Understanding Stunting: Impact, Causes, and Strategy to Accelerate Stunting Reduction—A Narrative Review
Abstract
1. Introduction
2. Methods of Search and Inclusion of Articles
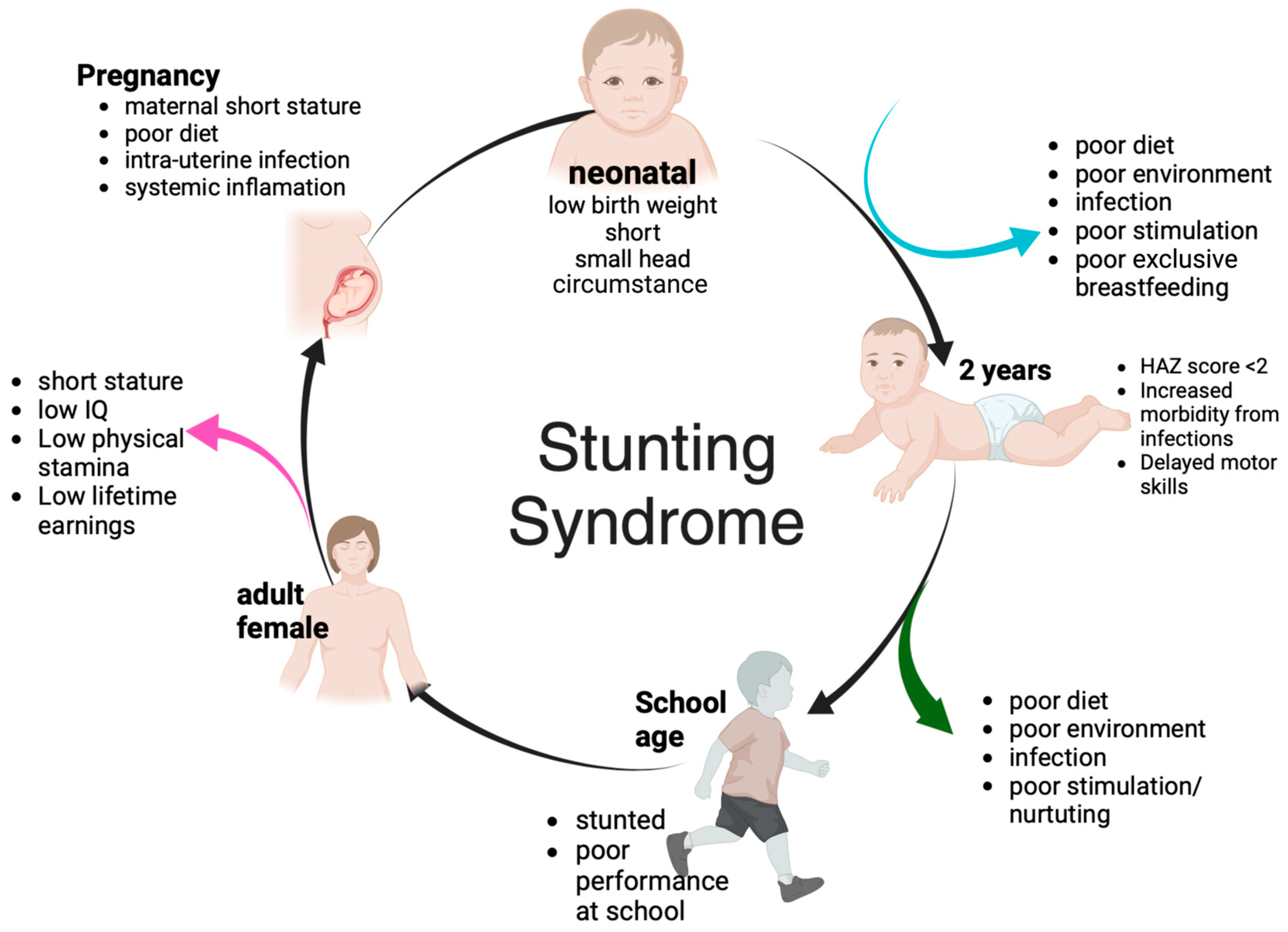
3. Stunting Impact and Its Causes
3.1. Growth Failure
3.2. Cognitive Impairment
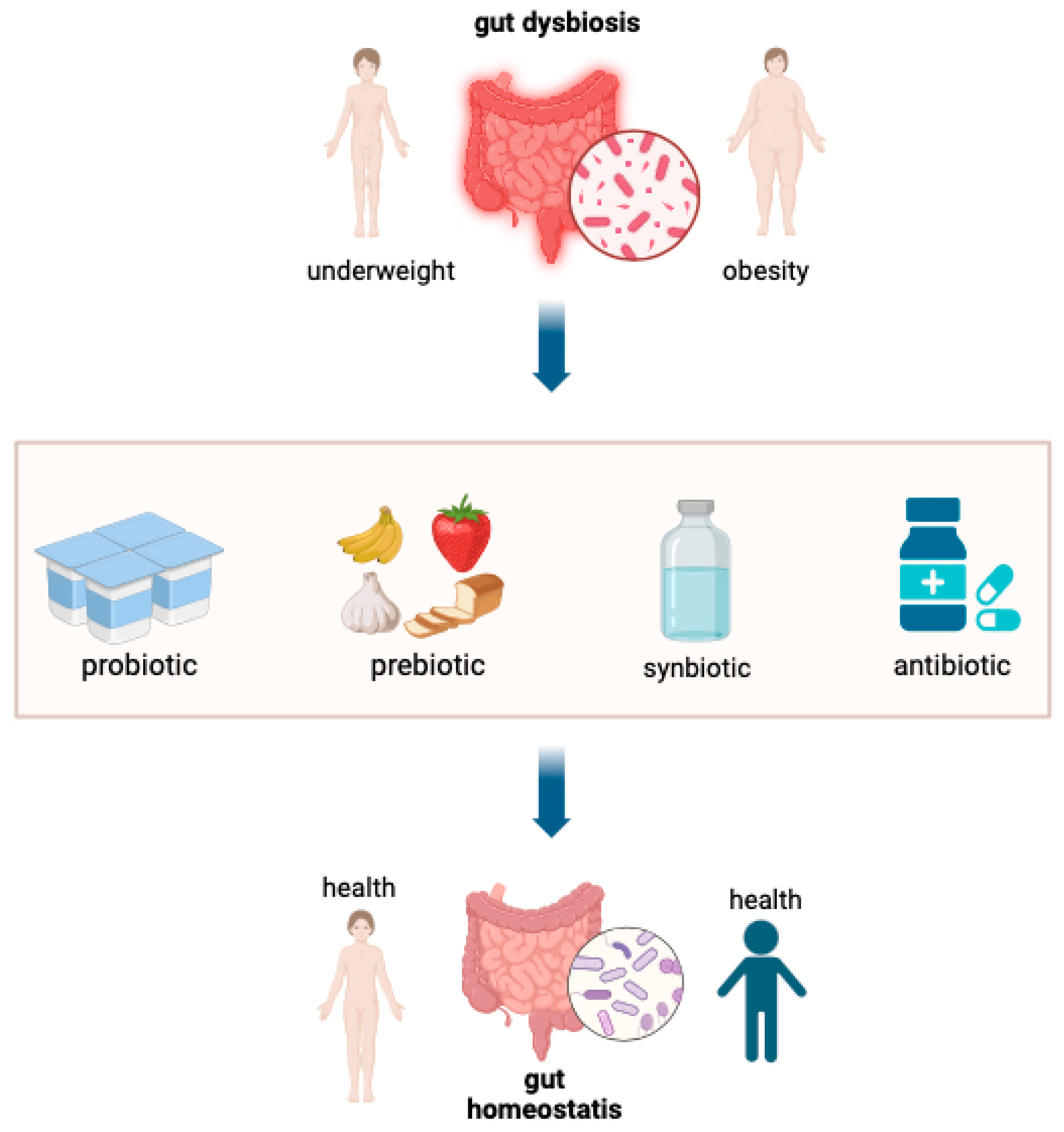
3.3. Dysbiosis (Infection, Enteric Environmental Dysfunction)
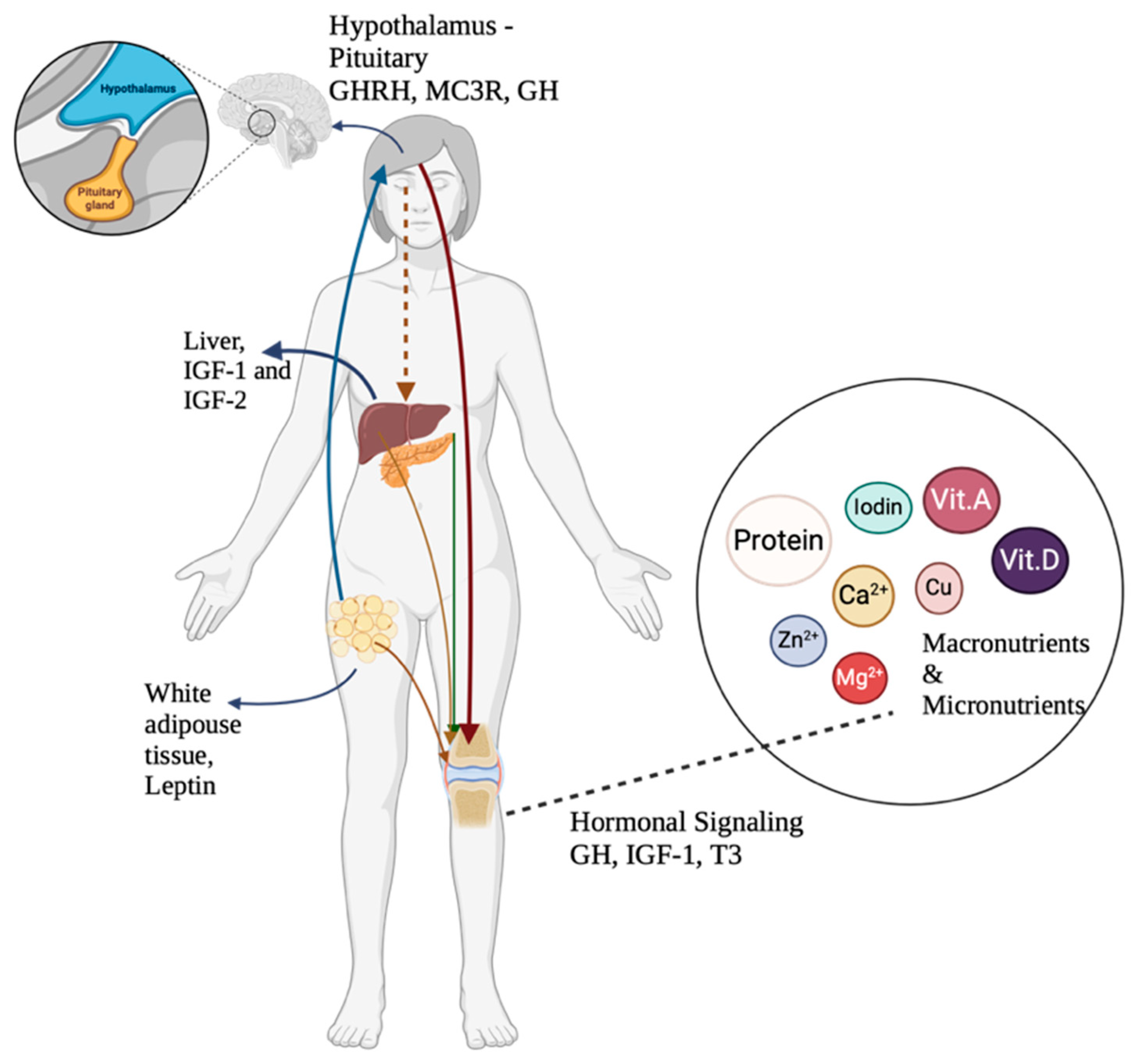
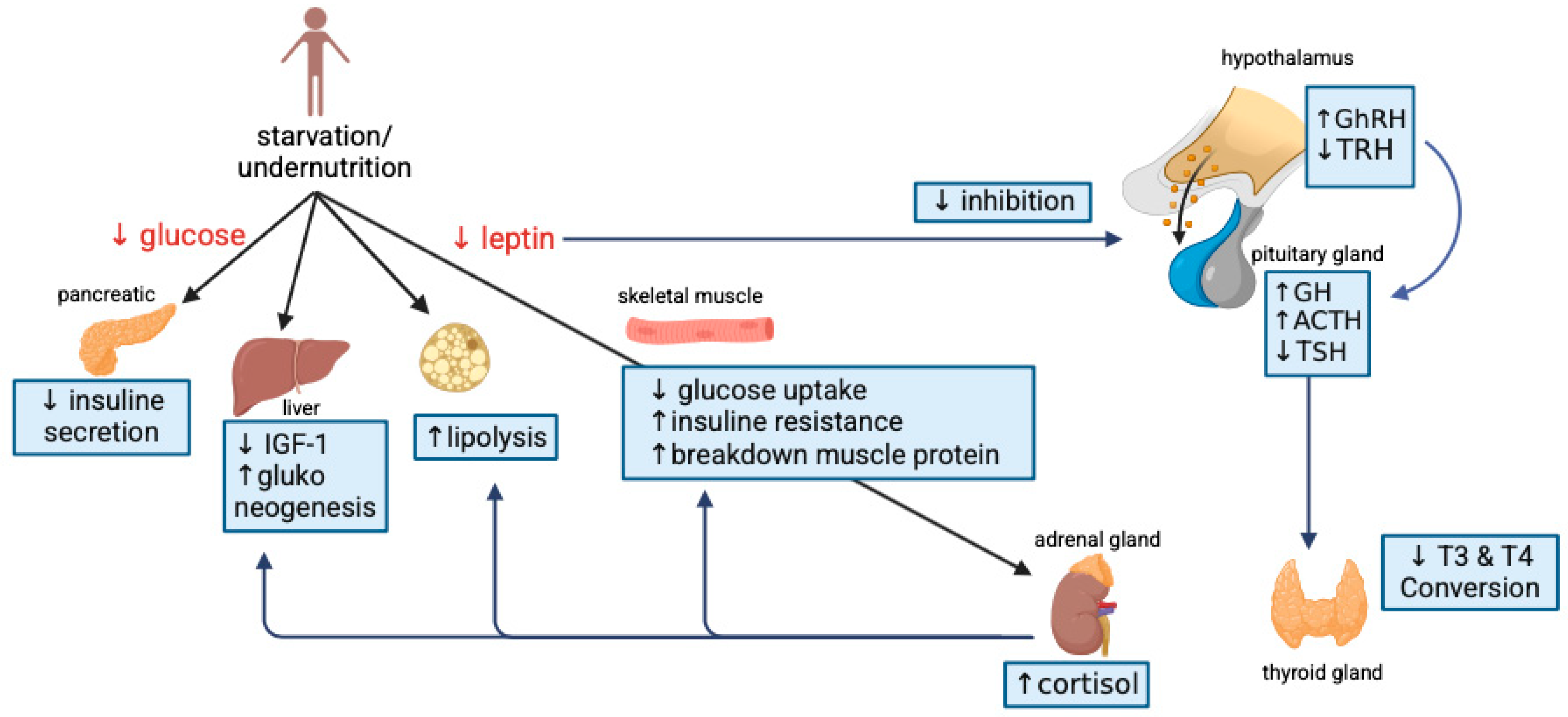
3.4. Endocrine Dysregulation
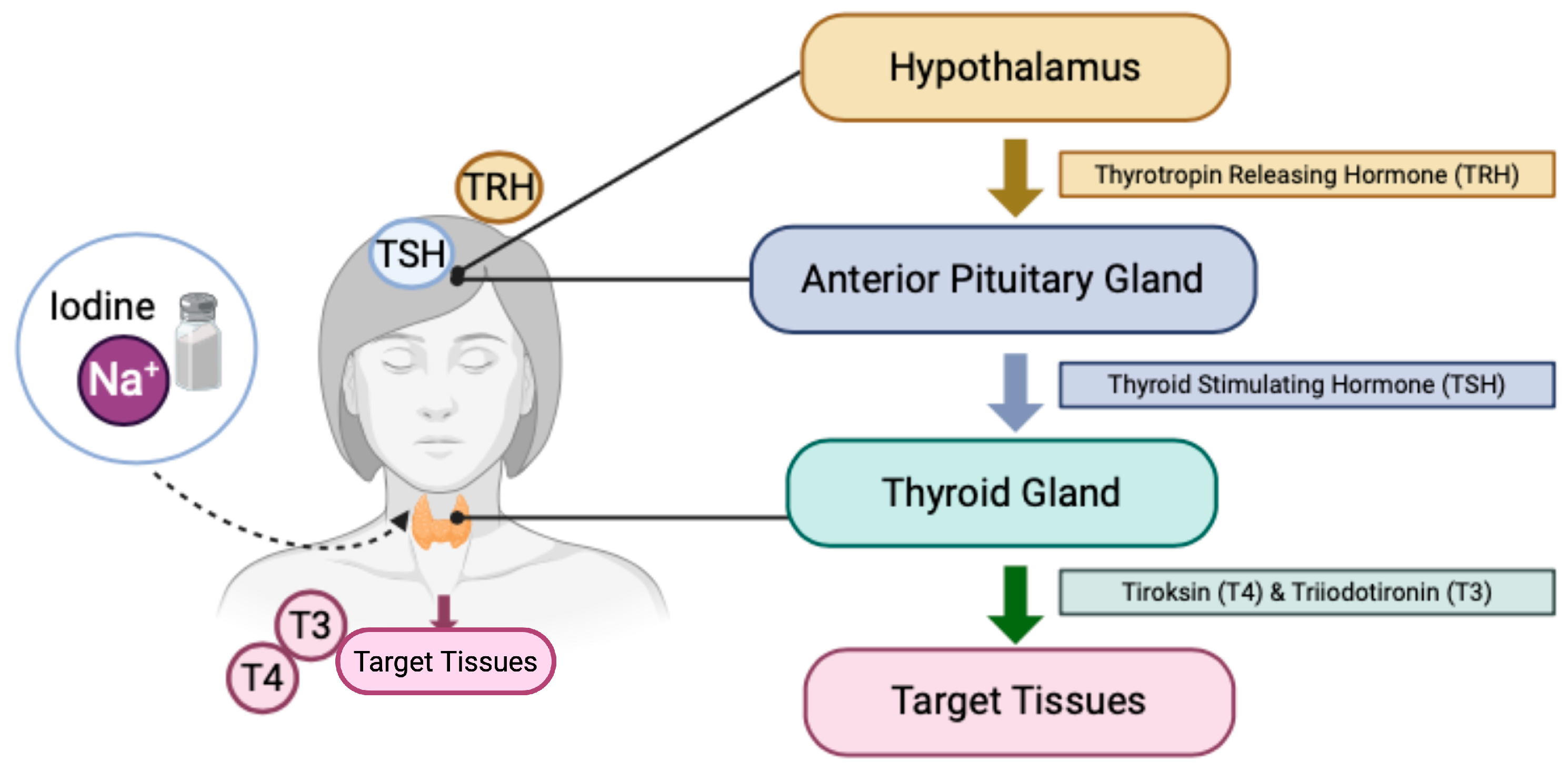
3.4.1. Hypothyroid
3.4.2. Diabetes Mellitus
3.4.3. Anemia
4. Accelerated Strategies to Reduce Stunting Prevalence as Part of the Sustainable Development Goal (SDG) of Countries
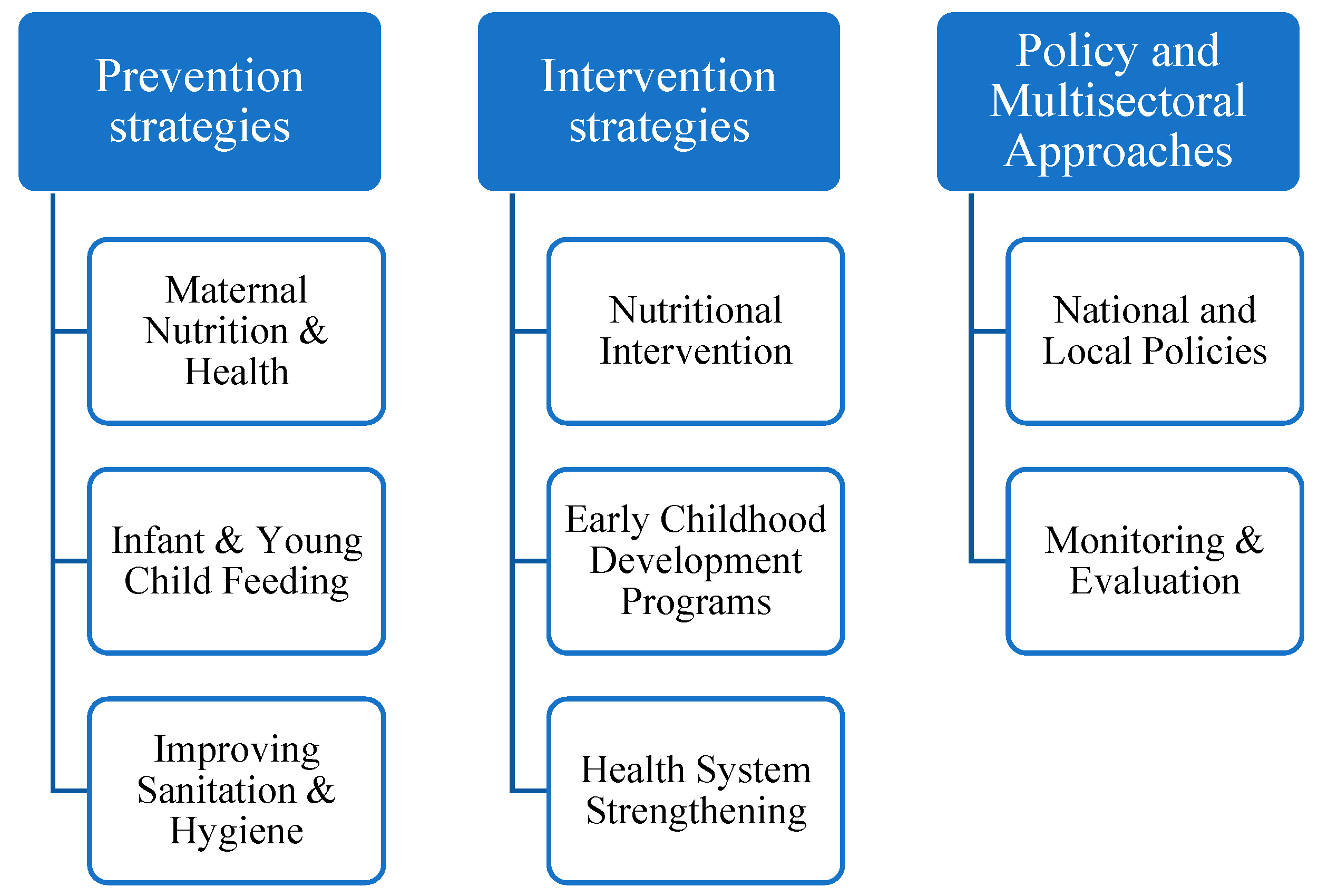
4.1. Prevention Strategies
4.1.1. Maternal Health and Nutrition
4.1.2. Infant and Young Child Feeding
4.1.3. Improvement of Sanitation and Hygiene
4.2. Intervention Strategies
4.2.1. Nutritional Intervention
Impaired Growth and Development
Gut Dysbiosis or Environmental Enteric Dysfunction (EED)
Hypothyroidism
Anemia
4.2.2. Early Childhood Development Programs
4.2.3. Strengthening the Health System
4.3. Multisectoral Policies and Approaches
4.3.1. Local and National Policies
4.3.2. Monitoring and Evaluation
5. Limitations and Future Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Number | Respondent Characteristics | Research Objectives | Main Findings | Ref. |
|---|---|---|---|---|
| 3.1. Growth Failure | ||||
| 1 | 185 children aged 6 to 59 months | To determine the prevalence of putative developmental delay and the variables linked to it in children under five in rural Pakistan suffering from severe acute malnutrition (SAM) without comorbidities. | Suspected global developmental delay is much more common in children with severe acute malnutrition, particularly in those who were not nursed exclusively and had a history of contact with TB-positive adults. | [39] |
| 2 | 78 children aged 5 to 12 months | To explore the correlation between linear growth, systemic inflammation, and gut damage in infants at risk of stunting. | Stunted children show differences in their fecal microbiota compared to non-stunted children, which are associated with increased systemic inflammatory markers. | [52] |
| 3 | 710 children aged 4 to 6 years | To evaluate the association between linear growth and hemoglobin concentrations during growth with motor, cognitive, and socio-emotional development in Ghana. | Birth length-for-age z-score (LAZ) is significantly associated with cognitive development and hemoglobin levels at 18 months; however, no significant correlation was found with motor or socio-emotional development. | [106] |
| 3.2. Impaired Cognition | ||||
| 1 | 51 children aged 1 month to 3 years | To assess cognitive development in children experiencing stunting and malnutrition compared to their well-nourished peers. | The tendency for cognitive, motor, and adaptive skills is significantly lower in stunted children compared to those with malnutrition and those with normal development. | [40] |
| 2 | 300 children aged 1 to 3 years | To investigate the cognitive development of stunted children and malnutrition compared to children with normal nutrition. | Children experiencing stunting are at a higher risk of cognitive developmental delays compared to children who are not stunted. | [107] |
| 3 | 4379 children aged 0 to 5 years | To analyze the correlation between stunted children and its impact on educational outcomes and cognitive performance in adulthood in Indonesia | Stunted children and those of relatively small stature are significantly associated with cognitive development, leading to poorer educational outcomes. | [45] |
| 3.3. Dysbiosis (Infection, Enteric Erectical Dysfunction) | ||||
| 1 | 224 children aged 24 to 59 months | To investigate the relationship between stunting, sanitation, intestinal infections, and environmental enteric dysfunction (EED) in Ethiopia. | Stunted children are significantly associated with hygiene, poor dietary patterns, inadequate sanitation, intestinal infections, and environmental enteric dysfunction (EED). | [108] |
| 2 | 42 children aged 24 to 59 months | To determine the association between intestinal infections, growth biomarkers (IGF-1), and gut microbiota composition in children with stunting in Indonesia | The gut microbiota profile of stunted children exhibits dysbiosis, characterized by an overabundance of taxa that induces inflammation, metabolic abnormalities, and unhealthy dietary patterns. | [16] |
| 3 | 82 children aged 6 to 59 months | To examine the relationship between intestinal inflammation and stunted children under 5 years old in Ethiopia. | A strong correlation exists between stunting and gastrointestinal inflammation, diarrhea, breastfeeding duration, environmental factors, and family size. | [109] |
| 4 | 131 children aged 3 to 5 years | To investigate alterations in gut microbiota composition between stunted children and those with normal nutritional status. | Prevotella 9 levels are significantly lower in stunted children, indicating lower fiber intake, which is supported by nutritional intake data compared to normal children. | [53] |
| 5 | 42 children aged 2 to 5 years | To investigate the gut microbiota composition and related variables that affect stunted and non-stunted children in Jakarta’s slums. | Stunted children exhibit a gut microbiota composition significantly different from non-stunted children. This composition is characterized by an increased prevalence of pathogenic bacteria and a reduced abundance of beneficial bacteria. | [99] |
| 3.4. Metabolic System Disorder | ||||
| 1 | 3046 school-aged children, 8 to 10 years old | To examine the prevalence of stunting and how iodine deficiency disorders (IDDs) might predict stunting in Aseer region primary school students. | IDDs may predict stunting, with students exhibiting clinical goiter and those with urinary iodine concentration (UIC) levels < 17 μg/L showing a significant association with stunting. | [63] |
| 2 | 1135 adults aged 30 to 89 years | To examine the correlation between birth weight and type 2 diabetes mellitus in the general population of Japan. | Low birth weight in the Japanese population is associated with an increased risk of acquiring type 2 diabetes mellitus, particularly among those who are overweight or obese. | [70] |
| 3 | 1682 adults aged 18 to 68 years | To explore the relationship between low stature and increased sitting height ratio (SHR) as indications of stunting and obesity in adults. | Short stature is identified as a risk factor for obesity in both adult men and women in Portugal. | [110] |
| 4 | 3534 adult men and women aged 30 years and above | Testing the association between childhood stunting and a higher risk of cardiometabolic disorders in adulthood. | Adults who experienced stunting during childhood tend to have less muscle mass and subcutaneous fat and may exhibit a higher tendency for visceral fat accumulation. | [111] |
| 5 | 3844 children and adolescents aged 7 to 18 years | To investigate the correlation between short stature, obesity, and cardiometabolic risk factors in children and adolescents in Iran. | Children and adolescents with short height are at an elevated risk of acquiring metabolic syndrome, abdominal obesity, and hypertension relative to their counterparts of normal size. | [81] |
| 3.5. Anemia | ||||
| 1 | 21,172 children aged 6–59 months | Investigating the prevalence of anemia and stunting and identifying the factors influencing these conditions in children aged 6–59 months in Ethiopia. | The prevalence of anemia and stunting is 24.4%, with a 95% Confidence Interval (CI) of (23.8–24.9%). Key risk factors contributing to this prevalence include the following: anemia in mothers, very short stature of mothers, low maternal education, poor hygiene practices, and living in rural areas. | [75] |
| 2 | 750 children aged 12–59 months | Assessing the status of micronutrients and the correlation with hemoglobin levels in children aged 12–59 months with stunting. | Two out of three children with stunting also experience anemia. Malaria and other infections, in addition to micronutrient deficiencies, significantly contribute to anemia in these children. | [112] |
| 3 | 21,918 children aged 6–59 months | Examining the relationship between malnutrition (stunting, wasting, underweight) and open defecation and how these relate to anemia in children aged 6–59 months in Ethiopia. | In Ethiopia, childhood malnutrition partially mediates the relationship between open defecation and anemia, highlighting thes importance of sanitation in addressing both issues. | [76] |
| Environment, Social, and Economy | ||||
| 1 | 750 children aged 1–5 years | Evaluating the socioeconomic factors, household conditions, anthropometric values, and clinical changes based on early childhood development (ECD) outcomes for stunted children aged 1–5 years. | Stunting is closely related to developmental outcomes. Children with severe stunting tend to have lower early childhood development (ECD) scores, and this gap persists or even worsens over time. | [113] |
Appendix B
| No. | Respondent Characteristics | Intervention | Main Findings | Ref |
|---|---|---|---|---|
| ||||
| 1 | 85 mothers with children aged 0–6 months | Education and simulation classes (principles of breastfeeding, introduction to complementary feeding), monthly home visits totalling 15 visits, growth monitoring, and sanitation monitoring. | Maternal nutritional literacy (MNL) plays a crucial role in the prevention of stunting, with a focus not only on stunted children but also on healthy children. Emphasizing exclusive breastfeeding for stunted children is central to MNL efforts. | [85] |
| 2 | 3120 mothers with children aged 0–5 months | Specific nutrition intervention programs will be implemented over 36 months, divided into 4 phases with gradual evaluations. These programs will include monitoring of development and cooking practices or demonstrations. | The Suchana program has demonstrated positive outcomes in promoting exclusive breastfeeding practices among children in Bangladesh, with exclusive breastfeeding being identified as a key factor in reducing stunting. | [114] |
| 3 | 408 children aged 6–24 months | The data collection consists of a quantitative survey examining breastfeeding practices, demographic and socioeconomic characteristics, and monthly family expenditures. The survey utilizes a structured questionnaire conducted by professional interviewers. | Exclusive breastfeeding can reduce the likelihood of children experiencing stunting. It provides a cost-effective and efficient solution for low-income households to address stunting. | [115] |
| 4 | 8451 caregivers with children aged 6–23 months | Supplementary feeding provision (including protein, fat, carbohydrates, vitamin A, B1, B2, B12, D3, folic acid, iron, zinc, and calcium) and complementary feeding counseling for caregivers. | Community-based complementary food supplements and dietary guidance can enhance feeding patterns and reduce the incidence of anemia. | [92] |
| ||||
| 1 | 2928 and 3205 households with children under 2 years of age | Pregnant and breastfeeding women received Super Cereal 7.5 kg (250 g/day) during pregnancy and for six months of breastfeeding. Children aged 6–23 months were given 30 sachets of lipid-based nutritional supplements (50 g/sachet/day) each month. | The use of small-quantity nutrient supplements (SQNS) alongside social and behavioral change communication (SBCC) during the first 1000 days of life is linked to a decrease in stunting and malnutrition and improvements in infant and young child feeding practices for children under 2 years old. | [93] |
| 2 | 6674 children under 5 years of age | Female healthcare workers distributed a soy–wheat blend to pregnant and breastfeeding women, as well as micronutrient powder to children aged 6–23 months and 24–59 months. | Short-term nutritional absorption through the Wawamum intervention is not effective in reducing stunting. Proven communication about behavior change must be supported to enhance complementary feeding behaviors. | [116] |
| 3 | 110 children aged 6–23 months | Daily consumption of Wawamum, consisting of one sachet of 50 g, was given to children over 12 months. | Wawamum (LNS-MQ) has proven its efficacy in enhancing micronutrient status, hemoglobin levels, and growth metrics in children aged 6 to 23 months. | [102] |
| 4 | 4011 women in early pregnancy (under 20 weeks) and 1552 adolescents |
| Providing LNS before birth may reduce newborn stunting and low birth weight, significantly reducing wasting compared to IFA supplementation. | [88] |
| 5 | 971 children aged 6–72 months | ‘Chispuditos®’, a hot beverage enriched with micronutrients (atole + MN), providing 9 mg of zinc and 12.5 mg of iron, or lactose-free milk, was provided for 18 months. | Long-term micronutrient supplementation in beverages does not significantly impact growth or nutritional status. | [117] |
| 6 | 1059 children aged 15 months to 7 years, | Food supplements (NEWSUP), which contain plants rich in polyphenols and omega-3 fatty acids, along with various micronutrients and high-protein content, or fortified blended foods (FBFs) or traditional rice-based breakfast as a control food, were administered every morning for 23 weeks. | NEWSUP has been shown to improve working memory and reduce BMI compared to the normal group. Additionally, NEWSUP increases hemoglobin concentration in children with anemia. Supplementation over 23 weeks enhances executive function, brain health, and nutritional status in children. | [118] |
| 7 | 67 children aged 2 to 6 months, | Severely malnourished infants were provided with probiotics (Bifidobacterium infantis EVC001) or synbiotics (B. infantis EVC001 + Lacto-N-neotetraose [LNnT]) or a placebo (lactose) for four weeks, followed by a four-week follow-up after supplementation. | Severely malnourished infants exhibit higher weight gain when given probiotics. | [99] |
| 8 | 151 children aged 3 to 24 months | The intervention involving the commercial strain of B. infantis from a U.S. donor (EVC001) was administered daily for 28 days to the control group with or without human milk oligosaccharides (HMOs), lacto-N-neotetraose, or the placebo SYNERGIE. | The abundance of Bifidobacterium infantis increased in the feces of infants with severe acute malnutrition (SAM). However, the increase was 10 to 100 times lower than the abundance observed in healthy controls. | [98] |
| 9 | 304 children aged 29 to 49 months | Preschool children were given a dual-micronutrient powder or placebo fortification in their food six days per week (excluding Sundays and public holidays) for 8 months. | Providing micronutrients during the first 1000 days of life can reduce developmental delays and decrease anemia and iron deficiency. | [119] |
| 10 | 387 mothers with children under 2 years of age. | The intensive nutrition counseling intervention, including information on maternal nutrition, exclusive breastfeeding, and complementary feeding demonstration, was provided along with LNS for children aged 6 to 18 months as well as pregnant or breastfeeding women until the infant reached 6 months. | Children who received intensive nutrition counseling and lipid-based nutrient supplementation (LNS) for pregnant women until the child reached 18 months of age had a lower prevalence of anemia and iron-deficiency anemia than children in the control group. | [103] |
| 11 | 5920 preschool children aged 3 to 6 years | The questionnaire filled out by the child’s caregiver encompassed demographic attributes of the child, including age, gender, ethnicity, source of drinking water, geographic location, degree of urbanization, and exposure to secondhand smoking. | Iodine nutrition in preschool children and their physical growth is linked to the child’s age and height. | [120] |
| 12 | 834 mothers with children aged 6 to 59 months | Health educators conducted a behavioral intervention on iodine intake through food over 17 months. The program included education to increase the utilization of iodized salt, reduce the prevalence of iodine deficiency, and improve children’s linear growth. | The prevalence of stunting among children in the intervention group at the start of the study (40.5%) decreased by the end of the study (15.1%). Additionally, urinary iodine concentrations in the intervention group increased compared to the normal group. | [121] |
| 13 | 96,512 mothers with children under 2 years of age. | The study utilized a nationally representative survey on socio-demographic, health, and nutrition indicators. Exposure to antenatal iron–folic acid (IFA) supplementation was gathered retrospectively through maternal recall. | Antenatal iron–folic acid (IFA) supplementation was significantly linked to a reduced risk of below-average birth size, stunting, and severe stunting in children under 2 years of age in South Asia. | [87] |
| ||||
| 1 | 253 preschool children aged 3 to 5 years, | The 12-month health education program included education on nutrition deficiencies, demonstrations, and explanations on preparing nutritious meals and various healthy recipes that provide protein, energy, calcium, and iron to mothers. | Children who received the intervention showed improved cognitive development compared to the control group. Home-based, nutrition-focused foods contributed to this enhancement. | [122] |
| 2 | 2222 mothers with children aged 6 to 24 months. | Community health volunteers conducted 16 nutrition education and stimulation sessions (every two weeks) under a program named Msingi Bora. The Msingi Bora curriculum emphasized five key practices: responsive play, responsive communication, hygiene, nutrition, and fostering love and respect within the family. | Parenting interventions conducted by trained community health volunteers in the mother–child group effectively improved child development within the community. | [123] |
References
- de Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016, 16, 16–26. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Soliman, A.; De Sanctis, V.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N.; Soliman, N. Early and long-term consequences of nutritional stunting: From childhood to adulthood. Acta Biomed. 2021, 92, e2021168. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- van Zyl, C.; van Wyk, C. Exploring factors that could potentially have affected the first 1000 days of absent learners in South Africa: A qualitative study. Int. J. Environ. Res. Public Health 2021, 18, 2768. [Google Scholar] [CrossRef]
- Victora, C.G.; de Onis, M.; Shrimpton, R. Linear growth faltering should be assessed in absolute and relative terms. J. Nutr. 2014, 144, 2092–2093. [Google Scholar] [CrossRef] [PubMed]
- WHO. Stunting in a Nutshell. 2015. Available online: https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell (accessed on 16 January 2025).
- Millward, D.J. Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev. 2017, 30, 50–72. [Google Scholar] [CrossRef]
- WHO. Global Nutrition Targets 2025: Stunting Policy Brief. Available online: https://iris.who.int/handle/10665/149019 (accessed on 16 January 2025).
- Hörnell, A.; Lagström, H. Infant feeding—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 68, 10-29219. [Google Scholar] [CrossRef]
- Irarrázaval, B.; Barja, S.; Bustos, E.; Doirsaint, R.; Senethmm, G.; Guzmán, M.P.; Uauy, R. Influence of feeding practices on malnutrition in haitian infants and young children. Nutrients 2018, 10, 382. [Google Scholar] [CrossRef]
- UNICEF. Improving Young Children’s Diets during the Complementary Feeding Period. In UNICEF Programming Guidance; UNICEF: New York, NY, USA, 2020. [Google Scholar]
- Titaley, C.R.; Ariawan, I.; Hapsari, D.; Muasyaroh, A.; Dibley, M.J. Determinants of the stunting of children under two years old in Indonesia: A multilevel analysis of the 2013 Indonesia basic health survey. Nutrients 2019, 11, 1106. [Google Scholar] [CrossRef]
- Budge, S.; Parker, A.H.; Hutchings, P.T.; Garbutt, C. Environmental enteric dysfunction and child stunting. Nutr. Rev. 2019, 77, 240–253. [Google Scholar] [CrossRef]
- Rinanda, T.; Riani, C.; Artarini, A.; Sasongko, L. Correlation between gut microbiota composition, enteric infections and linear growth impairment: A case–control study in childhood stunting in Pidie, Aceh, Indonesia. Gut Pathog. 2023, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Vonaesch, P.; Randremanana, R.; Gody, J.-C.; Collard, J.-M.; Giles-Vernick, T.; Doria, M.; Vigan-Womas, I.; Rubbo, P.-A.; Etienne, A.; Andriatahirintsoa, E.J.; et al. Identifying the etiology and pathophysiology underlying stunting and environmental enteropathy: Study protocol of the AFRIBIOTA project. BMC Pediatr. 2018, 18, 236. [Google Scholar] [CrossRef]
- Dewey, K.G.; Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 2011, 7 (Suppl. 3), 5–18. [Google Scholar] [CrossRef]
- UNICEF; WHO; World Bank Group. United Nations Children’s Fund (UNICEF), World Health Organization (WHO), International Bank for Reconstruction and Development/The World Bank. In Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition; UNICEF: New York, NY, USA; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Kim, K.; Melough, M.; Kim, D.W.; Sakaki, J.; Lee, J.; Choi, K.; Chun, O. Nutritional adequacy and diet quality are associated with standardized height-for-age among U.S. children. Nutrients 2021, 13, 1689. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ren, Y.; Jia, Y. Effects of nutritional interventions on the physical development of preschool children: A systematic review and meta-analysis. Transl. Pediatr. 2023, 12, 991–1003. [Google Scholar] [CrossRef]
- Chiplonkar, S.; Kajale, N.A.; Sanwalka, N. A Review of Food-Based Intervention Strategies for Improving Micronutrient Status and Health During Childhood. Curr. Res. Nutr. Food Sci. 2022, 10, 407–426. [Google Scholar] [CrossRef]
- Allen, L.H.; Peerson, J.M.; Olney, D.K. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. J. Nutr. 2009, 139, 1022–1030. [Google Scholar] [CrossRef]
- Inzaghi, E.; Pampanini, V.; Deodati, A.; Cianfarani, S. The Effects of Nutrition on Linear Growth. Nutrients 2022, 14, 1752. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Speakman, J.R.; Soltani, S.; Djafarian, K. Effect of calorie restriction or protein intake on circulating levels of insulin like growth factor I in humans: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 1705–1716. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Klibanski, A. Determinants of GH resistance in malnutrition. J. Endocrinol. 2014, 220, R57–R65. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Bikle, D.D.; Tahimic, C.; Chang, W.; Wang, Y.; Philippou, A.; Barton, E.R. Role of IGF-I signaling in muscle bone interactions. Bone 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
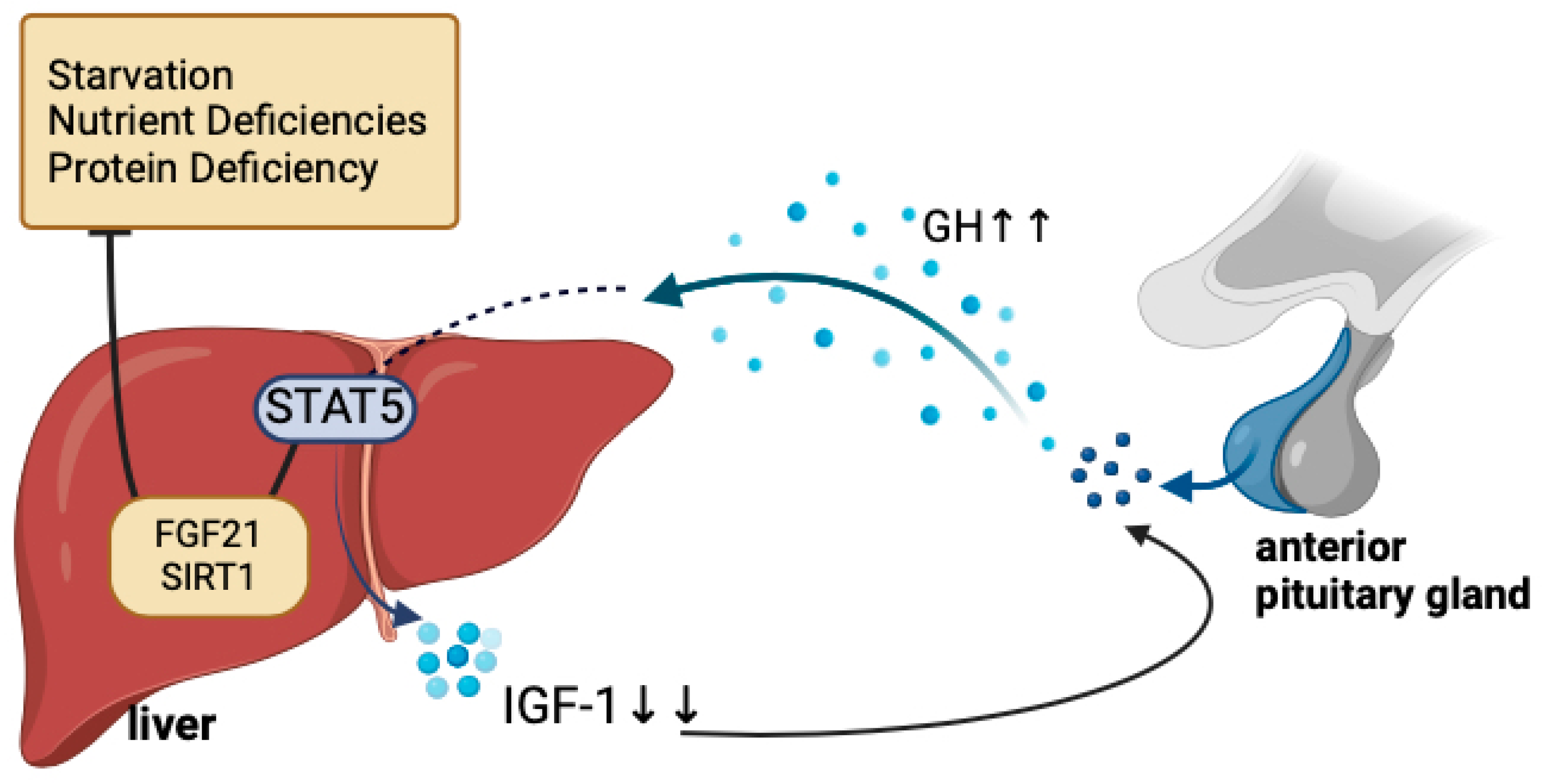
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of Growth Hormone Signaling by the Fasting-Induced Hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- LeRoith, D.; Yakar, S. Mechanisms of Disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef]
- Moøller, N.; Joørgensen, J.O.L. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Oscarsson, J.; Johannsson, G.; Johansson, J.; Lundberg, P.; Lindstedt, G.; Bengtsson, B. Diurnal variation in serum insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations during daily subcutaneous injections of recombinant human growth hormone in GH-deficient adults. Clin. Endocrinol. 1997, 46, 63–68. [Google Scholar] [CrossRef]
- Alam, A.; Richard, S.A.; Fahim, S.M.; Mahfuz, M.; Nahar, B.; Das, S.; Shrestha, B.; Koshy, B.; Mduma, E.; Seidman, J.C.; et al. Impact of early-onset persistent stunting on cognitive development at 5 years of age: Results from a multi-country cohort study. PLoS ONE 2020, 15, e0227839. [Google Scholar] [CrossRef]
- Woldehanna, T.; Behrman, J.R.; Araya, M.W. The Effect of Early Childhood Stunting on Children’s Cognitive Achievements: Evidence from Young Lives Ethiopia. 2017. Available online: www.younglives.org.uk (accessed on 24 December 2024).
- Saleem, J.; Zakar, R.; Zakar, M.Z.; Belay, M.; Rowe, M.; Timms, P.M.; Scragg, R.; Martineau, A.R. High-dose Vitamin D 3 in the treatment of severe acute malnutrition: A multicenter double-blind randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Zakar, R.; Bukhari, G.M.J.; Fatima, A.; Fischer, F. Developmental delay and its predictors among children under five years of age with uncomplicated severe acute malnutrition: A cross-sectional study in rural Pakistan. BMC Public Health 2021, 21, 1397. [Google Scholar] [CrossRef] [PubMed]
- Handryastuti, S.; Pusponegoro, H.D.; Nurdadi, S.; Chandra, A.; Pramita, F.A.; Soebadi, A.; Widjaja, I.R.; Rafli, A. Comparison of Cognitive Function in Children with Stunting and Children with Undernutrition with Normal Stature. J. Nutr. Metab. 2022, 2022, 75727. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Benton, D. Micronutrient status, cognition and behavioral problems in childhood. Eur. J. Nutr. 2008, 47, 38–50. [Google Scholar] [CrossRef]
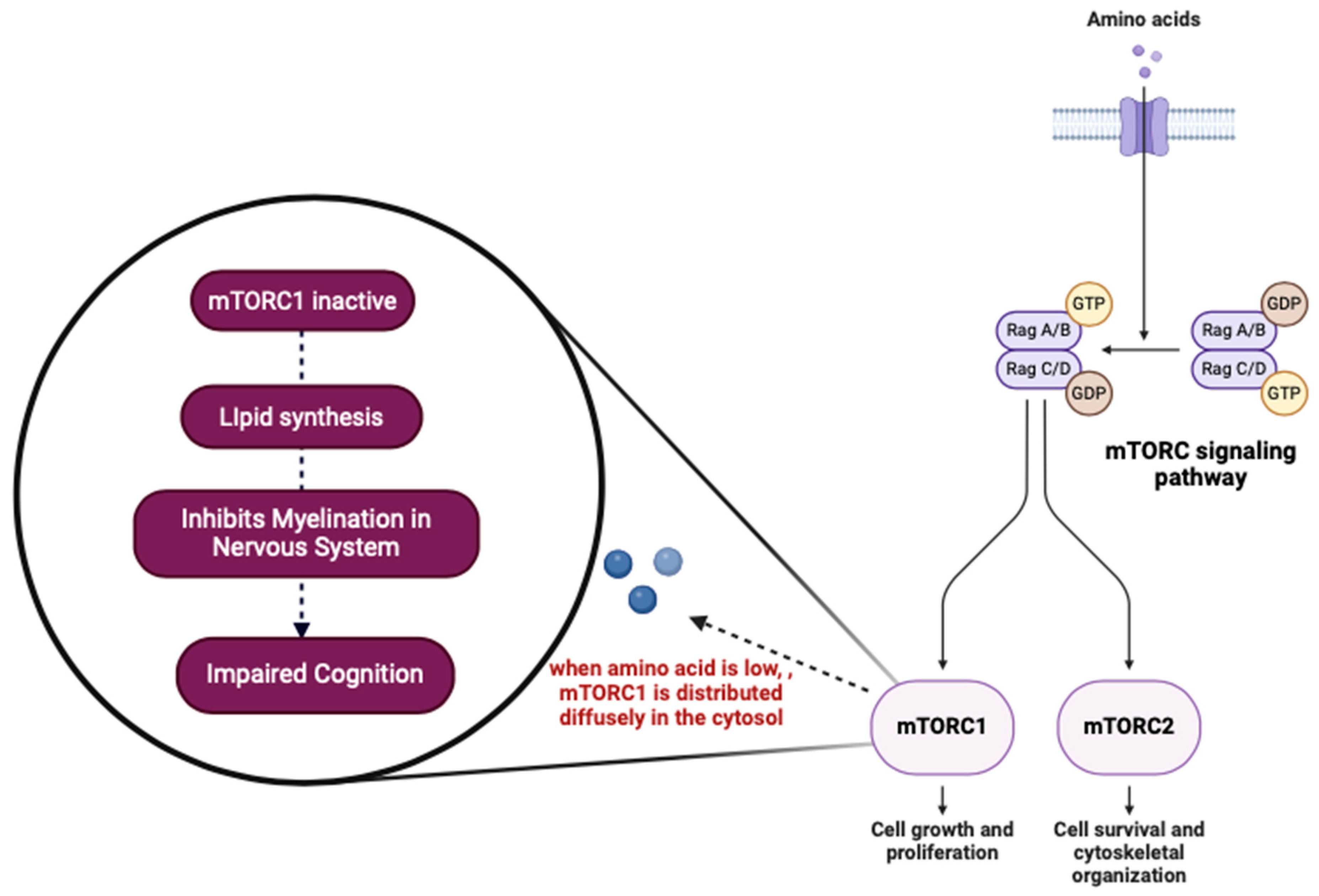
- Semba, R.D.; Trehan, I.; Gonzalez-Freireetal, M. Perspective: The potential role of essential amino acids and the mechanistic target of rapamycin complex 1 (mTORC1) pathway in the pathogenesis of child stunting. Adv. Nutr. 2016, 7, 853–865. [Google Scholar] [CrossRef]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef]
- Lestari, E.; Siregar, A.; Hidayat, A.K.; Yusuf, A.A. Stunting and its association with education and cognitive outcomes in adulthood: A longitudinal study in Indonesia. PLoS ONE 2024, 19, e0295380. [Google Scholar] [CrossRef]
- Demetriades, C.; Doumpas, N.; Teleman, A.A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 2014, 156, 786–799. [Google Scholar] [CrossRef]
- Beal, T.; Tumilowicz, A.; Sutrisna, A.; Izwardy, D.; Neufeld, L.M. A review of child stunting determinants in Indonesia. Matern. Child Nutr. 2018, 14, e12617. [Google Scholar] [CrossRef]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Morkl, S.; Berding, K.; Ritz, N.L.; Strain, C.; Patangia, D.; Patel, S.; Stanton, C.; et al. The gut microbiome in social anxiety disorder: Evidence of altered composition and function. Transl. Psychiatry 2023, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bonete, M.J.; Rajan, A.; Suriano, F.; Layunta, E. The Underrated Gut Microbiota Helminths, Bacteriophages, Fungi, and Archaea. Life 2023, 13, 1765. [Google Scholar] [CrossRef]
- Dinh, D.M.; Ramadass, B.; Kattula, D.; Sarkar, R.; Braunstein, P.; Tai, A.; Wanke, C.A.; Hassoun, S.; Kane, A.V.; Naumova, E.N.; et al. Longitudinal analysis of the intestinal microbiota in persistently stunted young children in south India. PLoS ONE 2016, 11, e0155405. [Google Scholar] [CrossRef] [PubMed]
- Thahir, A.I.A.; Gordon, A.; Salam, A. Does gut microbiome associate with the growth of infants? A review of the literature. Enferm. Clin. 2020, 30, 66–70. [Google Scholar] [CrossRef]
- Zambruni, M.; Ochoa, T.J.; Somasunderam, A.; Cabada, M.M.; Morales, M.L.; Mitreva, M.; Rosa, B.A.; Acosta, G.J.; Vigo, N.I.; Riveros, M.; et al. Stunting is preceded by intestinal mucosal damage and microbiome changes and is associated with systemic inflammation in a cohort of Peruvian infants. Am. J. Trop. Med. Hyg. 2019, 101, 1009–1017. [Google Scholar] [CrossRef]
- Surono, I.S.; Widiyanti, D.; Kusumo, P.D.; Venema, K. Gut microbiota profile of Indonesian stunted children and children with normal nutritional status. PLoS ONE 2021, 16, e02453991. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Campos-Ponce, M.; Taddei, C.R.; Doak, C.M. Microbiome, growth retardation and metabolism: Are they related? Ann. Hum. Biol. 2017, 44, 201–207. [Google Scholar] [CrossRef]
- Krebs, N.F. Overview of Zinc Absorption and Excretion in the Human Gastrointestinal Tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Wang, Z.; Chen, Y.; Tang, S.; Wang, S.; Su, L.; Huang, X.; Long, D.; Wang, L.; et al. Alteration in Gut Microbiota Associated with Zinc Deficiency in School-Age Children. Nutrients 2022, 14, 2895. [Google Scholar] [CrossRef]
- Danaei, G.; Andrews, K.G.; Sudfeld, C.R.; Fink, G.; McCoy, D.C.; Peet, E.; Sania, A.; Smith Fawzi, M.C.; Ezzati, M.; Fawzi, W.W. Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels. PLoS Med. 2016, 13, e1002164. [Google Scholar] [CrossRef]
- Ademas, A.; Adane, M.; Keleb, A.; Berihun, G.; Tesfaw, G. Water, sanitation, and hygiene as a priority intervention for stunting in under-five children in northwest Ethiopia: A community-based cross-sectional study. Ital. J. Pediatr. 2021, 47, 174. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Braegger, C.P. The Role of Iodine for Thyroid Function in Lactating Women and Infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The Effects of Iodine Deficiency in Pregnancy and Infancy. Paediatr. Perinat. Epidemiol. 2012, 26, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Velasco, I.; Bath, S.C.; Rayman, M.P. Iodine as essential nutrient during the first 1000 days of life. Nutrients 2018, 10, 290. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Toro, F.; Jialal, I. Physiology, Thyroid Stimulating Hormone; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Abbag, F.I.; Abu-Eshy, S.A.; Mahfouz, A.A.; Alsaleem, M.A.; Alsaleem, S.A.; Patel, A.A.; Mirdad, T.M.; Shati, A.A.; Awadalla, N.J. Iodine deficiency disorders as a predictor of stunting among primary school children in the aseer region, southwestern Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 7644. [Google Scholar] [CrossRef]
- Salazar, P.; Cisternas, P.; Codocedo, J.F.; Inestrosa, N.C. Induction of hypothyroidism during early postnatal stages triggers a decrease in cognitive performance by decreasing hippocampal synaptic plasticity. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 870–883. [Google Scholar] [CrossRef]
- Amano, I.; Takatsuru, Y.; Khairinisa, M.A.; Kokubo, M.; Haijima, A.; Koibuchi, N. Effects of Mild Perinatal Hypothyroidism on Cognitive Function of Adult Male Offspring. Endocrinology 2018, 159, 1910–1921. [Google Scholar] [CrossRef]
- Stricker, R.; Echenard, M.; Eberhart, R.; Chevailler, M.-C.; Perez, V.; Quinn, F.A. Evaluation of maternal thyroid function during pregnancy: The importance of using gestational age-specific reference intervals. Eur. J. Endocrinol. 2007, 157, 509–514. [Google Scholar] [CrossRef]
- Moog, N.K.; Entringer, S.; Heim, C.; Wadhwa, P.D.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [Google Scholar] [CrossRef]
- Sartika, R.A.D.; Sigit, F.S.; Purwanto, E.; Aris, N.; Marjan, A.Q.; Putra, W.K.Y.; Hastono, S.P. Association of birth weight with risk of diabetes mellitus in adolescence and early adulthood: Analysis of the Indonesian Family Life Survey. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y. Adult-onset diseases in low birth weight infants: Association with adipose tissue maldevelopment. J. Atheroscler. Thromb. 2020, 27, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Saito, I.; Ueno, M.; Kato, H.; Yoshida, A.; Kawamura, R.; Maruyama, K.; Takata, Y.; Osawa, H.; Tanigawa, T.; et al. Low birthweight is associated with type 2 diabetes mellitus in Japanese adults: The Toon Health Study. J. Diabetes Investig. 2020, 11, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Hill, T.G. Maternal diet during pregnancy and adaptive changes in the maternal and fetal pancreas have implications for future metabolic health. Front. Endocrinol. 2024, 15, 1456629. [Google Scholar] [CrossRef]
- Santos, C.D.D.L.; Clemente, A.P.G.; Martins, V.J.B.; Albuquerque, M.P.; Sawaya, A.L. Adolescents with mild stunting show alterations in glucose and insulin metabolism. J. Nutr. Metab. 2010, 2010, 943070. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Vollenweider, P.; Bochud, M.; Mooser, V.; Waeber, G.; Marques-Vidal, P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc. Diabetol. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Elzalabany, M.M.; Salama, M.; Ansari, B.M. Serum Leptin Concentrations During Severe Protein-Energy Malnutrition: Correlation with Growth Parameters and Endocrine Function. Metabolism 2000, 49, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Sahiledengle, B.; Mwanri, L.; Petrucka, P.; Agho, K.E. Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 6251. [Google Scholar] [CrossRef] [PubMed]
- Sahiledengle, B.; Petrucka, P.; Desta, F.; Sintayehu, Y.; Mesfin, T.; Mwanri, L. Childhood undernutrition mediates the relationship between open defecation with anemia among Ethiopian children: A nationally representative cross-sectional study. BMC Public Health 2024, 24, 1484. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Tielsch, J.M.; Black, M.M.; Black, R.E. Iron supplementation in early childhood: Health benefits and risks 1,2,3. Am. J. Clin. Nutr. 2006, 84, 1261–1276. [Google Scholar] [CrossRef]
- Mara, D. The elimination of open defecation and its adverse health effects: A moral imperative for governments and development professionals. J. Water Sanit. Hyg. Dev. 2017, 7, washdev2017027. [Google Scholar] [CrossRef]
- Wirth, J.P.; Rohner, F.; Woodruff, A.B.; Chiwile, F.; Yankson, H.; Koroma, A.S.; Russel, F.; Sesay, F.; Dominguez, E.; Petry, N.; et al. Anemia, micronutrient deficiencies, and malaria in children and women in Sierra Leone prior to the Ebola outbreak—Findings of a cross-sectional study. PLoS ONE 2016, 11, e0155031. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Balarajan, Y.; Ramakrishnan, U.; Özaltin, E.; Shankar, A.H. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2158. [Google Scholar] [CrossRef]
- Safari, O.; Ejtahed, H.-S.; Namazi, N.; Heshmat, R.; Arjmand, R.; Saleh, S.K.; Seif, E.; Shahsanai, A.; Motlagh, M.E.; Abdar, M.E.; et al. Association of short stature and obesity with cardio-metabolic risk factors in Iranian children and adolescents: The CASPIAN-V study. J. Diabetes Metab. Disord. 2021, 20, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Sekretariat Wakil Presiden Republik Indonesia. Strategi Nasional Percepatan Pencegahan Anak Kerdil (Stunting); Sekretariat Wakil Presiden Republik Indonesia: Jakarta, India, 2019.
- UNICEF. Improving Child Nutrition, The Achievable Imperative for Global Progress; UNICEF: New York, NY, USA, 2013. [Google Scholar]
- World Health Organization. Reducing Stunting in Children: Equity Considerations for Achieving the Global Nutrition Targets 2025. Available online: https://iris.who.int/handle/10665/260202 (accessed on 5 January 2025).
- Sirajuddin; Sirajuddin, S.; Razak, A.; Ansariadi; Thaha, R.M.; Sudargo, T. The Intervention of Maternal Nutrition Literacy Has the Potential to Prevent Childhood Stunting: Randomized Control Trials. J. Public Health Res. 2021, 10, jphr-2021. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendons on Antenatal Care for a Positive Pregnancy Experience. Available online: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/ (accessed on 5 January 2025).
- Nisar, Y.B.; Aguayo, V.M.; Billah, S.M.; Dibley, M.J. Antenatal Iron-Folic Acid Supplementation Is Associated with Improved Linear Growth and Reduced Risk of Stunting or Severe Stunting in South Asian Children Less than Two Years of Age: A Pooled Analysis from Seven Countries. Nutrients 2020, 12, 2632. [Google Scholar] [CrossRef]
- Dewey, K.G.; Arimond, M. Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition? PLoS Med. 2012, 9, e1001314. [Google Scholar] [CrossRef]
- Kawai, K.; Spiegelman, D.; Shankar, A.H.; Fawzi, W.W. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: Meta-analysis and meta-regression. Bull. World Health Organ. 2011, 89, 402–411. [Google Scholar] [CrossRef]
- de Onis, M.; Dewey, K.G.; Borghi, E.; Onyango, A.W.; Blössner, M.; Daelmans, B.; Piwoz, E.; Branca, F. The World Health Organization’s global target for reducing childhood stunting by 2025: Rationale and proposed actions. Matern. Child Nutr. 2013, 9, 6–26. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.M.; Juan, C.S.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- Ratnayani; Hegar, B.; Sunardi, D.; Fadilah, F.; Gunardi, H.; Fahmida, U.; Vidiawati, D. Association of Gut Microbiota Composition with Stunting Incidence in Children under Five in Jakarta Slums. Nutrients 2024, 16, 3444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q.; Wang, W.; van Velthoven, M.H.; Chang, S.; Han, H.; Xing, M.; Chen, L.; Scherpbier, R.W. Effectiveness of complementary food supplements and dietary counselling on anaemia and stunting in children aged 6–23 months in poor areas of Qinghai Province, China: A controlled interventional study. BMJ Open 2016, 6, e011234. [Google Scholar] [CrossRef]
- Soofi, S.B.; Khan, G.N.; Sajid, M.; Hussainyar, M.A.; Shams, S.; Shaikh, M.; Ouma, C.; Azami, S.; Naeemi, M.; Hussain, A.; et al. Specialized nutritious foods and behavior change communication interventions during the first 1000 d of life to prevent stunting: A quasi-experimental study in Afghanistan. Am. J. Clin. Nutr. 2024, 120, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Das, J.K.; Khan, G.N.; Najmi, R.; Shah, M.M.; Soofi, S.B. Food supplements to reduce stunting in Pakistan: A process evaluation of community dynamics shaping uptake. BMC Public Health 2020, 20, 1046. [Google Scholar] [CrossRef]
- Adriani, M.; Wirjatmadi, B. The effect of adding zinc to vitamin A on IGF-1, bone age and linear growth in stunted children. J. Trace Elem. Med. Biol. 2014, 28, 431–435. [Google Scholar] [CrossRef]
- Pekmez, C.T.; Dragsted, L.O.; Brahe, L.K. Gut microbiota alterations and dietary modulation in childhood malnutrition—The role of short chain fatty acids. Clin. Nutr. 2019, 38, 615–630. [Google Scholar] [CrossRef]
- Barratt, M.J.; Nuzhat, S.; Ahsan, K.; Frese, S.A.; Arzamasov, A.A.; Alam Sarker, S.; Islam, M.M.; Palit, P.; Islam, R.; Hibberd, M.C.; et al. Bifidobacterium infantis treatment promotes weight gain in Bangladeshi infants with severe acute malnutrition. Sci. Transl. Med. 2022, 14, eabk1107. [Google Scholar] [CrossRef]
- Nuzhat, S.; Hasan, S.M.T.; Palit, P.; Islam, R.; Mahfuz, M.; Islam, M.M.; Alam, A.; Flannery, R.L.; Kyle, D.J.; Sarker, S.A.; et al. Effects of probiotic and synbiotic supplementation on ponderal and linear growth in severely malnourished young infants in a randomized clinical trial. Sci. Rep. 2023, 13, 1845. [Google Scholar] [CrossRef]
- Fallah, R.; Du, L.; Braverman, L.E.; He, X.; Segura-Harrison, M.; Yeh, M.W.; Pearce, E.N.; Chiu, H.K.; Mittelman, S.D.; Leung, A.M. Iodine Nutrition in Weaning Infants in the United States. Thyroid 2019, 29, 573–576. [Google Scholar] [CrossRef]
- Wang, L.; Huo, J.; Wei, Y.; Tang, Y.; Sun, J.; Huang, J. Yingyangbao Reduced Anemia among Infants and Young Children Aged 6–23 Months When Delivered through a Large-Scale Nutrition Improvement Program for Children in Poor Areas in China from 2015 to 2020. Nutrients 2023, 15, 2634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, A.; Ul-Haq, Z.; Fazid, S.; Fatima, S.; Muhammad, N.; Ahmed, J.; Manoharadas, S.; Safi, S.Z.; Habib, I.; Garzon, C.; et al. Effectiveness of locally produced ready to use supplementary food on hemoglobin, anthropometrics, and plasma micronutrients concentrations of 6 to 23 months age children: A non-randomized community-based trial from Pakistan. Front. Nutr. 2023, 10, 1176778. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Fernald, L.C.H.; Weber, A.M.; Arnold, C.; Galasso, E. Lipid-Based Nutrient Supplementation Reduces Child Anemia and Increases Micronutrient Status in Madagascar: A Multiarm Cluster-Randomized Controlled Trial. J. Nutr. 2020, 150, 958–966. [Google Scholar] [CrossRef]
- Anderson, L.M.; Shinn, C.; Fullilove, M.T.; Scrimshaw, S.C.; Fielding, E.J.; Normand, J.; Carande-Kulis, V.G. The effectiveness of early childhood development programs. Am. J. Prev. Med. 2003, 24, 32–46. [Google Scholar] [CrossRef]
- World Health Organization. Everybody Business: Strengthening Health Systems to Improve Health Outcomes: WHO’s Framework for Action; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- Ocansey, M.E.; Adu-Afarwuah, S.; Kumordzie, S.M.; Okronipa, H.; Young, R.R.; Tamakloe, S.M.; Oaks, B.M.; Arimond, M.; Dewey, K.G.; Prado, E.L. The association of early linear growth and haemoglobin concentration with later cognitive, motor, and social–emotional development at preschool age in Ghana. Matern. Child Nutr. 2019, 15, e12834. [Google Scholar] [CrossRef] [PubMed]
- Mustakim, M.R.D.; Irawan, R.; Irmawati, M.; Setyoboedi, B. Impact of Stunting on Development of Children between 1–3 Years of Age. Ethiop. J. Health Sci. 2022, 32, 569. [Google Scholar] [CrossRef]
- Gizaw, Z.; Yalew, A.W.; Bitew, B.D.; Lee, J.; Bisesi, M. Stunting among children aged 24–59 months and associations with sanitation, enteric infections, and environmental enteric dysfunction in rural northwest Ethiopia. Sci. Rep. 2022, 12, 19293. [Google Scholar] [CrossRef]
- Lefebo, B.K.; Kassa, D.H.; Tarekegn, B.G. Factors associated with stunting: Gut inflammation and child and maternal-related contributors among under-five children in Hawassa City, Sidama Region, Ethiopia. BMC Nutr. 2023, 9, 54. [Google Scholar] [CrossRef]
- Henriques, A.; Teixeira, V.; Cardoso, H.F.V.; Azevedo, A. The influence of stunting on obesity in adulthood: Results from the EPIPorto cohort. Public Health Nutr. 2018, 21, 1819–1826. [Google Scholar] [CrossRef]
- Rolfe, E.D.L.; de França, G.V.A.; Vianna, C.A.; Gigante, D.P.; Miranda, J.J.; Yudkin, J.S.; Horta, B.L.; Ong, K.K. Associations of stunting in early childhood with cardiometabolic risk factors in adulthood. PLoS ONE 2018, 13, e0192196. [Google Scholar] [CrossRef]
- Mutumba, R.; Mbabazi, J.; Pesu, H.; Greibe, E.; Olsen, M.F.; Briend, A.; Mølgaard, C.; Ritz, C.; Mupere, E.; Filteau, S.; et al. Micronutrient Status and Other Correlates of Hemoglobin among Children with Stunting: A Cross-Sectional Study in Uganda. Nutrients 2023, 15, 3785. [Google Scholar] [CrossRef]
- Mbabazi, J.; Pesu, H.; Mutumba, R.; McCray, G.; Ritz, C.; Filteau, S.; Briend, A.; Mupere, E.; Grenov, B.; Friis, H.; et al. Predictors of change in early child development among children with stunting: Secondary analysis of a randomized trial in Uganda. PLoS Glob. Public Health 2024, 4, e0003456. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Wahid, B.Z.; Farzana, F.D.; Ahmed, S.M.T.; Ali, M.; Naz, F.; Rahman, S.S.; Siddiqua, T.J.; Faruque, A.S.G.; Choudhury, N.; et al. Influence of the Suchana intervention on exclusive breastfeeding and stunting among children aged under 6 months in the Sylhet region of Bangladesh. Matern. Child Nutr. 2023, 19, e13535. [Google Scholar] [CrossRef]
- Hadi, H.; Fatimatasari, F.; Irwanti, W.; Kusuma, C.; Alfiana, R.D.; Asshiddiqi, M.I.N.; Nugroho, S.; Lewis, E.C.; Gittelsohn, J. Exclusive breastfeeding protects young children from stunting in a low-income population: A study from eastern Indonesia. Nutrients 2021, 13, 4264. [Google Scholar] [CrossRef]
- Ashraf, K.; Huda, T.M.; Ikram, J.; Ariff, S.; Sajid, M.; Khan, G.N.; Umer, M.; Ahmed, I.; Dibley, M.J.; Soofi, S.B. The Effectiveness of Nutritional Interventions Implemented through Lady Health Workers on the Reduction of Stunting in Children under 5 in Pakistan: The Difference-in-Difference Analysis. Nutrients 2024, 16, 2149. [Google Scholar] [CrossRef]
- Mayén, V.A.; Ogunlusi, A.; Wright, C.M.; Garcia, A.L. Childhood stunting and micronutrient status unaffected by RCT of micronutrient fortified drink. Matern. Child Nutr. 2022, 18, e13256. [Google Scholar] [CrossRef]
- Roberts, S.B.; Franceschini, M.A.; Silver, R.E.; Taylor, S.F.; De Sa, A.B.; Có, R.; Sonco, A.; Krauss, A.; Taetzsch, A.; Webb, P.; et al. Effects of food supplementation on cognitive function, cerebral blood flow, and nutritional status in young children at risk of undernutrition: Randomized controlled trial. BMJ 2020, 370, m2397. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Fernandez-Rao, S.; Nair, K.M.; Balakrishna, N.; Tilton, N.; Radhakrishna, K.V.; Ravinder, P.; Harding, K.B.; Reinhart, G.; Yimgang, D.P.; et al. A Randomized Multiple Micronutrient Powder Point-of-Use Fortification Trial Implemented in Indian Preschools Increases Expressive Language and Reduces Anemia and Iron Deficiency. J. Nutr. 2021, 151, 2029–2042. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.-X.; Shen, X.-L.; Wang, Y.-Q.; Yan, C.-H. A national survey of iodine nutrition in children aged 3-6 years in China and its relationship with children’s physical growth. Matern. Child Nutr. 2024, 20, e13685. [Google Scholar] [CrossRef]
- Ferede, A.; Wordofa, M.A.; Belachew, T. Behavior change intervention to sustain iodide salt utilization in households in Ethiopia and study of the effect of iodine status on the growth of young children: Community trial. PeerJ 2024, 12, e16849. [Google Scholar] [CrossRef]
- Ansuya; Nayak, B.S.; Unnikrishnan, B.; Shashidhara, Y.N.; Mundkur, S.C. Effect of nutrition intervention on cognitive development among malnourished preschool children: Randomized controlled trial. Sci. Rep. 2023, 13, 10636. [Google Scholar] [CrossRef]
- Luoto, J.E.; Garcia, I.L.; Aboud, F.E.; Singla, D.R.; Fernald, L.C.H.; Pitchik, H.O.; Saya, U.Y.; Otieno, R.; Alu, E. Group-based parenting interventions to promote child development in rural Kenya: A multi-arm, cluster-randomised community effectiveness trial. Lancet Glob. Health 2021, 9, e309–e319. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyani, A.T.; Khairinisa, M.A.; Khatib, A.; Chaerunisaa, A.Y. Understanding Stunting: Impact, Causes, and Strategy to Accelerate Stunting Reduction—A Narrative Review. Nutrients 2025, 17, 1493. https://doi.org/10.3390/nu17091493
Mulyani AT, Khairinisa MA, Khatib A, Chaerunisaa AY. Understanding Stunting: Impact, Causes, and Strategy to Accelerate Stunting Reduction—A Narrative Review. Nutrients. 2025; 17(9):1493. https://doi.org/10.3390/nu17091493
Chicago/Turabian StyleMulyani, Aisyah Tri, Miski Aghnia Khairinisa, Alfi Khatib, and Anis Yohana Chaerunisaa. 2025. "Understanding Stunting: Impact, Causes, and Strategy to Accelerate Stunting Reduction—A Narrative Review" Nutrients 17, no. 9: 1493. https://doi.org/10.3390/nu17091493
APA StyleMulyani, A. T., Khairinisa, M. A., Khatib, A., & Chaerunisaa, A. Y. (2025). Understanding Stunting: Impact, Causes, and Strategy to Accelerate Stunting Reduction—A Narrative Review. Nutrients, 17(9), 1493. https://doi.org/10.3390/nu17091493





