Impact of a Chokeberry (Aronia melanocarpa (Michx.) Elliott) Supplementation on Cardiometabolic Outcomes: A Critical Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. The Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Assessment of Risk of Bias and Certainty of Evidence
2.5. Statistical Analysis
3. Results
3.1. Literature Search and Characteristics of Included Studies
| Study (Year), Country | Study Design | Participants Characteristics | (Poly)phenols Intake | Investigational Approach | Duration | Measured Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End of trial | Type | (n) | Intervention | Control | |||||
| Naruszewicz et al. (2007), Poland [66] | Randomized, double-blinded, placebo-controlled, parallel trial | CVD subjects Age: 65.97 ± 8.01 years BMI: 26.70 ± 3.01 kg/m2 Sex: mixed (11 F/33 M) | ND | ND | Extract, capsules | Intervention: 22 Control: 22 | 255 mg/d (127.5 proanthocyanidins, 63.75 mg anthocyanins, 22.95 mg phenolic acids) | Placebo (maltodextrin) | 6 wk | BMI, TAG, TC, LDL-C, HDL-C, FBG, SBP, DBP |
| Loo et al. (2016), Finland [62] | Randomized, single-blinded, placebo-controlled, crossover trial | Pre- or mild hypertensive subjects Age: 55.80 (41–69) years BMI: 25.90 ± 3.30 kg/m2 Sex: mixed (23 F/14 M) | Intervention and control (mg): - Phenolic acids = 630 ± 327; - Quercetin = 3.6 ± 2.5; - Total (poly)phenols = 633.6. | Intervention (mg): - Phenolic acids = 597 ± 281; Quercetin = 3.6 ± 2.8; - Total (poly)phenols = 600.6. Control (mg): - Phenolic acids = 586 ± 297; Quercetin = 3.9 ± 4.0; - Total (poly)phenols = 589.9. | Juice and chokeberry powder | Intervention: 37 Control: 37 | 300 mL/d (966 mg anthocyanins, 576 mg proanthocyanidins, 351 mg phenolic acids, and 55 mg flavonoids) and 3 g/d powder (169 mg proanthocyanidins, 58 mg anthocyanins, 16 mg phenolic acids, and 3.3 mg flavonoids) | Placebo juice (water, sugar syrup [12 g], and table sugar [7 g]) and placebo powder (wheat flour [0.8 g], rice powder [1.7 g], and cane sugar [0.5 g]) | 8 wk | BW, BMI, TAG, TC, LDL-C, HDL-C, FBG, SBP, DBP |
| Xie et al. (2017), USA [64] | Randomized, double-blinded, placebo-controlled, parallel trial | Healthy participants, former smokers Age: 34.95 ± 3.68 years BMI: 26.29 ± 1.27 kg/m2 Sex: mixed (25 F/24 M) | Intervention (mg/d): - Isoflavones = 8.5 ± 2.6; - Flavanols = 81.7 ± 18.7; - Flavones = 50.3 ± 27.2; - Flavanones = 22.4 ± 10.7; - Flavan-3-ols = 26.0 ± 6.9; - Anthocyanins = 26.6 ± 4.8; - Proanthocyanins = 418 ± 150; - Total (poly)phenols = 633.5. Control (mg/d): - Isoflavones = 3.9 ± 2.7; - Flavanols = 73.9 ± 20.0; - Flavones = 54.1 ± 29.1; - Flavanones = 28.8 ± 11.4; - Flavan-3-ols = 20.6 ± 7.4; - Anthocyanins = 18.2 ± 5.2; - Proanthocyanins = 222 ± 162; - Total (poly)phenols = 421.5. | Extract, capsules | Intervention: 25 Control: 24 | 500 mg/d (45.1 mg anthocyanins, 41.9 mg proanthocyanidins, and 35.7 mg hydroxycinnamic acids) | Placebo (rice powder with 0.2% beet juice concentrate) | 12 wk | BW, BMI, WC, TAG, TC, LDL-C, HDL-C, SBP, DBP | |
| Istas et al. (2019), New Zealand [59] | Randomized, double-blinded, placebo-controlled, parallel trial | Healthy subjects Age: 24 ± 5.30 years BMI: 23 ± 2.10 kg/m2 Sex: males (66) | Arm 1 (mg): - Total (poly)phenols = 606 ± 76; - Anthocyanins = 34 ± 8.7; - Flavan-3-ols = 130 ± 20; - Proanthocyanidins = 78 ± 16; - Flavonols = 42 ± 6.1; - Flavones = 3 ± 0.6; - Phenolic acids = 209 ± 45; - Stilbenes = 0.1 ± 0.02; - Other = 102 ± 6.2. Arm 2 (mg): - Total (poly)phenols = 568 ± 92; - Anthocyanins = 20 ± 5.5; - Flavan-3-ols = 153 ± 40; - Proanthocyanidins = 52 ± 10; - Flavonols = 45 ± 9; - Flavones = 3.2 ± 0.7; - Phenolic acids = 180 ± 36; - Stilbenes = 0.2 ± 0.1; - Other = 115 ± 11. Control (mg): - Total (poly)phenols = 419 ± 52; - Anthocyanins = 11 ± 4; - Flavan-3-ols = 71 ± 17; - Proanthocyanidins = 46 ± 12; - Flavonols = 37 ± 4.2; - Flavones = 3.8 ± 0.8; - Phenolic acids = 157 ± 36; - Stilbenes = 0.1 ± 0.05; - Other = 93 ± 5.9. | Arm 1 (mg): - Total (poly)phenols = 465 ± 73; - Anthocyanins = 31 ± 9.3; - Flavan-3-ols = 74 ± 13; - Proanthocyanidins = 87 ± 24; - Flavonols = 36 ± 0.7; - Flavones = 3.6 ± 35; - Phenolic acids = 149 ± 6.7; - Stilbenes = 0.1 ± 0.03; - Other = 84 ± 8.7. Arm 2 (mg): - Total (poly)phenols = 584 ± 84; - Anthocyanins = 24 ± 7.3; - Flavan-3-ols = 163 ± 37; - Proanthocyanidins = 70 ± 13; - Flavonols = 44 ± 0.7; - Flavones = 3.4 ± 34; - Phenolic acids = 156 ± 5.2; - Stilbenes = 0.3 ± 0.2; - Other = 123 ± 14. Control (mg): - Total (poly)phenols = 553 ± 86; - Anthocyanins = 19 ± 7; - Flavan-3-ols = 139 ± 44; -Proanthocyanidins = 56 ± 11; - Flavonols = 39 ± 1; - Flavones = 5 ± 42; - Phenolic acids = 192 ± 5; - Stilbenes = 0.2 ± 0; - Other = 103 ± 4. | Extract and powder, capsules | Arm 1: 23 Arm 2: 23 Control: 20 | Arm 1: 500 mg/d extract (71 mg phenols, 30 mg anthocyanins, 16 mg proanthocyanidins) Arm 2: 500 mg/d powder (4.8 mg phenols, 3.6 mg anthocyanins, 3.3 mg proanthocyanidins) | Placebo (maltodextrin) | 12 wk | BW, TAG, TC, HDL-C, LDL-C, FBG, SBP, DBP |
| Pokimica et al. (2019), Serbia [63] | Randomized, double-blinded, placebo-controlled, parallel trial | High CVD risk subjects Age: 40.6 ± 7.1 years BMI: 27.43 (2.81) kg/m2 Sex: mixed (52 F/32 M) | ND | ND | Juice | Arm 1: 27 Arm 2: 28 Control: 29 | Arm 1: 100 mL/d (1177.11 GAE, 113.3 C3GE) Arm 2: 100 mL/d (294.28 GAE, 28.3 mg C3GE) | Placebo (similar appearance [color, flavor], the same nutritional composition [sugars, minerals, vitamins, organic acids], without (poly)phenols) | 4 wk | BMI, WC, TAG, TC, LDL-C, HDL-C, FBG, SBP, DBP |
| Ahles et al. (2020), The Netherlands [57] | Randomized, double-blinded, placebo-controlled, parallel trial | Healthy subjects Age: 53 ± 5.75 years BMI: 29.40 ± 2.69 kg/m2 Sex: mixed (65 F/36 M) | ND | ND | Extract, capsules | Arm 1: 35 Arm 2: 34 Control: 32 | Arm 1: 150 mg/d (27 mg anthocyanins) Arm 2: 90 mg/d (16 mg anthocyanins) | Placebo (maltodextrin) | 24 wk | SBP, DBP |
| Le Sayec et al. (2022), United Kingdom [61] | Randomized, double-blinded, placebo-controlled, parallel trial | Healthy prehypertensive subjects Age: 56.20 ± 8.81 years BMI: 24.70 ± 3.16 kg/m2 Sex: mixed (55 F/47 M) | Intervention (mg): - Anthocyanins = 77 ± 62.1; - Flavan-3-ols = 272 ± 367; - Proanthocyanins = 211 ± 153; - Flavanones = 20.4 ± 27.7; - Flavones = 4.5 ± 4.5; - Flavonols = 74 ± 53.2; - Isoflavonoids = 5.5 ± 13.4; - Lignans = 16.9 ± 38.7; - Stilbenes = 0.4 ± 0.6; - Phenolic acids = 583 ± 365; - Other (poly)phenols = 99.1 ± 266; - Total (poly)phenols = 1364 ± 705. Control (mg): - Anthocyanins = 59.2 ± 68.9; - Flavan-3-ols = 334 ± 281; - Proanthocyanins = 308 ± 424; - Flavanones = 20.9 ± 24.2; Flavones = 5 ± 7.5; - Flavonols = 85.1 ± 73.4; - Isoflavonoids = 1.9 ± 4; - Lignans = 9.6 ± 22.5; - Stilbenes = 0.3 ± 0.4; - Phenolic acids = 691 ± 585; - Other (poly)phenols = 222 ± 916; - Total (poly)phenols = 1738 ± 1287. | Intervention (mg): - Anthocyanins = 61.2 ± 56.9; - Flavan-3-ols = 232 ± 233; - Proanthocyanins = 241 ± 184; - Flavanones = 17.8 ± 30; - Flavones = 6.8 ± 7.3; - Flavonols = 56.7 ± 34.5; - Isoflavonoids = 5.8 ± 11.3; - Lignans = 9.4 ± 16.8; - Stilbenes = 0.5 ± 0.6; - Phenolic acids = 581 ± 547; - Other (poly)phenols = 46.2 ± 84.1; - Total (poly)phenols = 1258 ± 652. Control (mg): - Anthocyanins = 73.3 ± 85.7; - Flavan-3-ols = 322 ± 283; - Proanthocyanins = 320 ± 281; - Flavanones = 17 ± 25.6; -Flavones = 5.8 ± 11.6; - Flavonols = 72.8 ± 41.9; - Isoflavonoids = 3.7 ± 9.3; - Lignans = 21.3 ± 51.5; - Stilbenes = 0.3 ± 0.6; - Phenolic acids = 695 ± 706; - Other (poly)phenols = 55.4 ± 70.7; - Total (poly)phenols = 1586 ± 998. | Extract, capsules | Intervention: 51 Control: 51 | 500 mg/d (31.8 mg proanthocyanidins, 23.25 anthocyanins) | Placebo (maltodextrin) | 12 wk | BW, BMI, TAG, TC, LDL-C, HDL-C, FBG, SBP, DBP |
| Sangild et al. (2023), Denmark [65] | Randomized, double-blinded, placebo-controlled, crossover trial | Healthy pre-hypercholesterolemia subjects Age: 49.8 ± 9.7 years BMI: 27.2 ± 3.3 kg/m2 | ND | ND | Powder, capsules | Intervention: 51 Control: 44 | 5 g/d (150 mg anthocyanins) | Placebo (microcrystalline cellulose) | 12 wk | FBG, HbA1c, HOMA-IR, SBP, DBP |
| Chamberlin et al. (2024), USA [58] | Randomized, double-blinded, placebo-controlled, parallel trial | Healthy subjects Age: 33.70 ± 7.25 years BMI: 25.95 ± 5.03 kg/m2 Sex: mixed (8 F/6 M) | ND | ND | Juice | Intervention: 7 Control: 7 | 100 mL/d (623.1 mg phenolic acids, 85.02 mg anthocyanins, 8.84 mg flavonols) | Placebo (matched in taste and color, low in (poly)phenols) | 4 wk | TAG, TC, LDL-C, HDL-C, FBG |
| Lackner et al. (2024), The Netherlands [60] | Randomized, single-blinded, placebo-controlled, parallel trial | Healthy subjects Age: 25.75 ± 4.85 years BMI: 21.21 ± 2.15 kg/m2 Sex: females (37) | Intervention (mg): total (poly)phenols = 663.70 ± 502.06 Control (mg): total (poly)phenols = 771.60 ± 646.22 | Intervention (mg): total (poly)phenols = 557.40 ± 490.28 Control (mg): total (poly)phenols = 899.10 ± 1126.71 | Juice | Intervention: 19 Control: 18 | 200 mL/d (1666 mg (poly)phenols) | Placebo (similar in taste, color, smell, and texture, (poly)phenol-free) | 6 wk | BW, WC |
3.2. Impact of Chokeberry on Anthropometric Parameters
3.3. Impact of Chokeberry on FBG
3.4. Impact of Chokeberry on Lipid Profile
3.5. Impact of Chokeberry on Blood Pressure
3.6. Risk of Bias, TSA, and Certainty of Evidence
4. Discussion
4.1. Impact of Chokeberry Supplementation on Glycemia
4.2. Impact of Chokeberry Supplementation on Cholesterol Levels
4.3. Impact of Chokeberry Supplementation on Systolic Blood Pressure and Body Weight
4.4. Chokeberry Proanthocyanidins and Evaluated Cardiometabolic Outcomes
4.5. (Poly)phenol Intake Inconsistency
4.6. Randomized Versus Non-Randomized Intervention Trials of Chokeberry Supplementation
4.7. Meta-Analysis Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. GBD 2017 Causes of Death Collaborators. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Karoney, E.M.; Molelekoa, T.; Bill, M.; Siyoum, N.; Korsten, L. Global Research Network Analysis of Fresh Produce Postharvest Technology: Innovative Trends for Loss Reduction. Postharvest Biol. Technol. 2024, 208, 112642. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of Postharvest Water Loss in Fruits: Mechanisms, Influencing Factors, and Effective Control Strategies—A Comprehensive Review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Xu, L.; Tian, Z.; Chen, H.; Zhao, Y.; Yang, Y. Anthocyanins, Anthocyanin-Rich Berries, and Cardiovascular Risks: Systematic Review and Meta-Analysis of 44 Randomized Controlled Trials and 15 Prospective Cohort Studies. Front. Nutr. 2021, 8, 747884. [Google Scholar] [CrossRef]
- Jang, H.H.; Hwang, I.G.; Lee, Y.M. Effects of Anthocyanin Supplementation on Blood Lipid Levels: A Systematic Review and Meta-Analysis. Front. Nutr. 2023, 10, 1207751. [Google Scholar] [CrossRef] [PubMed]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and by-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- USDA. Black Chokeberry (Aronia melanocarpa Michx. Ell.). Available online: https://www.nrcs.usda.gov/plantmaterials/ndpmcpg8351.pdf (accessed on 23 March 2024).
- Wawer, I.; Wolniak, M.; Paradowska, K. Solid State NMR Study of Dietary Fiber Powders from Aronia, Bilberry, Black Currant and Apple. Solid State Nucl. Magn. Reson. 2006, 30, 106–113. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa Phenolics and Their Antioxidant Activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Hudec, J.; Bakoš, D.; Mravec, D.; Kobida, L.; Burdová, M.; Turianica, I.; Hlušek, J. Content of Phenolic Compounds and Free Polyamines in Black Chokeberry (Aronia melanocarpa) after Application of Polyamine Biosynthesis Regulators. J. Agric. Food Chem. 2006, 54, 3625–3628. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Rohn, S.; Mocan, A. Fermented Black Chokeberry (Aronia melanocarpa (Michx.) Elliott) Products—A Systematic Review on the Composition and Current Scientific Evidence of Possible Health Benefits. Food Res. Int. 2024, 196, 115094. [Google Scholar] [CrossRef]
- Hellström, J.K.; Shikov, A.N.; Makarova, M.N.; Pihlanto, A.M.; Pozharitskaya, O.N.; Ryhänen, E.L.; Kivijärvi, P.; Makarov, V.G.; Mattila, P.H. Blood Pressure-Lowering Properties of Chokeberry (Aronia mitchurinii, Var. Viking). J. Funct. Foods 2010, 2, 163–169. [Google Scholar] [CrossRef]
- Kim, B.; Ku, C.S.; Pham, T.X.; Park, Y.; Martin, D.A.; Xie, L.; Taheri, R.; Lee, J.; Bolling, B.W. Aronia melanocarpa (Chokeberry) Polyphenol-Rich Extract Improves Antioxidant Function and Reduces Total Plasma Cholesterol in Apolipoprotein E Knockout Mice. Nutr. Res. 2013, 33, 406–413. [Google Scholar] [CrossRef]
- Kim, N.H.; Jegal, J.; Kim, Y.N.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black Chokeberry (Aronia melanocarpa) Polyphenols Reveal Different Antioxidant, Antimicrobial and Neutrophil-Modulating Activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Kumkum, R.; Aston-Mourney, K.; McNeill, B.A.; Hernández, D.; Rivera, L.R. Bioavailability of Anthocyanins: Whole Foods versus Extracts. Nutrients 2024, 16, 1403. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of Anthocyanins and Derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Wu, T.; Grootaert, C.; Voorspoels, S.; Jacobs, G.; Pitart, J.; Kamiloglu, S.; Possemiers, S.; Heinonen, M.; Kardum, N.; Glibetic, M.; et al. Aronia (Aronia melanocarpa) Phenolics Bioavailability in a Combined in Vitro Digestion/Caco-2 Cell Model Is Structure and Colon Region Dependent. J. Funct. Foods 2017, 38, 128–139. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: In Vitro and in Vivo Evidences and Possible Mechanisms of Action: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia Melanocarpa Extract Reduces Blood Pressure, Serum Endothelin, Lipid, and Oxidative Stress Marker Levels in Patients with Metabolic Syndrome. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, CR28-34. [Google Scholar]
- Kardum, N.; Ristic, A.K.; Zec, M.; Kojadinovic, M.; Petrovic-Oggiano, G.; Zekovic, M.; Kroon, P.A.; Glibetic, M. Design, Formulation and Sensory Evaluation of a Polyphenol-Rich Food Placebo: An Example of Aronia Juice for Food Intervention Studies. Int. J. Food Sci. Nutr. 2017, 68, 742–749. [Google Scholar] [CrossRef]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black Chokeberry Aronia melanocarpa Extract Reduces Blood Pressure, Glycemia and Lipid Profile in Patients with Metabolic Syndrome: A Prospective Controlled Trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily Supplementation with Aronia Melanocarpa (Chokeberry) Reduces Blood Pressure and Cholesterol: A Meta Analysis of Controlled Clinical Trials. J. Diet. Suppl. 2021, 18, 517–530. [Google Scholar] [CrossRef]
- Rahmani, J.; Clark, C.; Kord Varkaneh, H.; Lakiang, T.; Vasanthan, L.T.; Onyeche, V.; Mousavi, S.M.; Zhang, Y. The Effect of Aronia Consumption on Lipid Profile, Blood Pressure, and Biomarkers of Inflammation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Effects of Aronia melanocarpa on Cardiometabolic Diseases: A Systematic Review of Quasi-Design Studies and Randomized Controlled Trials. Rev. Diabet. Stud. 2022, 18, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Choosing Effect Measures and Computing Estimates of Effect. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 143–176. ISBN 9781119536604. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Riberholt, C.G.; Olsen, M.H.; Gluud, C. Research Note: Trial Sequential Analysis in Systematic Reviews with Meta-Analysis. J. Physiother. 2024, 70, 243–246. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Nowicka, A.; Koter-Michalak, M.; Broncel, M. In Vivo Influence of Extract from Aronia melanocarpa on the Erythrocyte Membranes in Patients with Hypercholesterolemia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, CR569-74. [Google Scholar] [CrossRef]
- Sikora, J.; Broncel, M.; Markowicz, M.; Chałubiński, M.; Wojdan, K.; Mikiciuk-Olasik, E. Short-Term Supplementation with Aronia melanocarpa Extract Improves Platelet Aggregation, Clotting, and Fibrinolysis in Patients with Metabolic Syndrome. Eur. J. Nutr. 2012, 51, 549–556. [Google Scholar] [CrossRef]
- Kardum, N.; Konić-Ristić, A.; Šavikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of Polyphenol-Rich Chokeberry Juice on Antioxidant/pro-Oxidant Status in Healthy Subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Petrović-Oggiano, G.; Takic, M.; Glibetić, N.; Zec, M.; Debeljak-Martacic, J.; Konić-Ristić, A. Effects of Glucomannan-Enriched, Aronia Juice-Based Supplement on Cellular Antioxidant Enzymes and Membrane Lipid Status in Subjects with Abdominal Obesity. Sci. World J. 2014, 2014, 869250. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Broncel, M.; Mikiciuk-Olasik, E. Aronia melanocarpa Elliot Reduces the Activity of Angiotensin I-Converting Enzyme—In Vitro and EX Vivo Studies. Oxidative Med. Cell. Longev. 2014, 2014, 739721. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial Effects of Polyphenol-Rich Chokeberry Juice Consumption on Blood Pressure Level and Lipid Status in Hypertensive Subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef]
- Milutinović, M.; Radovanović, R.V.; Šavikin, K.; Radenković, S.; Arvandi, M.; Pešić, M.; Kostić, M.; Miladinović, B.; Branković, S.; Kitić, D. Chokeberry Juice Supplementation in Type 2 Diabetic Patients—Impact on Health Status. J. Appl. Biomed. 2019, 17, 218–224. [Google Scholar] [CrossRef]
- Rahn, C.; Bakuradze, T.; Stegmüller, S.; Galan, J.; Niesen, S.; Winterhalter, P.; Richling, E. Polyphenol-Rich Beverage Consumption Affecting Parameters of the Lipid Metabolism in Healthy Subjects. Int. J. Mol. Sci. 2023, 24, 841. [Google Scholar] [CrossRef]
- Tjelle, T.E.; Holtung, L.; Bøhn, S.K.; Aaby, K.; Thoresen, M.; Wiik, S.Å.; Paur, I.; Karlsen, A.S.; Retterstøl, K.; Iversen, P.O.; et al. Polyphenol-Rich Juices Reduce Blood Pressure Measures in a Randomised Controlled Trial in High Normal and Hypertensive Volunteers. Br. J. Nutr. 2015, 114, 1054–1063. [Google Scholar] [CrossRef]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia Melanocarpa Juice as Part of the Dietary Regimen in Patients with Diabetes Mellitus. Folia Med. 2002, 44, 20–23. [Google Scholar]
- Pedret, A.; Companys, J.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Salamanca, P.; Sandoval-Ramírez, B.-A.; Catalán, Ú.; Fernández-Castillejo, S.; Yuste, S.; et al. A Red-Fleshed Apple Rich in Anthocyanins Improves Endothelial Function, Reduces Inflammation, and Modulates the Immune System in Hypercholesterolemic Subjects: The AppleCOR Study. Food Funct. 2024, 15, 5825–5841. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.-J.; Ha, K.-C.; Baek, H.-I.; Yang, H.-J.; Kim, M.-J.; Park, S.-J. Efficacy and Safety of Aronia, Red Ginseng, Shiitake Mushroom, and Nattokinase Mixture on Insulin Resistance in Prediabetic Adults: A Randomized, Double-Blinded, Placebo-Controlled Trial. Foods 2021, 10, 1558. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. The Impact of an 8-Week Supplementation with Fermented and Non-Fermented Aronia Berry Pulp on Cardiovascular Risk Factors in Individuals with Type 2 Diabetes. Nutrients 2023, 15, 5094. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Aronia in the Type 2 Diabetes Treatment Regimen. Nutrients 2023, 15, 4188. [Google Scholar] [CrossRef] [PubMed]
- Stojković, L.; Zec, M.; Zivkovic, M.; Bundalo, M.; Bošković, M.; Glibetić, M.; Stankovic, A. Polyphenol-Rich Aronia melanocarpa Juice Consumption Affects LINE-1 DNA Methylation in Peripheral Blood Leukocytes in Dyslipidemic Women. Front. Nutr. 2021, 8, 689055. [Google Scholar] [CrossRef]
- Petrovic, S.; Arsic, A.; Glibetic, M.; Cikiriz, N.; Jakovljevic, V.; Vucic, V. The Effects of Polyphenol-Rich Chokeberry Juice on Fatty Acid Profiles and Lipid Peroxidation of Active Handball Players: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Can. J. Physiol. Pharmacol. 2016, 94, 1058–1063. [Google Scholar] [CrossRef]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; Groot, E.D.; Plat, J. The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Chamberlin, M.L.; Peach, J.T.; Wilson, S.M.G.; Miller, Z.T.; Bothner, B.; Walk, S.T.; Yeoman, C.J.; Miles, M.P. Polyphenol-Rich Aronia melanocarpa Fruit Beneficially Impact Cholesterol, Glucose, and Serum and Gut Metabolites: A Randomized Clinical Trial. Foods 2024, 13, 2768. [Google Scholar] [CrossRef]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Lackner, S.; Mahnert, A.; Moissl-Eichinger, C.; Madl, T.; Habisch, H.; Meier-Allard, N.; Kumpitsch, C.; Lahousen, T.; Kohlhammer-Dohr, A.; Mörkl, S.; et al. Interindividual Differences in Aronia Juice Tolerability Linked to Gut Microbiome and Metabolome Changes-Secondary Analysis of a Randomized Placebo-Controlled Parallel Intervention Trial. Microbiome 2024, 12, 49. [Google Scholar] [CrossRef]
- Le Sayec, M.; Xu, Y.; Laiola, M.; Gallego, F.A.; Katsikioti, D.; Durbidge, C.; Kivisild, U.; Armes, S.; Lecomte, M.; Fança-Berthon, P.; et al. The Effects of Aronia Berry (Poly)Phenol Supplementation on Arterial Function and the Gut Microbiome in Middle Aged Men and Women: Results from a Randomized Controlled Trial. Clin. Nutr. 2022, 41, 2549–2561. [Google Scholar] [CrossRef]
- Loo, B.M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of Chokeberry (Aronia Mitschurinii) Products Modestly Lowered Blood Pressure and Reduced Low-Grade Inflammation in Patients with Mildly Elevated Blood Pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef]
- Pokimica, B.; García-Conesa, M.-T.; Zec, M.; Debeljak-Martačić, J.; Ranković, S.; Vidović, N.; Petrović-Oggiano, G.; Konić-Ristić, A.; Glibetić, M. Chokeberry Juice Containing Polyphenols Does Not Affect Cholesterol or Blood Pressure but Modifies the Composition of Plasma Phospholipids Fatty Acids in Individuals at Cardiovascular Risk. Nutrients 2019, 11, 850. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia Berry Polyphenol Consumption Reduces Plasma Total and Low-Density Lipoprotein Cholesterol in Former Smokers without Lowering Biomarkers of Inflammation and Oxidative Stress: A Randomized Controlled Trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Sangild, J.; Faldborg, A.; Schousboe, C.; Fedder, M.D.K.; Christensen, L.P.; Lausdahl, A.K.; Arnspang, E.C.; Gregersen, S.; Jakobsen, H.B.; Knudsen, U.B.; et al. Effects of Chokeberries (Aronia Spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial. J. Clin. Med. 2023, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Laniewska, I.; Millo, B.; Dluzniewski, M. Combination Therapy of Statin with Flavonoids Rich Extract from Chokeberry Fruits Enhanced Reduction in Cardiovascular Risk Markers in Patients after Myocardial Infraction (MI). Atherosclerosis 2007, 194, E179–E184. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Effect of Citrus Fruit and Juice Consumption on Risk of Developing Type 2 Diabetes: Evidence on Polyphenols from Epidemiological and Intervention Studies. Trends Food Sci. Technol. 2021, 115, 133–146. [Google Scholar] [CrossRef]
- Pickering, R.T.; Bradlee, M.L.; Singer, M.R.; Moore, L.L. Baseline Diet Modifies the Effects of Dietary Change. Br. J. Nutr. 2020, 123, 951. [Google Scholar] [CrossRef]
- Alkhaldy, A.; Edwards, C.A.; Combet, E. The Urinary Phenolic Acid Profile Varies between Younger and Older Adults after a Polyphenol-Rich Meal despite Limited Differences in in Vitro Colonic Catabolism. Eur. J. Nutr. 2018, 58, 1095. [Google Scholar] [CrossRef]
- Howick, J.; Hoffmann, T. How Placebo Characteristics Can Influence Estimates of Intervention Effects in Trials. Can. Med. Assoc. J. 2018, 190, E908. [Google Scholar] [CrossRef]
- Tiwari, V.; Sharma, S.; Tiwari, A.; Sheoran, B.; Kaur, S.; Sharma, A.; Yadav, M.; Bhatnagar, A.; Garg, M. Effect of Dietary Anthocyanins on Biomarkers of Type 2 Diabetes and Related Obesity: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2024, 64, 7517–7534. [Google Scholar] [CrossRef]
- Pan, J.; Liang, J.; Xue, Z.; Meng, X.; Jia, L. Effect of Dietary Anthocyanins on the Risk Factors Related to Metabolic Syndrome: A Systematic Review and Meta-Analysis. PLoS ONE 2025, 20, e0315504. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as Promising Molecules and Dietary Bioactive Components against Diabetes—A Review of Recent Advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S. Macronutrients, Food Groups, and Eating Patterns in the Management of DiabetesA Systematic Review of the Literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of Dietary Fiber on Glycemic Control and Insulin Sensitivity in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- Raimundo, A.F.; Félix, F.; Andrade, R.; García-Conesa, M.T.; González-Sarrías, A.; Gilsa-Lopes, J.; do Ó, D.; Raimundo, A.; Ribeiro, R.; Rodriguez-Mateos, A.; et al. Combined Effect of Interventions with Pure or Enriched Mixtures of (Poly)Phenols and Anti-Diabetic Medication in Type 2 Diabetes Management: A Meta-Analysis of Randomized Controlled Human Trials. Eur. J. Nutr. 2020, 59, 1329–1343. [Google Scholar] [CrossRef]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-Rich Diets Improve Glucose Metabolism in People at High Cardiometabolic Risk: A Controlled Randomised Intervention Trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin Supplementation at Different Doses Improves Cholesterol Efflux Capacity in Subjects with Dyslipidemia—A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Fielding, C.J.; Fielding, P.E. Molecular Physiology of Reverse Cholesterol Transport. J. Lipid Res. 1995, 36, 211–228. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.Y.; Cao, L.; Ling, W.H. Anthocyanin Supplementation Improves Serum LDL- and HDL-Cholesterol Concentrations Associated with the Inhibition of Cholesteryl Ester Transfer Protein in Dyslipidemic Subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef]
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of Anthocyanin Supplementation on Cardio-Metabolic Biomarkers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2019, 38, 1153–1165. [Google Scholar] [CrossRef]
- Yang, L.P.; Ling, W.H.; Du, Z.C.; Chen, Y.M.; Li, D.; Deng, S.Z.; Liu, Z.M.; Yang, L.L. Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, Z.; Miao, H.; Zhang, Y. Effect of Weight Loss on Blood Pressure Changes in Overweight Patients: A Systematic Review and Meta-analysis. J. Clin. Hypertens. 2023, 25, 404. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.L.; Jiang, Z.Z.; Long, Y.; Gao, C.L.; Huang, W.; Tan, X.Z.; Ma, X.M.; Xu, Y. Effect of Proanthocyanidins on Blood Lipids: A Systematic Review and Meta-Analysis. Phytother. Res. 2024, 38, 2154–2164. [Google Scholar] [CrossRef]
- Ren, J.; An, J.; Chen, M.; Yang, H.; Ma, Y. Effect of Proanthocyanidins on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2021, 165, 105329. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Li, D.; Liu, X. Anthocyanin and Proanthocyanidin from Aronia melanocarpa (Michx.) Ell.: Purification, Fractionation, and Enzyme Inhibition. Food Sci. Nutr. 2023, 11, 3911. [Google Scholar] [CrossRef]
- Ou, K.; Percival, S.S.; Zou, T.; Khoo, C.; Gu, L. Transport of Cranberry A-Type Procyanidin Dimers, Trimers, and Tetramers across Monolayers of Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2012, 60, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Steck, J.; Junker, F.; Eichhöfer, H.; Bunzel, M. Chemically Different but Often Mistaken Phenolic Polymers of Food Plants: Proanthocyanidins and Lignin in Seeds. J. Agric. Food Chem. 2022, 70, 11704–11714. [Google Scholar] [CrossRef]
- Liu, Z.; De Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal Interactions between Epigallocatechin-3-Gallate (EGCG) and Human Gut Microbiota In Vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild Blueberry Proanthocyanidins Shape Distinct Gut Microbiota Profile and Influence Glucose Homeostasis and Intestinal Phenotypes in High-Fat High-Sucrose Fed Mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Ioannidis, J.P.A. Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Adv. Nutr. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.A.; Sarti, F.M.; Coutinho, C.P.; Hassimotto, N.M.; Marchioni, D.M.; Lotufo, P.A.; Bensenor, I.M.; Lajolo, F.M. Associations between Polyphenol Intake, Cardiometabolic Risk Factors and Metabolic Syndrome in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J. Nutr. 2024, 155, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Lanuza, F.; Zamora-Ros, R.; Bondonno, N.P.; Meroño, T.; Rostgaard-Hansen, A.L.; Riccardi, G.; Tjønneland, A.; Landberg, R.; Halkjær, J.; Andres-Lacueva, C. Dietary Polyphenols, Metabolic Syndrome and Cardiometabolic Risk Factors: An Observational Study Based on the DCH-NG Subcohort. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1167–1178. [Google Scholar] [CrossRef]
- Tao, L.; Wang, J.; Chen, J.; Chen, M. Letter to the Editor: Comment on “The Effects of Aronia Berry (Poly)Phenol Supplementation on Arterial Function and the Gut Microbiome in Middle Aged Men and Women: Results from a Randomized Controlled Trial”. Clin. Nutr. 2023, 42, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Adjoian, T.K.; Firestone, M.J.; Eisenhower, D.; Yi, S.S. Validation of Self-Rated Overall Diet Quality by Healthy Eating Index-2010 Score among New York City Adults, 2013. Prev. Med. Rep. 2016, 3, 127. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G. Evaluating Non-Randomised Intervention Studies. Health Technol. Assess. 2003, 7, iii-173. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Assessing Risk of Bias in a Non-Randomized Study. Cochrane Handb. Syst. Rev. Interv. 2019, 23, 621–641. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, Y.; Chen, Q.; Fu, X.; Huang, Q.; Zhang, B.; Dong, H.; Li, C. Exploring the Synergistic Benefits of Insoluble Dietary Fiber and Bound Phenolics: Unveiling the Role of Bound Phenolics in Enhancing Bioactivities of Insoluble Dietary Fiber. Trends Food Sci. Technol. 2024, 149, 104554. [Google Scholar] [CrossRef]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of Food Processing on Antioxidants, Their Bioavailability and Potential Relevance to Human Health. Food Chem. X 2022, 14, 100334. [Google Scholar] [CrossRef]



| Study, Year [Reference] | Random Sequence Generation | Carry-Over Effect | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Overall Assessment of Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Ahles et al. (2020) [57] | Low | - | Low | Low | Low | Low | Low | Low |
| Chamberlin et al. (2024) [58] | Low | - | Low | Low | Low | Low | Low | Low |
| Istas et al. (2019) [59] | Low | - | Low | Low | Low | Low | Low | Low |
| Lackner et al. (2024) [60] | Low | - | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Le Sayec et al. (2022) [61] | Low | - | Low | Low | Low | Low | Low | Low |
| Loo et al. (2016) [62] | Low | High | Unclear | Unclear | Unclear | Low | Low | High |
| Naruszewicz et al. (2007) [66] | Unclear | - | Unclear | Low | Low | Low | Low | Unclear |
| Pokimica et al. (2019) [63] | Low | - | Unclear | Low | Low | Low | Low | Unclear |
| Sangild et al. (2023) [65] | Unclear | Low | Low | Low | Low | Unclear | Unclear | Unclear |
| Xie et al. (2017) [64] | Low | - | Unclear | Low | Low | Low | Low | Unclear |
| No of Studies | Certainty Assessment | No. of Patients | Effect | Certainty | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Treatment Group | Control Group | SMD (95% CI) | ||
| BW | ||||||||||
| 5 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 176 | 167 | 0.01 [−0.20, 0.22] | ⨁◯◯◯ Very low |
| BMI | ||||||||||
| 5 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 188 | 189 | 0.03 [−0.17, 0.23] | ⨁◯◯◯ Very low |
| WC | ||||||||||
| 3 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e Small number of included studies f | 99 | 100 | 0.02 [−0.26, 0.30] | ⨁◯◯◯ Very low |
| TAG | ||||||||||
| 7 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 241 | 236 | −0.02 [−0.20, 0.16] | ⨁◯◯◯ Very low |
| TC | ||||||||||
| 7 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 241 | 236 | −0.12 [−0.36, 0.12] | ⨁◯◯◯ Very low |
| LDL-C | ||||||||||
| 7 | RCTs | Serious a | Serious b | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 241 | 236 | −0.13 [−0.40, 0.15] | ⨁◯◯◯ Very low |
| HDL-C | ||||||||||
| 7 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 241 | 236 | −0.05 [−0.23, 0.13] | ⨁◯◯◯ Very low |
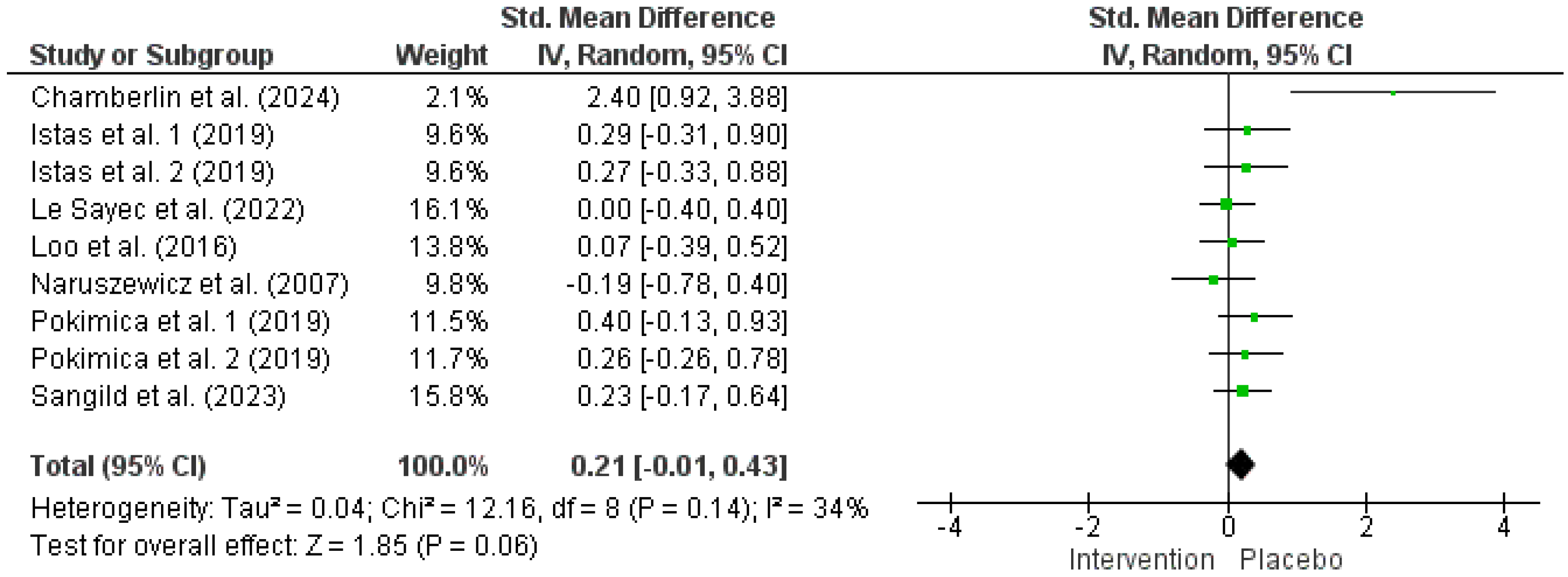
| FBG | ||||||||||
| 7 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 267 | 256 | 0.21 [−0.01, 0.43] | ⨁◯◯◯ Very low |
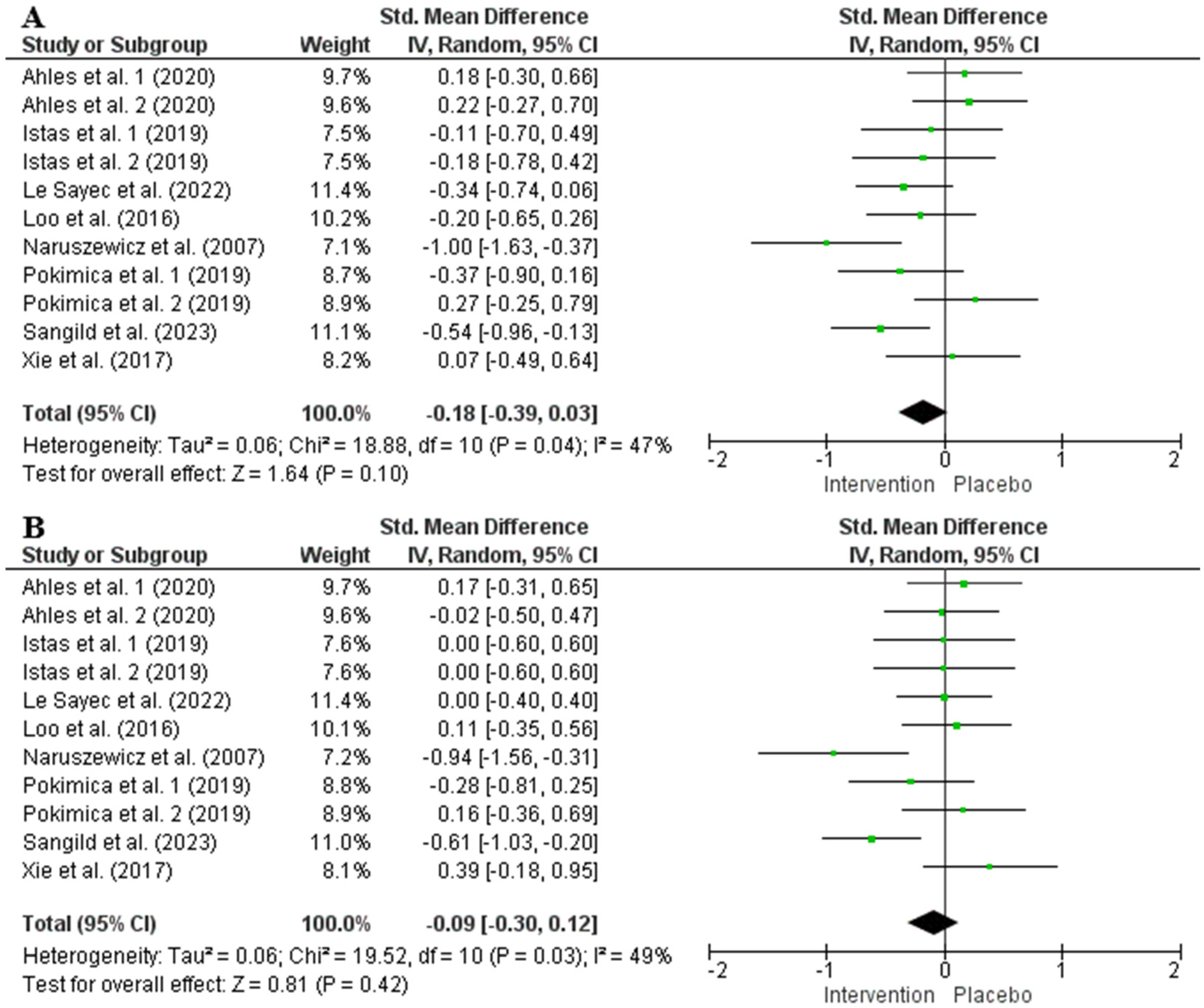
| SBP | ||||||||||
| 8 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 354 | 336 | −0.18 [−0.39, 0.03] | ⨁◯◯◯ Very low |
| DBP | ||||||||||
| 8 | RCTs | Serious a | Not serious | Not serious | Very serious c,d | (Poly)phenol intake inconsistency e | 354 | 336 | −0.09 [−0.30, 0.12] | ⨁◯◯◯ Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frumuzachi, O.; Mocan, A.; Rohn, S.; Gavrilaș, L. Impact of a Chokeberry (Aronia melanocarpa (Michx.) Elliott) Supplementation on Cardiometabolic Outcomes: A Critical Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 1488. https://doi.org/10.3390/nu17091488
Frumuzachi O, Mocan A, Rohn S, Gavrilaș L. Impact of a Chokeberry (Aronia melanocarpa (Michx.) Elliott) Supplementation on Cardiometabolic Outcomes: A Critical Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2025; 17(9):1488. https://doi.org/10.3390/nu17091488
Chicago/Turabian StyleFrumuzachi, Oleg, Andrei Mocan, Sascha Rohn, and Laura Gavrilaș. 2025. "Impact of a Chokeberry (Aronia melanocarpa (Michx.) Elliott) Supplementation on Cardiometabolic Outcomes: A Critical Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 17, no. 9: 1488. https://doi.org/10.3390/nu17091488
APA StyleFrumuzachi, O., Mocan, A., Rohn, S., & Gavrilaș, L. (2025). Impact of a Chokeberry (Aronia melanocarpa (Michx.) Elliott) Supplementation on Cardiometabolic Outcomes: A Critical Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 17(9), 1488. https://doi.org/10.3390/nu17091488








