Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Product
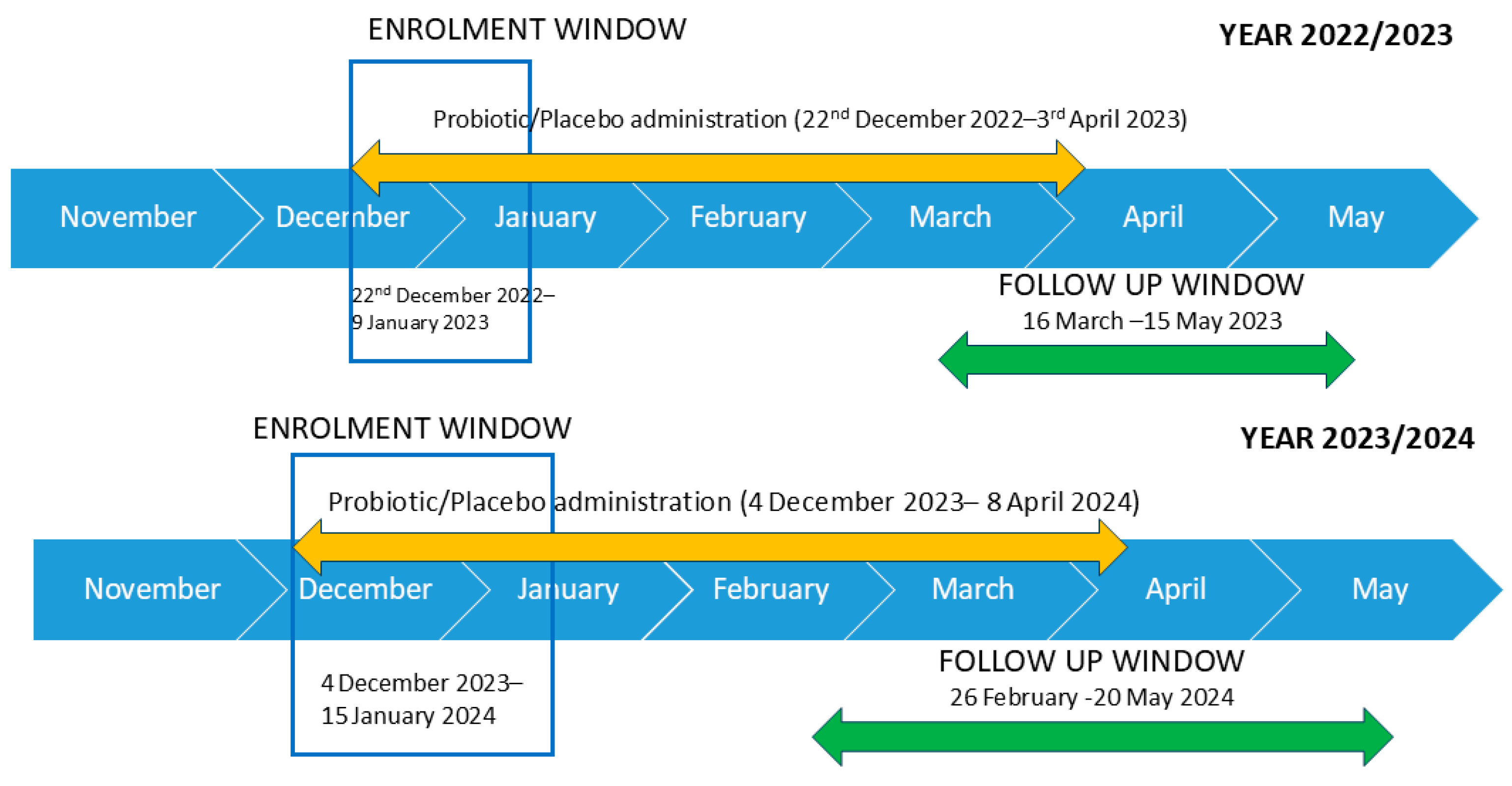
2.2. Study Design
2.3. Questionnaire
Wisconsin Upper Respiratory Symptom Survey (WURSS)
2.4. Measurement of Inflammatory Cytokines
2.5. Statistical Analysis
3. Results
3.1. Assessment of Cold Symptoms by WURSS-21
3.2. Assessment of the Total Number of Days with Cold Symptoms
3.3. Assessment of Typical Symptoms of Cold: Fever and Muscle Pain
3.4. Assessment of Subjects Using Specific Treatment for Cold and Without Any Symptoms of Cold
3.5. Cytokine Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| LAB | Lactic acid bacteria |
| QoL | Quality of life |
| RCT | Randomized clinical trial |
| RDBPCT | Randomized, double-blind, placebo-controlled trial |
| SCFAs | Short-chain fatty acids |
| URTIs | Upper respiratory tract infections |
References
- Pappas, D.E. 26—The Common Cold. In Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 199–202.e1. ISBN 978-0-323-40181-4. [Google Scholar]
- Kobatake, E.; Iwama, Y.; Arai, T.; Shioya, N.; Kise, M.; Kabuki, T. Intake of Lactobacillus Paragasseri SBT2055 Improves Subjective Symptoms of Common Cold during Winter Season in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Study. Front. Nutr. 2022, 9, 1063584. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.L.; Hatch-McChesney, A.; Small, S.D.; Allen, J.T.; Sullo, E.; Agans, R.T.; Fagnant, H.S.; Bukhari, A.S.; Karl, J.P. Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2022, 13, 2277–2295. [Google Scholar] [CrossRef]
- Eccles, R. Understanding the Symptoms of the Common Cold and Influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Kesson, A.M. Respiratory Virus Infections. Paediatr. Respir. Rev. 2007, 8, 240–248. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of a Bacteriocin-like Compound from Lactobacillus Plantarum with Antimicrobial Activity and Effects on Normal and Cancerogenic Human Intestinal Cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Corthésy, B.; Gaskins, H.R.; Mercenier, A. Cross-Talk between Probiotic Bacteria and the Host Immune System. J. Nutr. 2007, 137, 781S–790S. [Google Scholar] [CrossRef]
- de Vrese, M.; Winkler, P.; Rautenberg, P.; Harder, T.; Noah, C.; Laue, C.; Ott, S.; Hampe, J.; Schreiber, S.; Heller, K.; et al. Effect of Lactobacillus Gasseri PA 16/8, Bifidobacterium Longum SP 07/3, B. Bifidum MF 20/5 on Common Cold Episodes: A Double Blind, Randomized, Controlled Trial. Clin. Nutr. 2005, 24, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kemgang, T.S.; Kapila, S.; Shanmugam, V.P.; Kapila, R. Cross-Talk between Probiotic Lactobacilli and Host Immune System. J. Appl. Microbiol. 2014, 117, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a Fermented Dairy Product Containing the Probiotic Lactobacillus Casei DN-114001 Reduces the Duration of Respiratory Infections in the Elderly in a Randomised Controlled Trial. Br. J. Nutr. 2010, 103, 58–68. [Google Scholar] [CrossRef]
- Namba, K.; Hatano, M.; Yaeshima, T.; Takase, M.; Suzuki, K. Effects of Bifidobacterium Longum BB536 Administration on Influenza Infection, Influenza Vaccine Antibody Titer, and Cell-Mediated Immunity in the Elderly. Biosci. Biotechnol. Biochem. 2010, 74, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P. Ageing, Immunity and Influenza: A Role for Probiotics? Proc. Nutr. Soc. 2014, 73, 309–317. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Struszczak, L. Effects of Lactobacillus Casei Shirota Ingestion on Common Cold Infection and Herpes Virus Antibodies in Endurance Athletes: A Placebo-Controlled, Randomized Trial. Eur. J. Appl. Physiol. 2016, 116, 1555–1563. [Google Scholar] [CrossRef]
- Kolling, Y.; Salva, S.; Villena, J.; Marranzino, G.; Alvarez, S. Non-Viable Immunobiotic Lactobacillus Rhamnosus CRL1505 and Its Peptidoglycan Improve Systemic and Respiratory Innate Immune Response during Recovery of Immunocompromised-Malnourished Mice. Int. Immunopharmacol. 2015, 25, 474–484. [Google Scholar] [CrossRef]
- Shiraishi, T.; Yokota, S.; Fukiya, S.; Yokota, A. Structural Diversity and Biological Significance of Lipoteichoic Acid in Gram-Positive Bacteria: Focusing on Beneficial Probiotic Lactic Acid Bacteria. Biosci. Microbiota Food Health 2016, 35, 147–161. [Google Scholar] [CrossRef]
- Ghadimi, D.; Vrese, M.d.; Heller, K.J.; Schrezenmeir, J. Effect of Natural Commensal-Origin DNA on Toll-like Receptor 9 (TLR9) Signaling Cascade, Chemokine IL-8 Expression, and Barrier Integritiy of Polarized Intestinal Epithelial Cells. Inflamm. Bowel Dis. 2010, 16, 410–427. [Google Scholar] [CrossRef]
- Jijon, H.; Backer, J.; Diaz, H.; Yeung, H.; Thiel, D.; McKaigney, C.; De Simone, C.; Madsen, K. DNA from Probiotic Bacteria Modulates Murine and Human Epithelial and Immune Function. Gastroenterology 2004, 126, 1358–1373. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of Probiotic Actions—A Review. Int. J. Med. Microbiol. IJMM 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M. Immunomodulatory Mechanisms of Lactobacilli. Microb. Cell Factories 2011, 10, S17. [Google Scholar] [CrossRef]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the Probiotic Properties of New Lactobacillus and Bifidobacterium Strains and Their in Vitro Effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef]
- De Giani, A.; Sandionigi, A.; Zampolli, J.; Michelotti, A.; Tursi, F.; Labra, M.; Di Gennaro, P. Effects of Inulin-Based Prebiotics Alone or in Combination with Probiotics on Human Gut Microbiota and Markers of Immune System: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Microorganisms 2022, 10, 1256. [Google Scholar] [CrossRef]
- Dumville, J.C.; Hahn, S.; Miles, J.N.V.; Torgerson, D.J. The Use of Unequal Randomisation Ratios in Clinical Trials: A Review. Contemp. Clin. Trials 2006, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Brown, R.L.; Mundt, M.P.; Thomas, G.R.; Barlow, S.K.; Highstrom, A.D.; Bahrainian, M. Validation of a Short Form Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Health Qual. Life Outcomes 2009, 7, 76. [Google Scholar] [CrossRef]
- Lungaro, L.; Malfa, P.; Manza, F.; Costanzini, A.; Valentini, G.; Squarzanti, D.F.; Viciani, E.; Velichevskaya, A.; Castagnetti, A.; Barbalinardo, M.; et al. Clinical Efficacy of Probiotics for Allergic Rhinitis: Results of an Exploratory Randomized Controlled Trial. Nutrients 2024, 16, 4173. [Google Scholar] [CrossRef]
- Vicariotto, F.; Malfa, P.; Torricelli, M.; Lungaro, L.; Caio, G.; De Leo, V. Beneficial Effects of Limosilactobacillus Reuteri PBS072 and Bifidobacterium Breve BB077 on Mood Imbalance, Self-Confidence, and Breastfeeding in Women during the First Trimester Postpartum. Nutrients 2023, 15, 3513. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for Preventing Acute Upper Respiratory Tract Infections. Cochrane Database Syst. Rev. 2015, 21, CD006895. [Google Scholar] [CrossRef]
- Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; Jung, Y.-J.; et al. Lactobacillus Plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]
- Berggren, A.; Lazou Ahrén, I.; Larsson, N.; Önning, G. Randomised, Double-Blind and Placebo-Controlled Study Using New Probiotic Lactobacilli for Strengthening the Body Immune Defence against Viral Infections. Eur. J. Nutr. 2011, 50, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.; Gruenwald, J.; Dudek, S. Randomized, Double Blind and Placebo Controlled Study Using a Combination of Two Probiotic Lactobacilli to Alleviate Symptoms and Frequency of Common Cold. Food Nutr. Sci. 2013, 04, 13–20. [Google Scholar] [CrossRef]
- Lazou Ahrén, I.; Berggren, A.; Teixeira, C.; Martinsson Niskanen, T.; Larsson, N. Evaluation of the Efficacy of Lactobacillus Plantarum HEAL9 and Lactobacillus Paracasei 8700:2 on Aspects of Common Cold Infections in Children Attending Day Care: A Randomised, Double-Blind, Placebo-Controlled Clinical Study. Eur. J. Nutr. 2020, 59, 409–417. [Google Scholar] [CrossRef]
- Xu, C.; Hiraku, A.; Arai, S.; Iwabuchi, N.; Tanaka, M.; Nakamura, M. Probiotic Bifidobacterium Longum BB536 and Its Impact on Subjective Symptoms of Physical Conditions Associated with Common Cold-like Symptoms in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Funct. Foods 2024, 115, 106113. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Randomized Controlled Trial of Probiotics to Reduce Common Cold in Schoolchildren. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2012, 54, 682–687. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Kanmani, P.; Kim, H. Immunobiotic Strains Modulate Toll-Like Receptor 3 Agonist Induced Innate Antiviral Immune Response in Human Intestinal Epithelial Cells by Modulating IFN Regulatory Factor 3 and NF-κB Signaling. Front. Immunol. 2019, 10, 1536. [Google Scholar] [CrossRef]
- Altadill, T.; Espadaler-Mazo, J.; Liong, M.-T. Effects of a Lactobacilli Probiotic on Reducing Duration of URTI and Fever, and Use of URTI-Associated Medicine: A Re-Analysis of a Randomized, Placebo-Controlled Study. Microorganisms 2021, 9, 528. [Google Scholar] [CrossRef]
- Du, T.; Lei, A.; Zhang, N.; Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front. Immunol. 2022, 13, 908010. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C, and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C reduces the severity of common colds: A meta-analysis. BMC Public Health 2023, 23, 2468. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungaro, L.; Malfa, P.; Manza, F.; Negrelli, M.; Costanzini, A.; Squarzanti, D.F.; Lo Re, M.; Cariani, A.; Ghisellini, S.; Caputo, F.; et al. Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 1490. https://doi.org/10.3390/nu17091490
Lungaro L, Malfa P, Manza F, Negrelli M, Costanzini A, Squarzanti DF, Lo Re M, Cariani A, Ghisellini S, Caputo F, et al. Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2025; 17(9):1490. https://doi.org/10.3390/nu17091490
Chicago/Turabian StyleLungaro, Lisa, Patrizia Malfa, Francesca Manza, Matilde Negrelli, Anna Costanzini, Diletta Francesca Squarzanti, Marta Lo Re, Alessio Cariani, Sara Ghisellini, Fabio Caputo, and et al. 2025. "Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 17, no. 9: 1490. https://doi.org/10.3390/nu17091490
APA StyleLungaro, L., Malfa, P., Manza, F., Negrelli, M., Costanzini, A., Squarzanti, D. F., Lo Re, M., Cariani, A., Ghisellini, S., Caputo, F., De Giorgi, A., Mansueto, P., Carroccio, A., De Giorgio, R., & Caio, G. (2025). Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 17(9), 1490. https://doi.org/10.3390/nu17091490







