Protein Source, Dietary Fibre Intake, and Inflammation in Older Adults: A UK Biobank Study
Abstract
1. Introduction
2. Materials and Methods
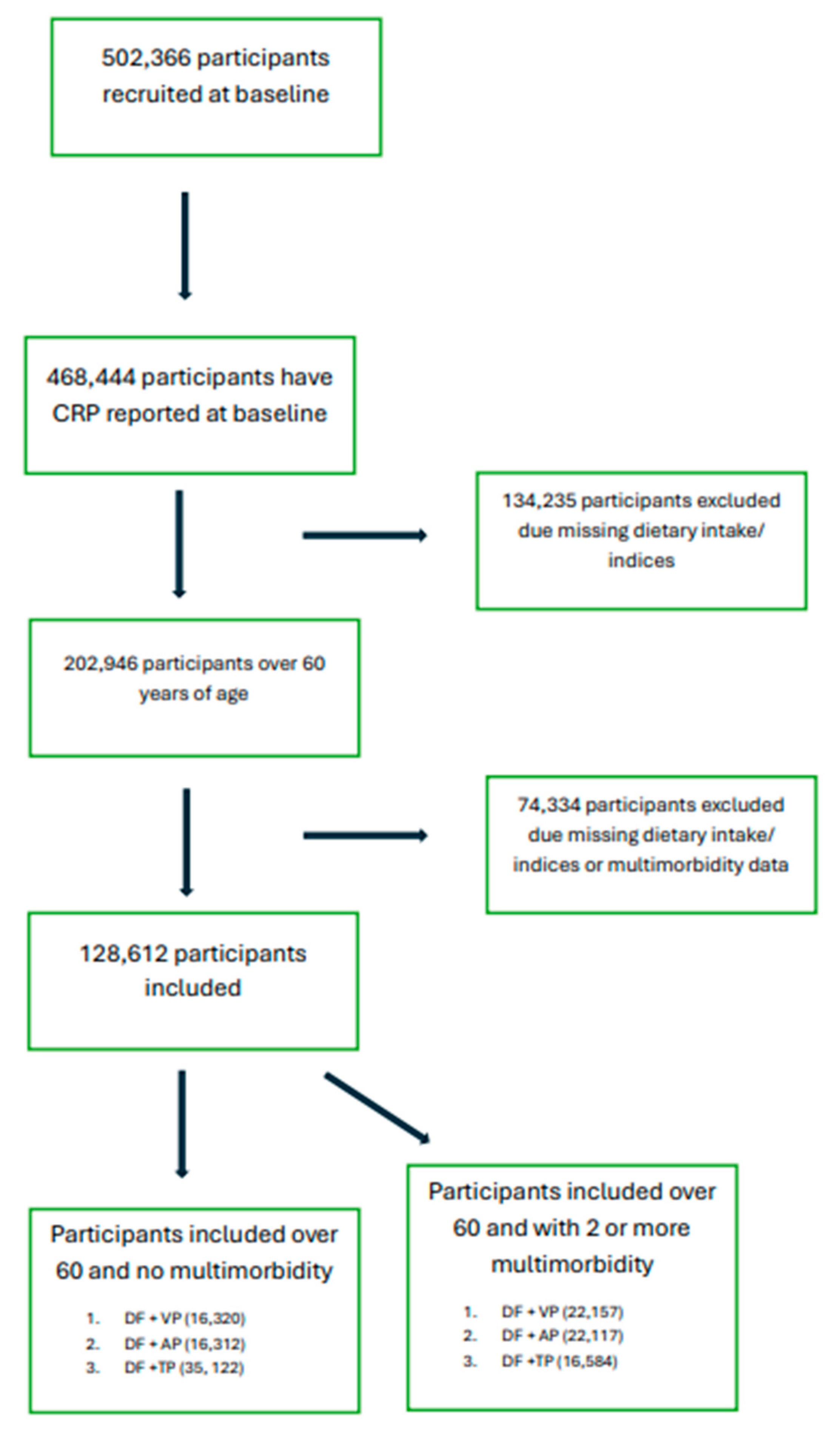
2.1. Inclusion Criteria
2.2. Diet
2.3. Dietary Protein Intake
2.4. Dietary Fibre Intake
2.5. C-Reactive Protein
2.6. Covariates
2.7. Ethical Approval
2.8. Statistical Analyses
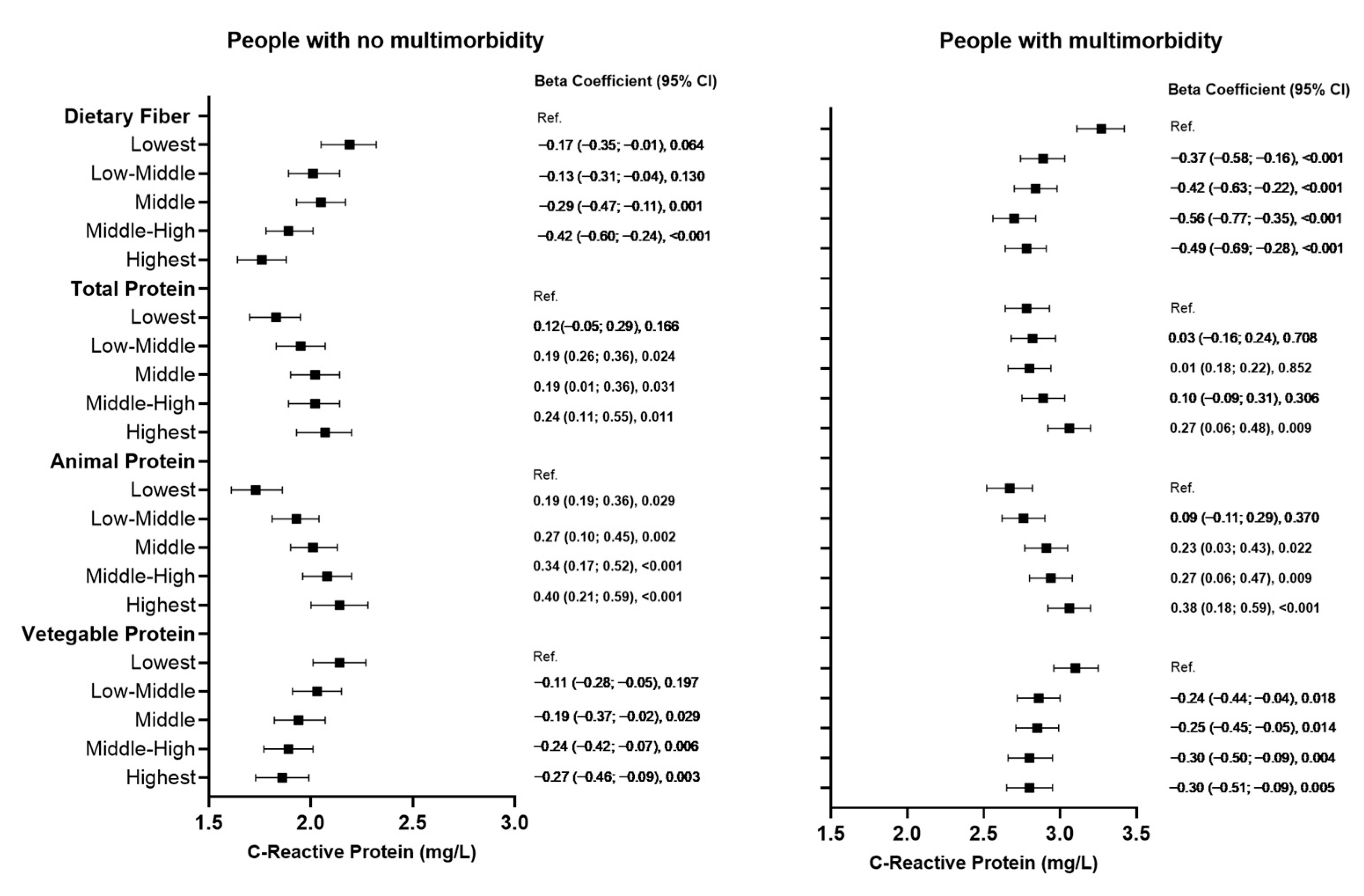
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowe, J.W.; Kahn, R.L. Human Aging: Usual and Successful. Science 1987, 237, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Records of Scotland N. Mid 2021 Population Estimates, Scotland; Report; National Records Scotland: Edinburgh, Scotland, 2022. [Google Scholar]
- Storey, A. Profile of the Older Population Living in England and Wales in 2021 and Changes Since 2011; Office for National Statistics: London, UK, 2023. [Google Scholar]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yao, Y.M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1032. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Strang, F.; Schunkert, H. C-reactive protein and coronary heart disease: All said--is not it? Mediat. Inflamm 2014, 2014, 757123. [Google Scholar] [CrossRef]
- Hruby, A.; Jacques, P.F. Dietary Protein and Changes in Biomarkers of Inflammation and Oxidative Stress in the Framingham Heart Study Offspring Cohort. Curr. Dev. Nutr. 2019, 3, nzz019. [Google Scholar] [CrossRef] [PubMed]
- Aycart, D.F.; Acevedo, S.; Eguiguren-Jimenez, L.; Andrade, J.M. Influence of Plant and Animal Proteins on Inflammation Markers among Adults with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1660. [Google Scholar] [CrossRef] [PubMed]
- Flight, I.; Clifton, P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary Fiber and Risk of Coronary Heart Disease. Arch. Intern. Med. 2004, 164, 370. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Alexander, C.; Swanson, K.S.; Fahey, G.C.; Garleb, K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrate Fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- King, D.E. Dietary fiber, inflammation, and cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Durazzo, M.; Guidi, S.; Carello, M.; Sacerdote, C.; Silli, B.; Rosato, R.; Cassader, M.; Gentile, L.; Pagano, G. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am. J. Clin. Nutr. 2006, 84, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J.; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Collins, R. What makes UK Biobank special? Lancet 2012, 379, 1173–1174. [Google Scholar] [CrossRef]
- Palmer, L.J. UK Biobank: Bank on it. Lancet 2007, 369, 1980–1982. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Perez-Cornago, A.; Pollard, Z.; Young, H.; van Uden, M.; Andrews, C.; Piernas, C.; Key, T.J.; Mulligan, A.; Lentjes, M. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur. J. Nutr. 2021, 60, 4019–4030. [Google Scholar] [CrossRef]
- McCance, R.A.; Widdowson, E.M. McCance and Widdowson’s The Composition of Foods: Royal Society of Chemistry; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7, e6. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef]
- Elliott, P.; Peakman, T.C.; Biobank, U.K. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, L.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Hosford-Donovan, A.; Nilsson, A.; Wåhlin-Larsson, B.; Kadi, F. Observational and mechanistic links between C-reactive protein and blood pressure in elderly women. Maturitas 2016, 89, 52–57. [Google Scholar] [CrossRef] [PubMed]
- de Rekeneire, N.; Peila, R.; Ding, J.; Colbert, L.H.; Visser, M.; Shorr, R.I.; Kritchevsky, S.B.; Kuller, L.H.; Strotmeyer, E.S.; Schwartz, A.V.; et al. Diabetes, Hyperglycemia, and Inflammation in Older Individuals. Diabetes Care 2006, 29, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Tracy, R.P.; Lemaitre, R.N.; Psaty, B.M.; Ives, D.G.; Evans, R.W.; Cushman, M.; Meilahn, E.N.; Kuller, L.H. Relationship of C-Reactive Protein to Risk of Cardiovascular Disease in the Elderly. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1121–1127. [Google Scholar] [CrossRef]
- Costello-White, R.; Ryff, C.D.; Coe, C.L. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Ageing Res. Rev. 2015, 37, 75. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.H.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory Markers and Onset of Cardiovascular Events. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef]
- Aryan, Z.; Ghajar, A.; Faghihi-Kashani, S.; Afarideh, M.; Nakhjavani, M.; Esteghamati, A. Baseline High-Sensitivity C-Reactive Protein Predicts Macrovascular and Microvascular Complications of Type 2 Diabetes: A Population-Based Study. Ann. Nutr. Metab. 2018, 72, 287–295. [Google Scholar] [CrossRef]
- Goodson, N.J.; Symmons, D.P.; Scott, D.G.; Bunn, D.; Lunt, M.; Silman, A.J. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum 2005, 52, 2293–2299. [Google Scholar] [CrossRef]
- Ferreira, G.D.; Simoes, J.A.; Senaratna, C.; Pati, S.; Timm, P.F.; Batista, S.R.; Nunes, B.P. Physiological markers and multimorbidity: A systematic review. J. Comorb. 2018, 8, 2235042X18806986. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Basora-Gallisa, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Escoda, R.; et al. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J. Epidemiol. Community Health 2009, 63, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Biggs, M.L.; Djoussé, L.; Durda, P.J.; Kizer, J.R.; Psaty, B.; Reiner, A.P.; Tracy, R.P.; Siscovick, D.; Mukamal, K.J. Intake and Sources of Dietary Fiber, Inflammation, and Cardiovascular Disease in Older US Adults. JAMA Netw. Open 2022, 5, e225012. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nat. Commun. 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef]
- Ryoo, H.; Choi, G.W.; Lee, H.S.; Choi, M.K.; Roh, Y.K. Association between Dietary Protein Intake and Serum High-Sensitivity C-Reactive Protein Level in the Korean Elderly with Diabetes: Based on the Korea National Health and Nutrition Examination Survey 2016–2018. Korean J. Fam. Pract. 2020, 10, 364–370. [Google Scholar] [CrossRef]
- Miki, A.; Hashimoto, Y.; Matsumoto, S.; Ushigome, E.; Fukuda, T.; Sennmaru, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. Protein Intake, Especially Vegetable Protein Intake, Is Associated with Higher Skeletal Muscle Mass in Elderly Patients with Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 7985728. [Google Scholar] [CrossRef]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Uffelman, C.; Hill, E.; Anderson, N.; Reed, J.; Olson, M.; Campbell, W. The Effects of Red Meat Intake on Inflammation Biomarkers in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Dev. Nutr. 2022, 6, 994. [Google Scholar] [CrossRef]
- Papier, K.; Hartman, L.; Tong, T.Y.N.; Key, T.J.; Knuppel, A. Higher Meat Intake Is Associated with Higher Inflammatory Markers, Mostly Due to Adiposity: Results from UK Biobank. J. Nutr. 2022, 152, 183–189. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Stadelmaier, J.; Eble, J.; Grummich, K.; Szczerba, E.; Kiesswetter, E.; Schlesinger, S.; Schwingshackl, L. Substitution of animal-based with plant-based foods on cardiometabolic health and all-cause mortality: A systematic review and meta-analysis of prospective studies. BMC Med. 2023, 21, 404. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Nosworthy, M.G.; Medina, G.; Lu, Z.H.; House, J.D. Plant Proteins: Methods of Quality Assessment and the Human Health Benefits of Pulses. Foods 2023, 12, 2816. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef]
- Yu, X.; Pu, H.; Voss, M. Overview of anti-inflammatory diets and their promising effects on non-communicable diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, M.; Celis-Morales, C.; Ozanne, S.E.; Burden, S.; Gray, S.R.; Morrison, D.J. Protein Source, Dietary Fibre Intake, and Inflammation in Older Adults: A UK Biobank Study. Nutrients 2025, 17, 1454. https://doi.org/10.3390/nu17091454
Jain M, Celis-Morales C, Ozanne SE, Burden S, Gray SR, Morrison DJ. Protein Source, Dietary Fibre Intake, and Inflammation in Older Adults: A UK Biobank Study. Nutrients. 2025; 17(9):1454. https://doi.org/10.3390/nu17091454
Chicago/Turabian StyleJain, Mahek, Carlos Celis-Morales, Susan E. Ozanne, Sorrel Burden, Stuart R. Gray, and Douglas J. Morrison. 2025. "Protein Source, Dietary Fibre Intake, and Inflammation in Older Adults: A UK Biobank Study" Nutrients 17, no. 9: 1454. https://doi.org/10.3390/nu17091454
APA StyleJain, M., Celis-Morales, C., Ozanne, S. E., Burden, S., Gray, S. R., & Morrison, D. J. (2025). Protein Source, Dietary Fibre Intake, and Inflammation in Older Adults: A UK Biobank Study. Nutrients, 17(9), 1454. https://doi.org/10.3390/nu17091454







