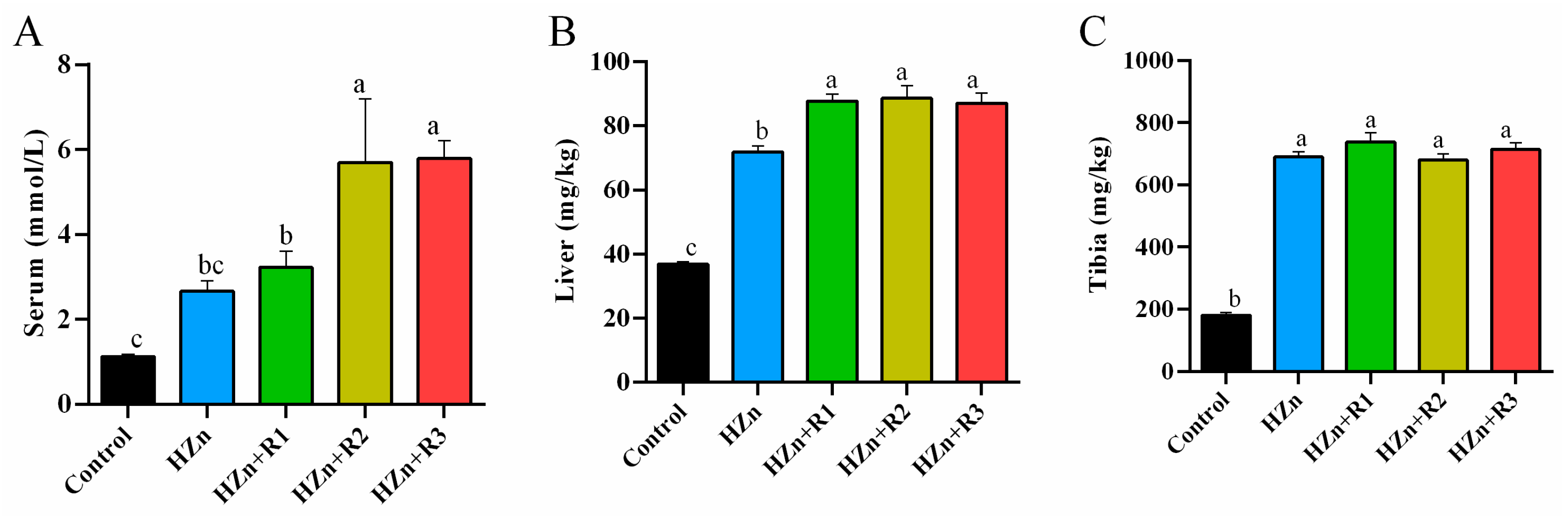
3.4. Zinc Concentrations in Serum, Liver, and Tibia
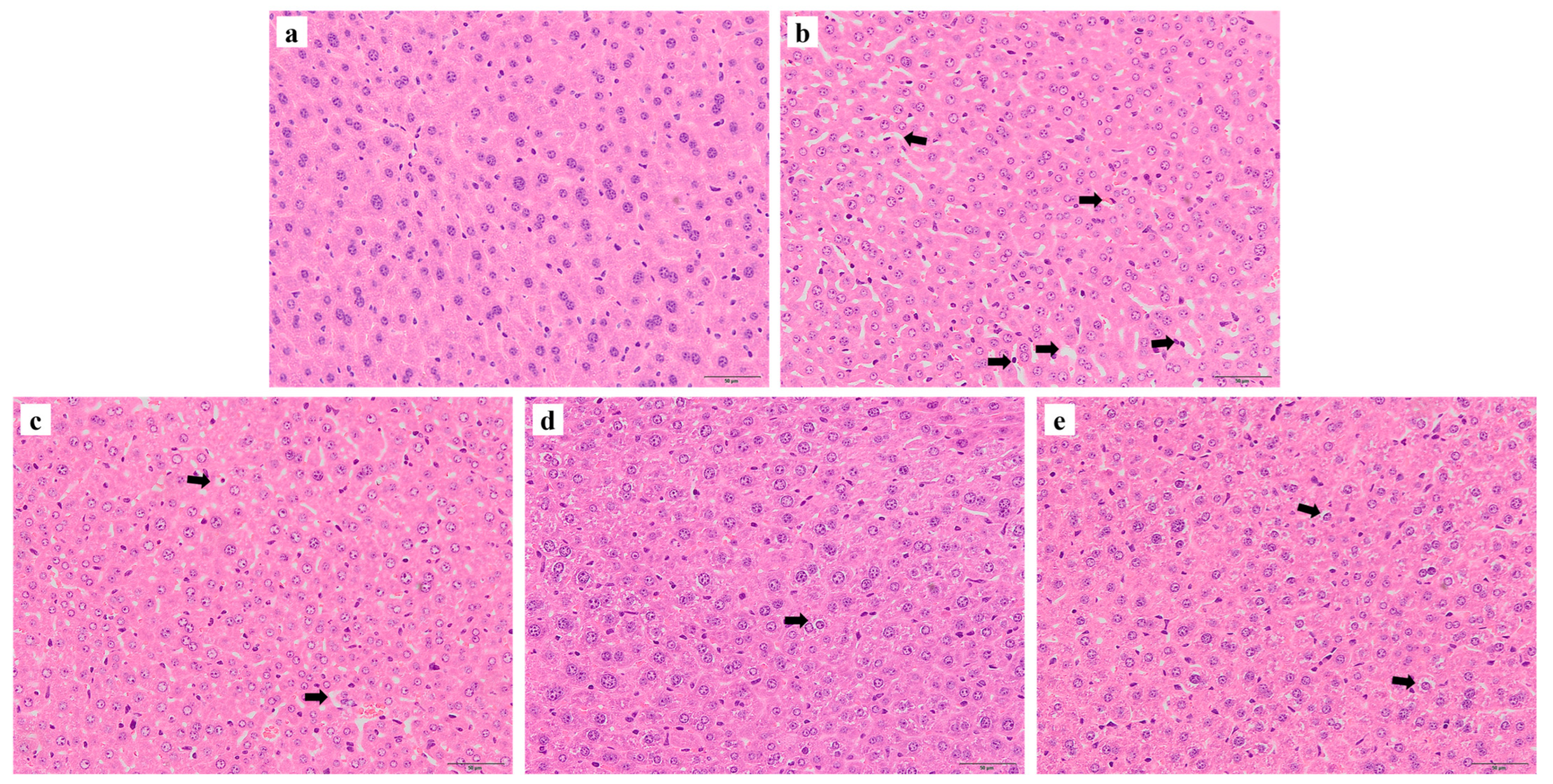
3.5. Histological Analysis of Liver
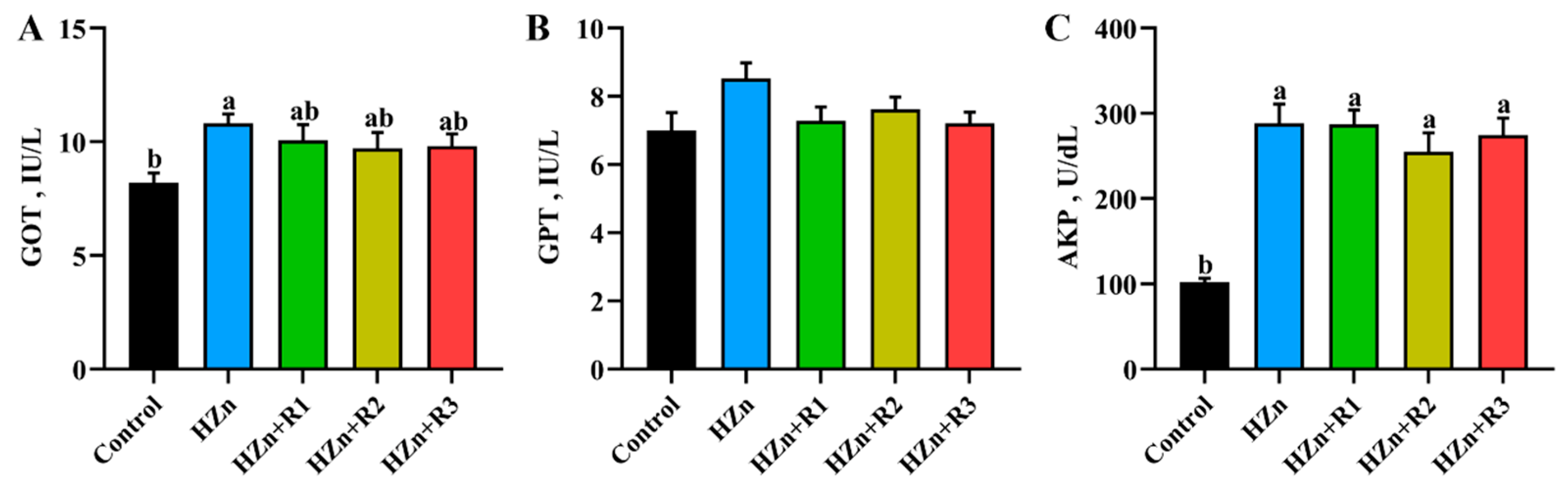
3.6. Serum Activities of GOT, GPT, and AKP
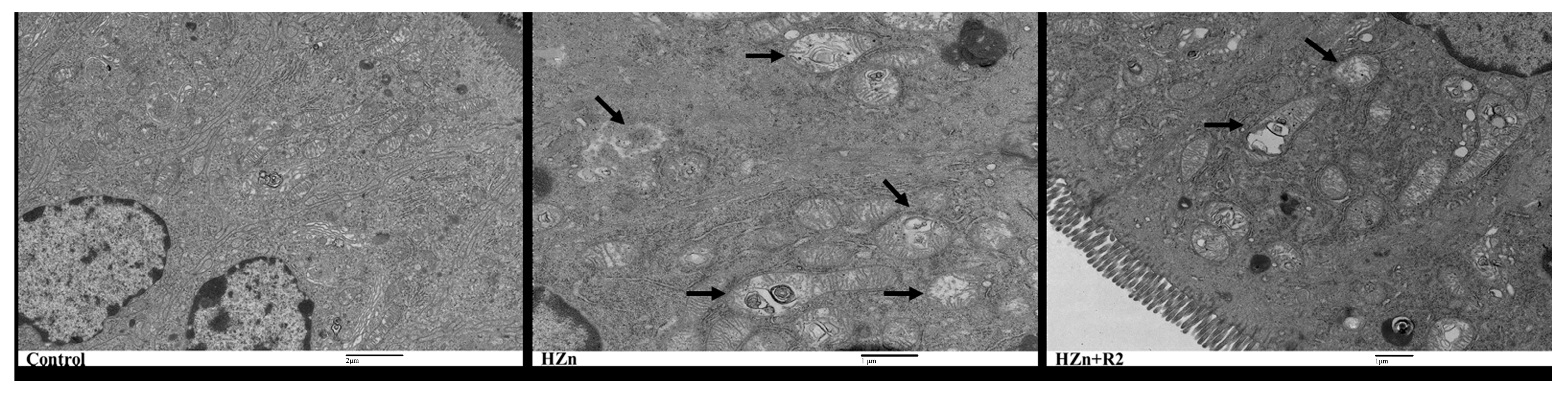
3.7. TEM Analysis of the Jejunum Histology
3.8. Serum and Jejunal Redox Status
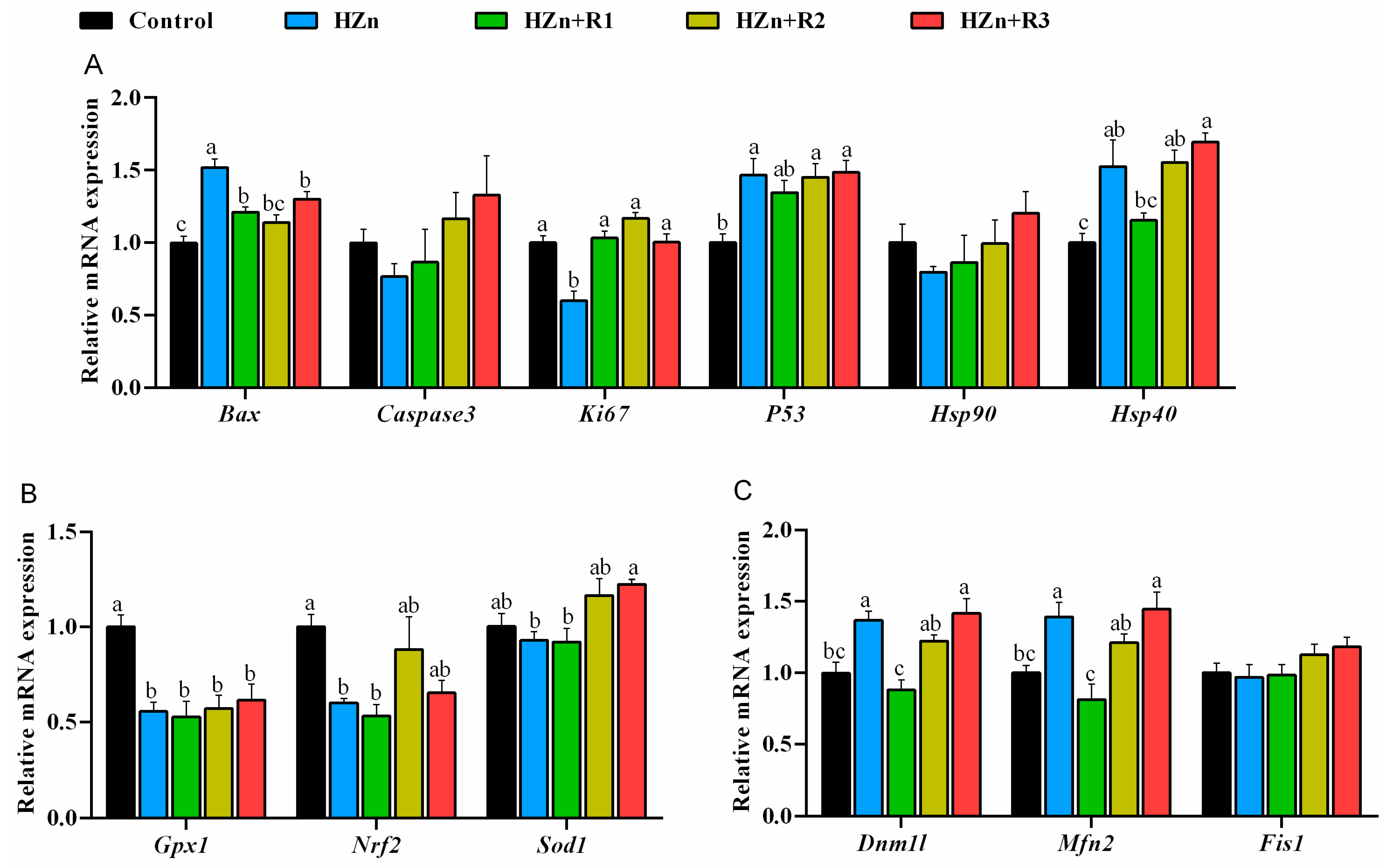
3.9. Gene Expression Involved in Antioxidant Capacity, Apoptosis, and Mitochondrial Function in Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | malondialdehyde |
| T-SOD | total superoxide dismutase |
| GOT | glutamic oxalacetic transaminase |
| GPT | glutamic pyruvic transaminase |
| AKP | alkaline phosphatase |
| T-AOC | total antioxidant capacity |
| Bax | BCL2-associated X protein |
| Hsp90 | heat shock protein 90 |
| Hsp40 | heat shock protein 40 |
| Nrf2 | nuclear factor erythroid-2-related factor 2 |
| Sod1 | superoxide dismutase 1 |
| Gpx1 | glutathione peroxidase 1 |
| Dnm1l | dynamin 1-like |
| Mfn2 | mitofusin 2 |
| Fis1 | fission, mitochondrial 1 |
References
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef]
- Gur, T.; Meydan, I.; Seckin, H.; Bekmezci, M.; Sen, F. Green synthesis, characterization and bioactivity of biogenic zinc oxide nanoparticles. Environ. Res. 2022, 204 Pt A, 111897. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, R.; Kumari, A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. EXCLI J. 2020, 19, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS ONE 2016, 11, e0164434. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, K.; Zhou, L.; He, J.; Zheng, X.; Zhang, L.; Zhong, X.; Wang, T. Evaluation of Long-Term Toxicity of Oral Zinc Oxide Nanoparticles and Zinc Sulfate in Mice. Biol. Trace Elem. Res. 2017, 178, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Milla, C.; Macías Macías, F.I.; Velázquez Delgado, K.A.; Herrera Rodríguez, M.A.; Colín-Val, Z.; Ramos-Godinez, M.D.P.; Cano-Martínez, A.; Vega-Miranda, A.; Robledo-Cadena, D.X.; Delgado-Buenrostro, N.L.; et al. Zinc oxide nanoparticles induce toxicity in H9c2 rat cardiomyoblasts. Int. J. Mol. Sci. 2022, 23, 12940. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Naqvi, Q.U.; Kanwal, A.; Qaseem, S.; Naeem, M.; Ali, S.R.; Shaffique, M.; Maqbool, M. Size-dependent inhibition of bacterial growth by chemically engineered spherical ZnO nanoparticles. J. Biol. Phys. 2019, 45, 147–159. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Manuelian, C.L.; Pitino, R.; Simoni, M.; Mavrommatis, A.; De Marchi, M.; Righi, F.; Tsiplakou, E. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on livestock mammals’ performances, health, and oxidative status: A review of the literature in the last 20 years. Antioxidants 2021, 10, 1461. [Google Scholar] [CrossRef]
- Goyal, J.; Verma, P.K. An Overview of Biosynthetic Pathway and Therapeutic Potential of Rutin. Mini Rev. Med. Chem. 2023, 23, 1451–1460. [Google Scholar] [CrossRef]
- Chitrakar, B.; Hou, Y.; Devahastin, S.; Zhang, M.; Sang, Y. Protocols for extraction and purification of rutin from leafy by-products of asparagus (Asparagus officinalis) and characterization of the purified product. Food Chem. 2023, 418, 136014. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.F.; Wu, Y.; Wang, Y.; et al. Rutin ameliorated lipid metabolism dysfunction of diabetic NAFLD via AMPK/SREBP1 pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Badr, G.M.; Sedky, A.; Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M. Rutin ameliorates carbon tetrachloride (CCl4)-induced hepatorenal toxicity and hypogonadism in male rats. PeerJ 2019, 7, e7011. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Kumar, S.; Kumar, P.; Bisht, S.; Kumar, A.; Maurya, A.K.; Pal, A.; Bawankule, D.U. Rutin ameliorates malaria pathogenesis by modulating inflammatory mechanism: An in vitro and in vivo study. Inflammopharmacology 2022, 30, 159–171. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Li, Z.; Li, J.; Chen, Y.; Wang, T.; Wang, C. Effects of zinc oxide nanoparticles on growth, intestinal barrier, oxidative status and mineral deposition in 21-day-old broiler chicks. Biol. Trace Elem. Res. 2022, 200, 1826–1834. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Zhang, J.; Liu, H.; Chen, S.; He, Y.; Wang, T.; Wang, C. Effects of Long-Term Dietary Zinc Oxide Nanoparticle on Liver Function, Deposition, and Absorption of Trace Minerals in Intrauterine Growth Retardation Pigs. Biol. Trace Elem. Res. 2023, 201, 4746–4757. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Mirsafi, F.S.; Leißner, T.; Akan, T.; Mishra, Y.K.; Kışla, D. Zinc oxide nanomaterials: Safeguarding food quality and sustainability. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70051. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of zinc oxide nanostructures with Mammalian cells: Cytotoxicity and photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, W.; Lu, X.; Chen, X.; Shan, Y.; Luo, X.; Li, Y.; Yang, X.; Li, C. Zinc oxide nanoparticles induces cell death and consequently leading to incomplete neural tube closure through oxidative stress during embryogenesis. Cell Biol. Toxicol. 2024, 40, 51. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, G.; Huang, J.; Zhang, Y.; Cheng, X.; Chuai, M.; Brand-Saberi, B.; Chen, G.; Jiang, X.; Yang, X. Zinc oxide nanoparticles exposure-induced oxidative stress restricts cranial neural crest development during chicken embryogenesis. Ecotoxicol. Environ. Saf. 2020, 194, 110415. [Google Scholar] [CrossRef]
- Rahmani, S.; Naraki, K.; Roohbakhsh, A.; Hayes, A.W.; Karimi, G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Sci. Nutr. 2022, 11, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lu, X.; Ji, Z.; Dong, L.; Jiang, J.; Tian, J.; Wen, H.; Xu, Z.; Xu, G.; Jiang, M. Preliminary study to assess the impact of dietary rutin on growth, antioxidant capacity, and intestinal health of yellow catfish, Pelteobagrus fulvidraco. Animals 2023, 13, 3386. [Google Scholar] [CrossRef]
- Shenbagam, M.; Nalini, N. Dose response effect of rutin a dietary antioxidant on alcohol-induced prooxidant and antioxidant imbalance—A histopathologic study. Fundam. Clin. Pharmacol. 2011, 25, 493–502. [Google Scholar] [CrossRef]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, B.; Liu, H.; Chen, S.; Zhang, J.; Wang, T.; Wang, C. Dietary rutin improves the antidiarrheal capacity of weaned piglets by improving intestinal barrier function, antioxidant capacity and cecal microbiota composition. J. Sci. Food Agric. 2024, 104, 6262–6275. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhang, J.; Zhou, B.; Zhuang, S.; He, X.; Wang, T.; Wang, C. Effects of different levels of rutin on growth performance, immunity, intestinal barrier and antioxidant capacity of broilers. Ital. J. Anim. Sci. 2022, 21, 1390–1401. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Raghu, P.; Nair, K.M. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J. Food Sci. 2010, 75, H123–H128. [Google Scholar] [CrossRef]
- Liao, X.; Ji, P.; Chi, K.; Chen, X.; Zhou, Y.; Chen, S.; Cheng, Y.; Flaumenhaft, R.; Yuan, C.; Huang, M. Enhanced inhibition of protein disulfide isomerase and anti-thrombotic activity of a rutin derivative: Rutin:Zn complex. RSC Adv. 2023, 13, 11464–11471. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. S1), 2–6. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhang, J.; Zhou, B.; He, X.; Wang, T.; Wang, C. Dietary rutin improves breast meat quality in heat-stressed broilers and protects mitochondria from oxidative attack via the AMPK/PINK1-Parkin pathway. J. Sci. Food Agric. 2023, 103, 2367–2377. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Li, D.; Lei, M.; Li, Q.; Liu, X.; Zhang, P. Dnm1l-related mitochondrial fission defects presenting as encephalopathy: A case report and literature review. Front. Pediatr. 2021, 9, 626657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, Z.; Yu, J.; Zhong, H.; Zhuo, X.; Jia, C.; Wan, Y. Mitofusin-2 restrains hepatic stellate cells’proliferation via PI3K/Akt signaling pathway and inhibits liver fibrosis in rats. J. Healthc. Eng. 2022, 2022, 6731335. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Dialdehyd malonowy (MDA) jako wskaźnik procesów peroksydacji lipidów w organizmie. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. (In Polish) [Google Scholar] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Elkomy, M.H.; Fahim, H.I.; Ashour, M.B.; Naguib, I.A.; Alghamdi, B.S.; Mahmoud, H.U.R.; Ahmed, N.A. Rutin and Quercetin Counter Doxorubicin-Induced Liver Toxicity in Wistar Rats via Their Modulatory Effects on Inflammation, Oxidative Stress, Apoptosis, and Nrf2. Oxid. Med. Cell. Longev. 2022, 2022, 2710607. [Google Scholar] [CrossRef] [PubMed]



| Gene | Accession No. | Sequence (5′ to 3′) | Size (bp) |
|---|---|---|---|
| β-actin | NM_007393 | F: AGCCATGTACGTAGCCATCC | 194 |
| R: CTCTCAGCTGTGGTGGTGAA | |||
| Bax | NM_007527.3 | F: TGCAGAGGATGATTGCTGAC | 173 |
| R: GATCAGCTCGGGCACTTTAG | |||
| Caspase-3 | XM_017312543.3 | F: GTGAGCCGGTGCGGTG | 250 |
| R: ACATCCGTACCAGAGCGAGA | |||
| Ki67 | XM_006507413.5 | F: CCATCATTGACCGCTCCTTT | 145 |
| R: TCACTCTTGTCAGGGTCAGC | |||
| P53 | AB021961.1 | F: AAGGATGCCCATGCTACAGAG | 223 |
| R: GAGTGGATCCTGGGGATTGTGTC | |||
| Hsp90 | NM_008302.3 | F: GCGGCAAAGACAAGAAAAAG | 167 |
| R: CAAGTGGTCCTCCCAGTCAT | |||
| Hsp40 | AB028272.1 | F: CCAATGGGTATGGGTGGCTT | 81 |
| R: ATCTTGCTTCTTCCGGGTGG | |||
| Nrf2 | NM_010902 | F: CTCGCTGGAAAAAGAAGTGG | 240 |
| R: CCGTCCAGGAGTTCAGAGAG | |||
| Sod1 | NM_011434.2 | F: CCAGTGCAGGACCTCATTTT | 197 |
| R: TTGTTTCTCATGGACCACCA | |||
| Gpx1 | NM_008160 | F: GTCCACCGTGTATGCCTTCT | 152 |
| R: TCTGCAGATCGTTCATCTCG | |||
| Dnm1l | NM_001360010.1 | F: GCCTCAGATCGTCGTAGTGG | 194 |
| R: TTTTCCATGTGGCAGGGTCA | |||
| Mfn2 | NM_001355590.1 | F: CTCCATTCAAGAAGCTTGGACAG | 229 |
| R: ACTTCAGCCATGTGTCGCTT | |||
| Fis1 | NM_001347504.1 | F: TACTCCTTCTACCCCGAGGC | 279 |
| R: TCCTTGCAGCTTCGTCTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Ma, L.; Zhang, J.; Zhou, B.; Chen, S.; Tu, M.; Cai, G.; Wang, T.; Wang, C. Dietary Rutin Ameliorates Nanoparticle Zinc Oxide-Induced Toxicity in Mice by Potentiating Antioxidant Defense Mechanisms. Nutrients 2025, 17, 1495. https://doi.org/10.3390/nu17091495
He X, Ma L, Zhang J, Zhou B, Chen S, Tu M, Cai G, Wang T, Wang C. Dietary Rutin Ameliorates Nanoparticle Zinc Oxide-Induced Toxicity in Mice by Potentiating Antioxidant Defense Mechanisms. Nutrients. 2025; 17(9):1495. https://doi.org/10.3390/nu17091495
Chicago/Turabian StyleHe, Xiaofang, Longfei Ma, Jiaqi Zhang, Binbin Zhou, Shun Chen, Minhang Tu, Gentan Cai, Tian Wang, and Chao Wang. 2025. "Dietary Rutin Ameliorates Nanoparticle Zinc Oxide-Induced Toxicity in Mice by Potentiating Antioxidant Defense Mechanisms" Nutrients 17, no. 9: 1495. https://doi.org/10.3390/nu17091495
APA StyleHe, X., Ma, L., Zhang, J., Zhou, B., Chen, S., Tu, M., Cai, G., Wang, T., & Wang, C. (2025). Dietary Rutin Ameliorates Nanoparticle Zinc Oxide-Induced Toxicity in Mice by Potentiating Antioxidant Defense Mechanisms. Nutrients, 17(9), 1495. https://doi.org/10.3390/nu17091495






