Crosstalk Among Gut Microbiota, Fecal Metabolites, and Amygdala Neuropathology Genes After Ginger Polyphenol Administration in Female Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Connection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, NP Induction, and Group Treatments
2.2. Mechanical (Hyper)Sensitivity Measurement
2.3. Anxiety-like Behavior Measurement
2.4. Sample Collection
2.5. Gut Microbiota Profiling via 16S rRNA Amplicon Sequencing
2.6. Hydrophilic Metabolites in Feces
2.7. Gene Expression Profiling Using a Neuropathology Panel
2.8. qRT-PCR Confirmation of Neuropathology Genes
2.9. Statistical Analysis
3. Results
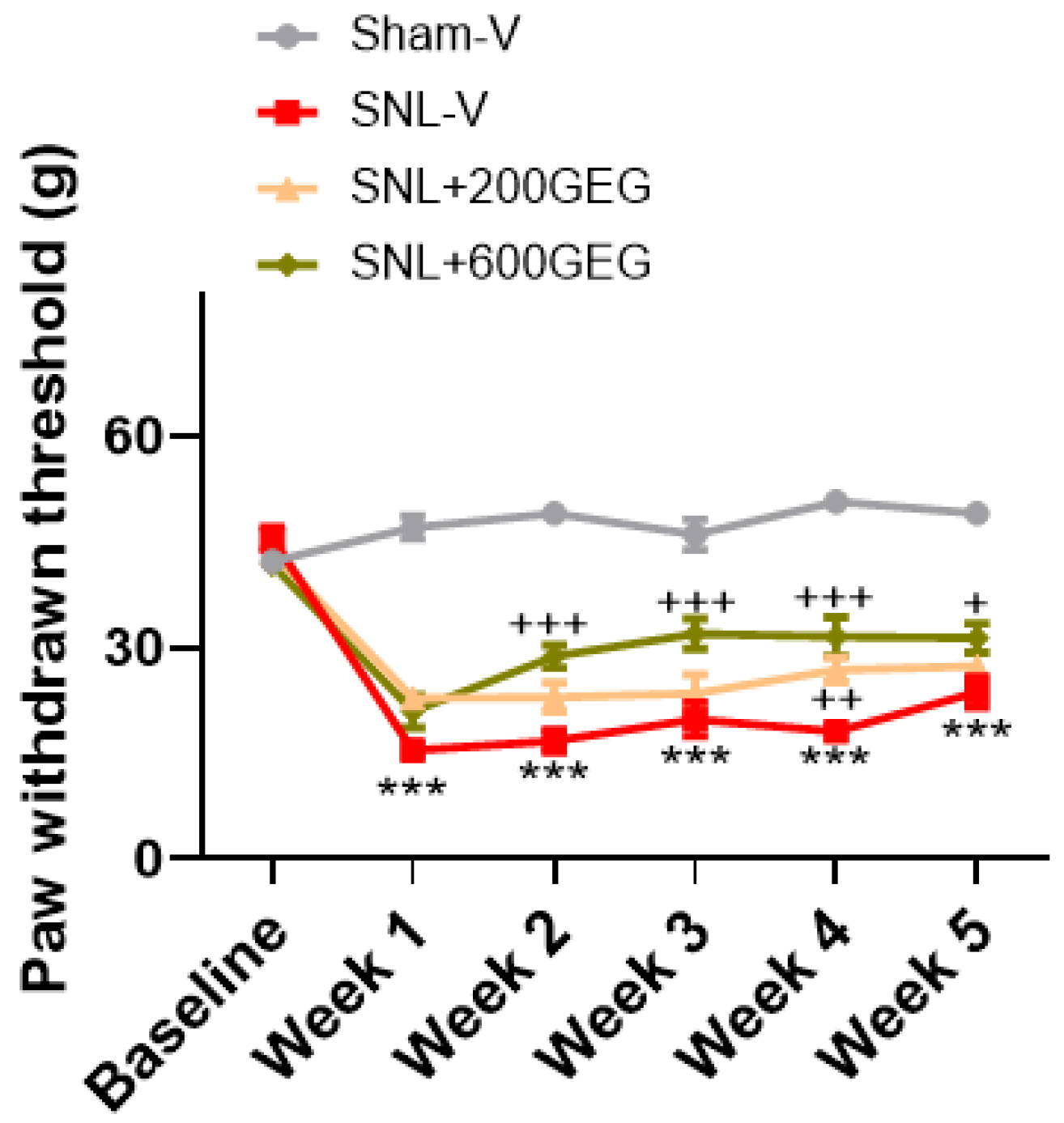
3.1. GEG Administration Mitigated Mechanical Hypersensitivity
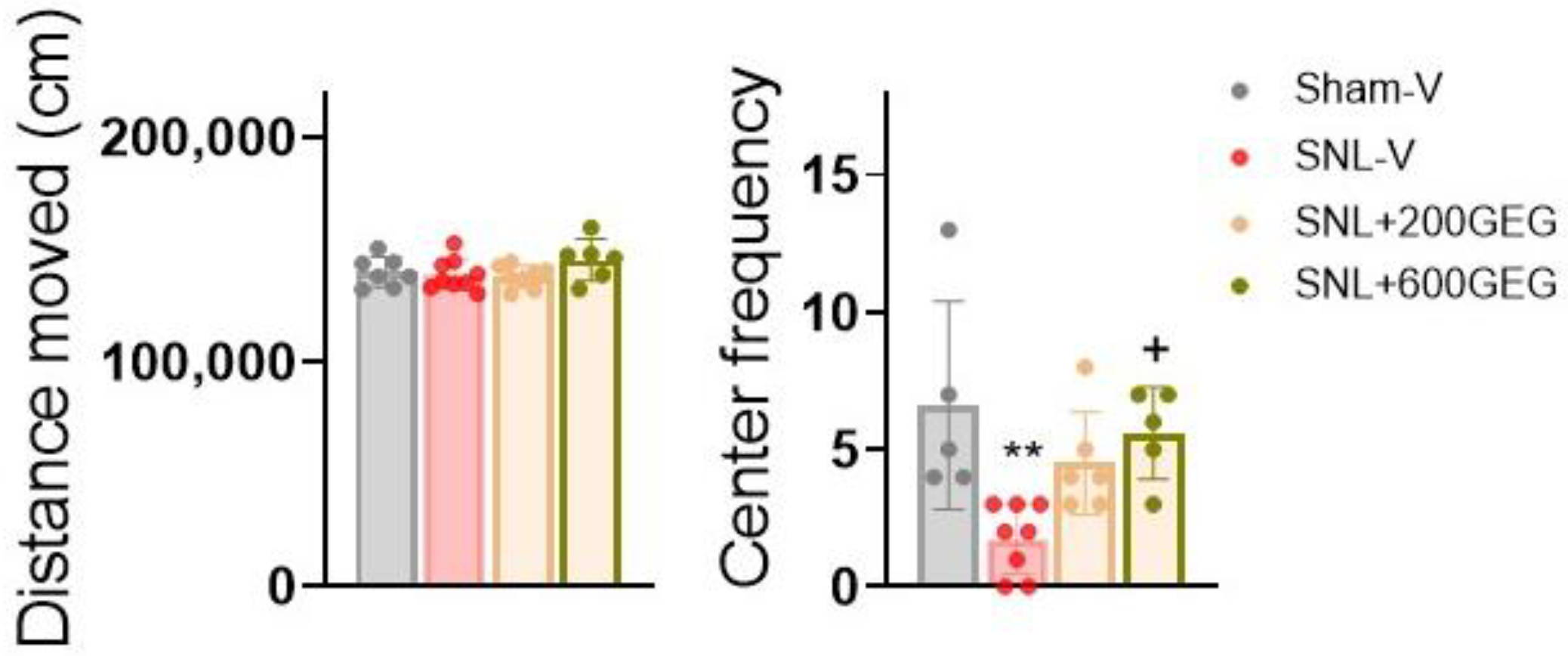
3.2. GEG Administration Alleviated Anxiety-like Behaviors
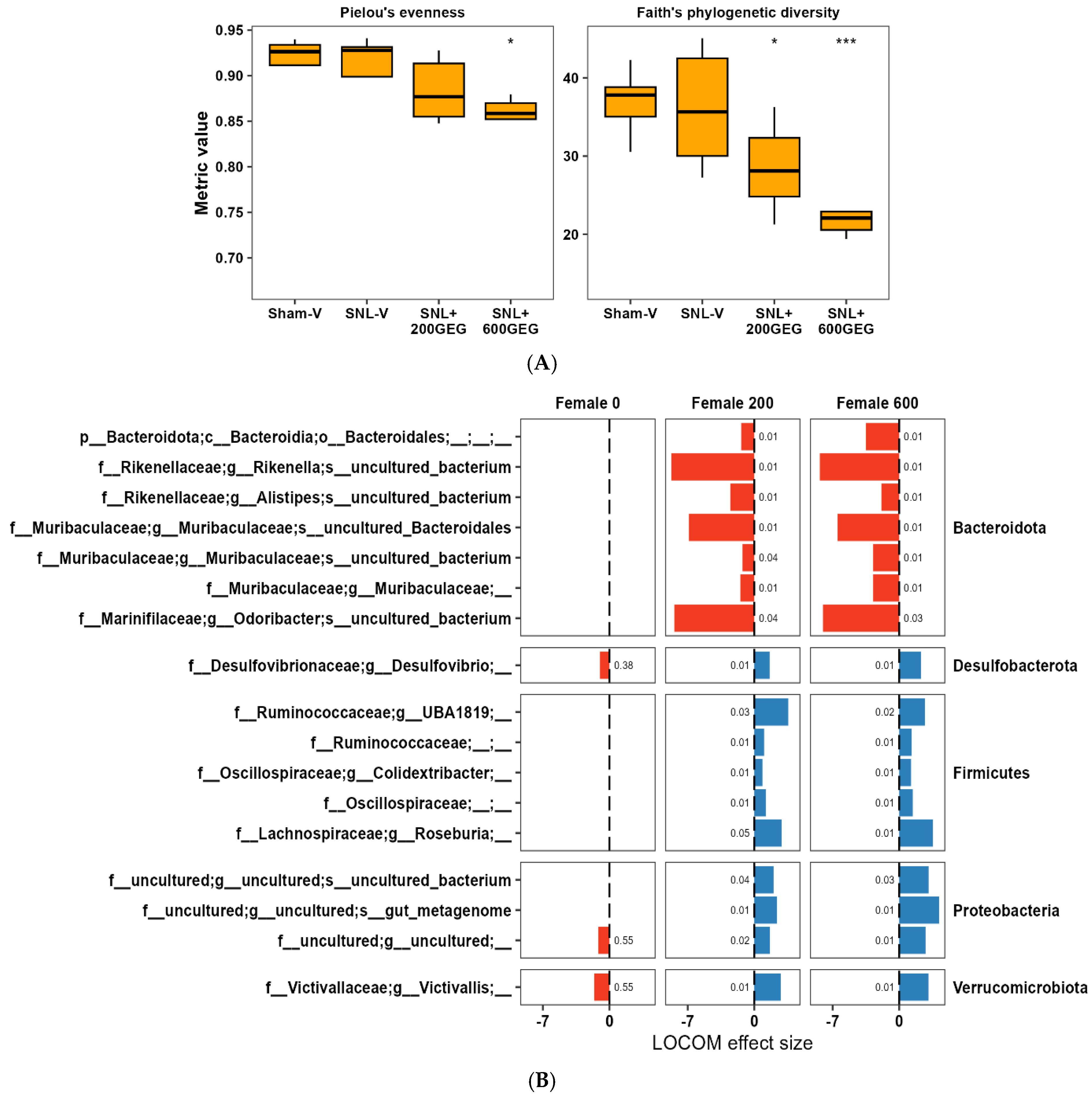
3.3. GEG Administration Modified Gut Microbiome Composition
3.4. GEG Administration Modified Fecal Metabolites
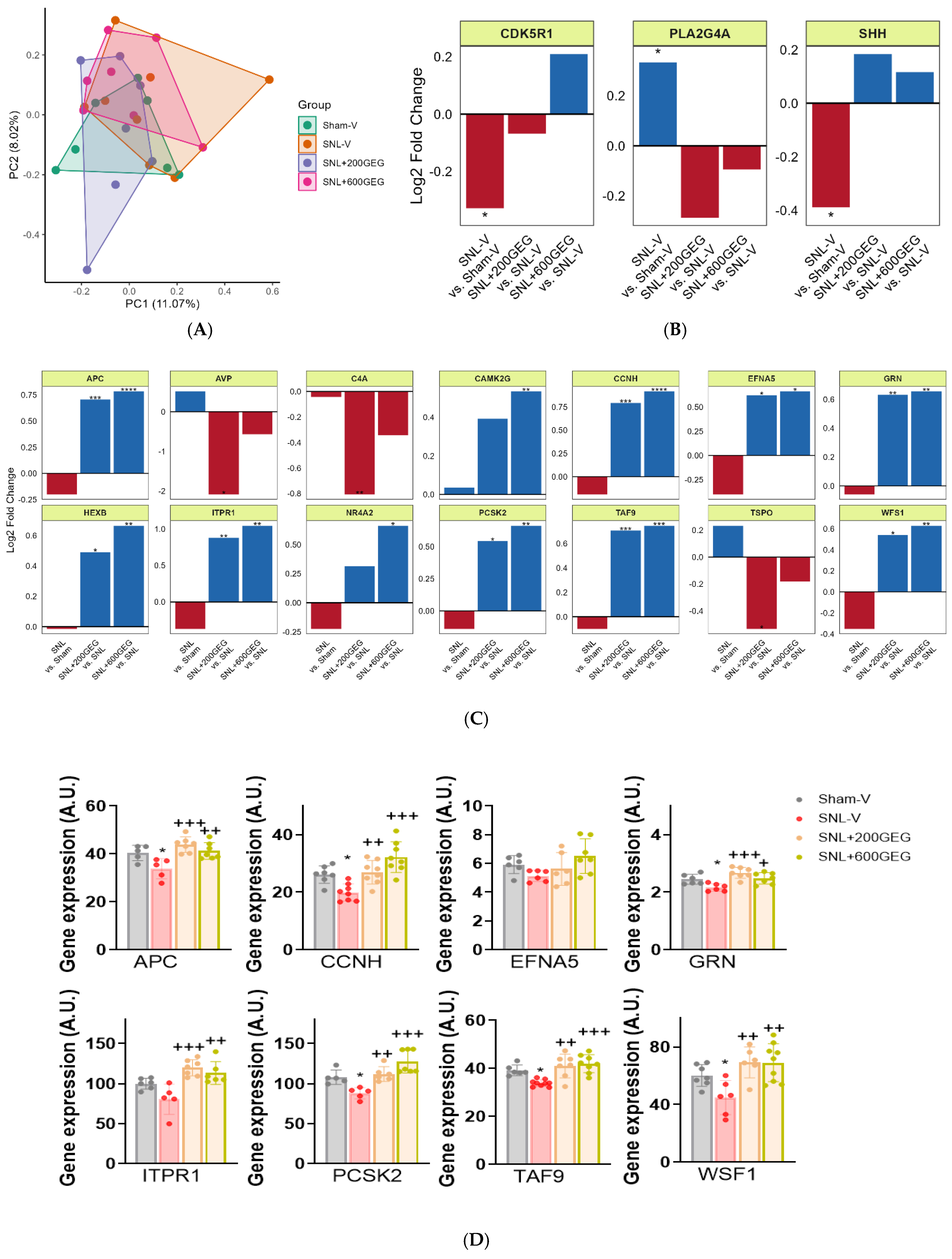
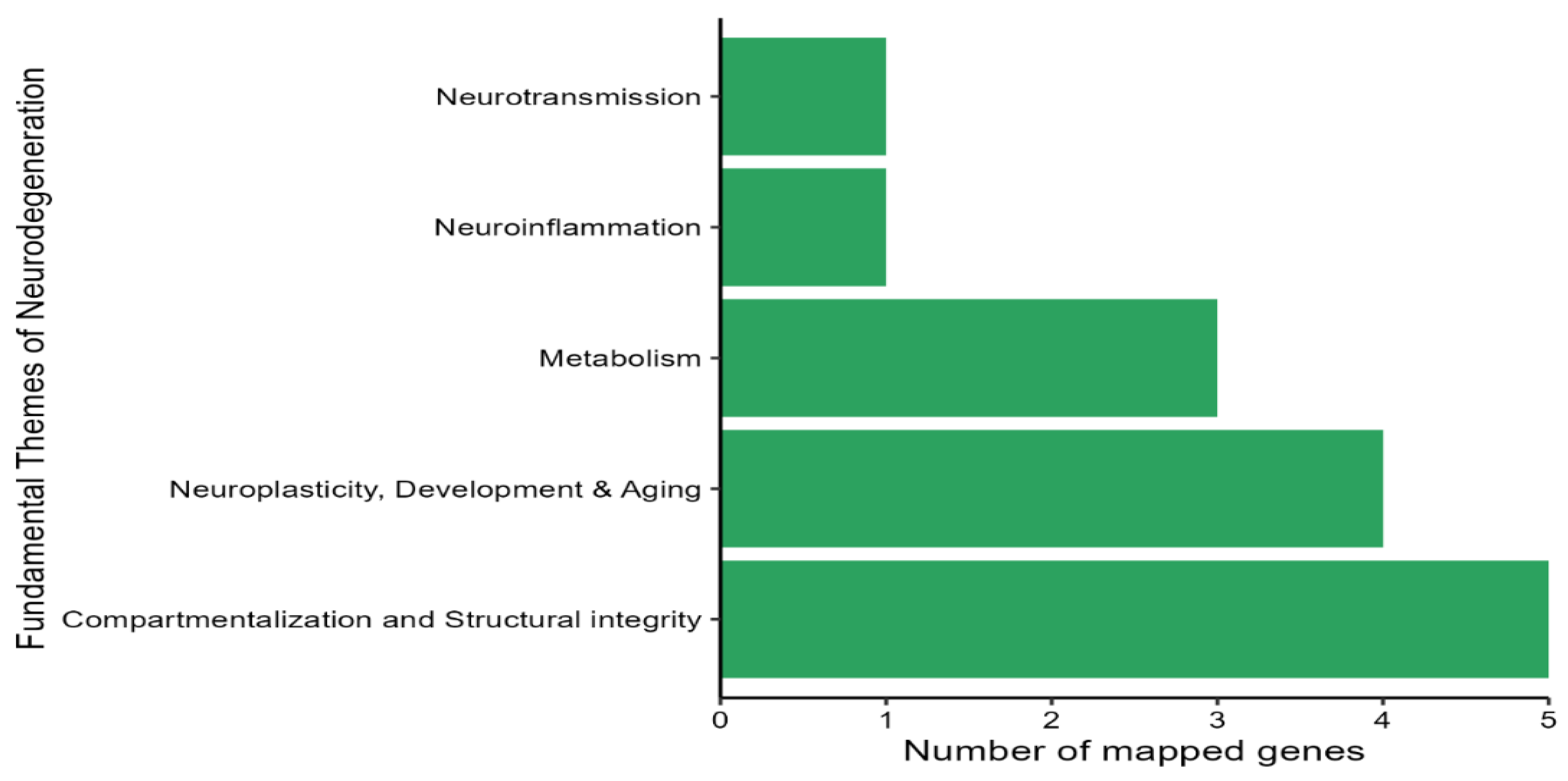
3.5. GEG Administration Modulated the Expression of Neuropathology Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A novel regulator of pain. J. Neural Transm. 2020, 127, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.B.; Wang, Y.T.; Zhang, P.; Yuan, Y.Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, R.; Ji, G.C.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.X.; Mirzaei, P.; Sang, S.M.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, R.; Santos, J.M.; Elmassry, M.M.; Stephens, E.; Kim, N.; Neugebauer, V. Ginger alleviates mechanical hypersensitivity and anxio-depressive behavior in rats with diabetic neuropathy through beneficial actions on gut microbiome composition, mitochondria, and neuroimmune cells of colon and spinal cord. Nutr. Res. 2024, 124, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, R.; Yakhnitsa, V.; Santos, J.M.; Watson, C.; Kiritoshi, T.; Ji, G.C.; Hamood, A.N.; Neugebauer, V. Gingerol-Enriched Ginger Supplementation Mitigates Neuropathic Pain via Mitigating Intestinal Permeability and Neuroinflammation: Gut-Brain Connection. Front. Pharmacol. 2022, 13, 912609. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008, 21, 570–579. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Beggs, S.; Liu, X.J.; Kwan, C.; Salter, M.W. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Mol. Pain 2010, 6, 74. [Google Scholar] [CrossRef]
- Echeverry, S.; Shi, X.Q.; Rivest, S.; Zhang, J. Peripheral Nerve Injury Alters Blood-Spinal Cord Barrier Functional and Molecular Integrity through a Selective Inflammatory Pathway. J. Neurosci. 2011, 31, 10819–10828. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Neugebauer, V. Amygdala Plasticity and Pain. Pain Res. Manag. 2017, 2017, 8296501. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.M.; Wilson, T.D.; Singh, S.; Torruella-Suárez, M.L.; Chaudhry, S.; Adke, A.P.; Becker, J.J.; Neugebauer, B.; Lin, J.L.; Gonzalez, S.M.; et al. The parabrachial to central amygdala pathway is critical to injury-induced pain sensitization in mice. Neuropsychopharmacology 2024, 49, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Yakhnitsa, V.; Thompson, J.; Ponomareva, O.; Ji, G.C.; Kiritoshi, T.; Mahimainathan, L.; Molehin, D.; Pruitt, K.; Neugebauer, V. Dysfunction of Small-Conductance Ca-Activated Potassium (SK) Channels Drives Amygdala Hyperexcitability and Neuropathic Pain Behaviors: Involvement of Epigenetic Mechanisms. Cells 2024, 13, 1055. [Google Scholar] [CrossRef]
- Raver, C.; Uddin, O.; Ji, Y.D.; Li, Y.; Cramer, N.; Jenne, C.; Morales, M.; Masri, R.; Keller, A. An Amygdalo-Parabrachial Pathway Regulates Pain Perception and Chronic Pain. J. Neurosci. 2020, 40, 3424–3442. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Ponomareva, O.; Presto, P.; John, J.; Neugebauer, V. Impaired amygdala astrocytic signaling worsens neuropathic pain-associated neuronal functions and behaviors. Front. Pharmacol. 2024, 15, 1368634. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Santos, J.M.; Elmassry, M.M.; Bhakta, V.; Driver, Z.; Ji, G.C.; Yakhnitsa, V.; Kiritoshi, T.; Lovett, J.; Hamood, A.N.; et al. Ginger Polyphenols Reverse Molecular Signature of Amygdala Neuroimmune Signaling and Modulate Microbiome in Male Rats with Neuropathic Pain: Evidence for Microbiota-Gut-Brain Axis. Antioxidants 2024, 13, 502. [Google Scholar] [CrossRef]
- Danaher, P.; Warren, S.; Dennis, L.; D'Amico, L.; White, A.; Disis, M.L.; Geller, M.A.; Odunsi, K.; Beechem, J.; Fling, S.P. Gene expression markers of Tumor Infiltrating Leukocytes. J. Immunother. Cancer 2017, 5, 18. [Google Scholar] [CrossRef]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Dell'istituto Super. Sanita 2016, 52, 184–189. [Google Scholar] [CrossRef]
- Coyle, D.E.; Sehlhorst, C.S.; Mascari, C. Female rats are more susceptible to the development of neuropathic pain using the partial sciatic nerve ligation (PSNL) model. Neurosci. Lett. 1995, 186, 135–138. [Google Scholar] [CrossRef]
- Presto, P.; Mazzitelli, M.; Junell, R.; Griffin, Z.; Neugebauer, V. Sex differences in pain along the neuraxis. Neuropharmacology 2022, 210, 109030. [Google Scholar] [CrossRef] [PubMed]
- Naji-Esfahani, H.; Vaseghi, G.; Safaeian, L.; Pilehvarian, A.A.; Abed, A.; Rafieian-Kopaei, M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab. Anim. 2016, 50, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Li, N.; Zhao, Y.; Zhang, L.J. Effects of female sex hormones on chemotherapeutic paclitaxel-induced neuropathic pain and involvement of inflammatory signal. J. Biol. Regul. Homeost. Agents 2018, 32, 1157–1163. [Google Scholar]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Ji, G.C.; Yakhnitsa, V.; Kiritoshi, T.; Presto, P.; Neugebauer, V. Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats. Mol. Pain 2018, 14, 1744806918804441. [Google Scholar] [CrossRef]
- Ji, L.L.; Peng, J.B.; Fu, C.H.; Tong, L.; Wang, Z.Y. Sigma-1 receptor activation ameliorates anxiety-like behavior through NR2A-CREB-BDNF signaling pathway in a rat model submitted to single-prolonged stress. Mol. Med. Rep. 2017, 16, 4987–4993. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Perkins, J.R.; Dawes, J.M.; McMahon, S.B.; Bennett, D.L.; Orengo, C.; Kohl, M. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genom. 2012, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Rubin, N.; Staley, C.; Onyeaghala, G.; Wen, Y.F.; Shaukat, A.; Milne, G.; Straka, R.J.; Church, T.R.; Prizment, A. Effect of ginger supplementation on the fecal microbiome in subjects with prior colorectal adenoma. Sci. Rep. 2024, 14, 2988. [Google Scholar] [CrossRef]
- Crichton, M.; Marshall, S.; Marx, W.; Isenring, E.; Vazquez-Campos, X.; Dawson, S.L.; Lohning, A. Effect of Ginger Root Powder on Gastrointestinal Bacteria Composition, Gastrointestinal Symptoms, Mental Health, Fatigue, and Quality of Life: A Double-Blind Placebo-Controlled Trial. J. Nutr. 2023, 153, 3193–3206. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, D.; Jiang, H.Q.; Zhang, S.; Pang, X.G.; Gao, S.J.; Zhang, H.M.; Zhang, S.Y.; Xiao, Q.Y.; Chen, L.Y.; et al. Gut Microbiota Variation With Short-Term Intake of Ginger Juice on Human Health. Front. Microbiol. 2021, 11, 576061. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, H.; Yan, E.; Zhang, X.; Wang, Y.; Huang, C.; He, T.; Miao, L.; Yang, L.; Jiang, R.; et al. Desulfovibrio confers resilience to the comorbidity of pain and anxiety in a mouse model of chronic inflammatory pain. Psychopharmacology 2023, 240, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fan, Y.X.; Zhang, G.L.; Cai, S.; Ma, Y.H.; Yang, L.J.; Wang, Y.M.; Yu, H.T.; Qiao, S.Y.; Zeng, X.F. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Zhang, W.; Zhang, S.; Chen, J.; He, K.; Zhang, C.; Yuan, X.; Xie, B. The gut microbiota and metabolite profiles are altered in patients with spinal cord injury. Mol. Brain 2023, 16, 26. [Google Scholar] [CrossRef]
- De Weirdt, R.; Van de Wiele, T. Micromanagement in the gut: Microenvironmental factors govern colon mucosal biofilm structure and functionality. npj Biofilms Microbiomes 2015, 1, 15026. [Google Scholar] [CrossRef]
- Tikunov, A.Y.; Fedorets, V.A.; Shrainer, E.V.; Morozov, V.V.; Bystrova, V.I.; Tikunova, N.V. Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation. J. Clin. Med. 2023, 12, 7702. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]
- Yang, J.P.; Li, Y.A.; Wen, Z.Q.; Liu, W.Z.; Meng, L.T.; Huang, H. Oscillospira-a candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Dominguez-Balmaseda, D.; Bressa, C.; Fernandez-Romero, A.; de Lucas, B.; Perez-Ruiz, M.; San Juan, A.F.; Roller, M.; Issaly, N.; Larrosa, M. Evaluation of a Zingiber officinale and Bixa orellana Supplement on the Gut Microbiota of Male Athletes: A Randomized Placebo-Controlled Trial. Planta Med. 2022, 88, 1245–1255. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Bai, X.L.; Cao, X.L.; Yue, W.J.; Jiang, W.W.; Yu, Z.H. Changes in intestinal microbiota and correlation with TLRs in ulcerative colitis in the coastal area of northern China. Microb. Pathog. 2021, 150, 104707. [Google Scholar] [CrossRef] [PubMed]
- Nurgaziyev, M.; Issilbayeva, A.; Bersimbaev, R.; Ilderbayev, O.; Vinogradova, E.; Jarmukhanov, Z.; Nurgozhina, A.; Sergazy, S.; Kozhabergen, N.; Akhmetova, Z.; et al. Gut microbiome-immune interactions and their role in rheumatoid arthritis development. PeerJ 2024, 12, e17477. [Google Scholar] [CrossRef]
- Abdugheni, R.; Wang, W.Z.; Wang, Y.J.; Du, M.X.; Liu, F.L.; Zhou, N.; Jiang, C.Y.; Wang, C.Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; Demuyser, T.; Pilitsis, J.G.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Gut dysbiosis in patients with chronic pain: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1342833. [Google Scholar] [CrossRef]
- Kang, J.N.; Sun, Z.F.; Li, X.Y.; Zhang, X.D.; Jin, Z.X.; Zhang, C.; Zhang, Y.; Wang, H.Y.; Huang, N.N.; Jiang, J.H.; et al. Alterations in gut microbiota are related to metabolite profiles in spinal cord injury. Neural Regen. Res. 2023, 18, 1076–1083. [Google Scholar] [CrossRef]
- Cobo, F.; Franco-Acosta, A.; Lara-Oya, A.; Pérez-Carrasco, V. Bacteremia caused by Alistipes finegoldii in a patient with peritonitis. Enfermedades Infecc. Microbiol. Clínica 2023, 41, 131–132. [Google Scholar] [CrossRef]
- Luo, L.M.; Chen, Y.R.; Ma, Q.T.; Huang, Y.; Xu, L.; Shu, K.; Zhang, Z.F.; Liu, Z.Y. Ginger volatile oil inhibits the growth of MDA-MB-231 in the bisphenol A environment by altering gut microbial diversity. Heliyon 2024, 10, e24388. [Google Scholar] [CrossRef]
- Chen, J.; Ding, X.Q.; Wu, R.Y.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.H.; Liu, Y.; Ren, B.R.; Li, J.; et al. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef]
- Caruso, R.; Mathes, T.; Martens, E.C.; Kamada, N.; Nusrat, A.; Inohara, N.; Nunez, G. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci. Immunol. 2019, 4, eaaw4341. [Google Scholar] [CrossRef]
- Kammeyer, A.; Peters, C.P.; Meijer, S.L.; Te Velde, A.A. Anti-inflammatory effects of urocanic Acid derivatives in models ex vivo and in vivo of inflammatory bowel disease. ISRN Inflamm. 2012, 2012, 898153. [Google Scholar] [CrossRef]
- Mravljak, J.; Slavec, L.; Hrast, M.; Sova, M. Synthesis and Evaluation of Antioxidant Properties of 2-Substituted Quinazolin-4(3H)-ones. Molecules 2021, 26, 6585. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.; Singla, R.K.; Shrivastava, B. Current updates on oxazolidinone and its significance. Int. J. Med. Chem. 2012, 2012, 159285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Wang, H.; Zhang, T.; He, M.; Liang, H.; Wang, H.; Xu, L.S.; Chen, S.; Xu, M.H. Inhibition of MicroRNA-195 Alleviates Neuropathic Pain by Targeting Patched1 and Inhibiting SHH Signaling Pathway Activation. Neurochem. Res. 2019, 44, 1690–1702. [Google Scholar] [CrossRef]
- Mapson, L.; Mawson, C. Stability of ascorbic acid in metaphosphoric acid extracts. Nature 1943, 151, 222–223. [Google Scholar] [CrossRef]
- Duan, Y.W.; Chen, S.X.; Li, Q.Y.; Zang, Y. Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-alpha-Necroptosis Pathway. Int. J. Mol. Sci. 2022, 23, 7191. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.C.; Liu, S.Y.; Zheng, H.P.; Ji, L.J.; Yi, N.; Zhu, X.M.; Sun, W.W.; Liu, X.X.; Zhang, S.; et al. Decreased amino acids in the brain might contribute to the progression of diabetic neuropathic pain. Diabetes Res. Clin. Pract. 2021, 176, 108790. [Google Scholar] [CrossRef]
- Li, J.J.; Xu, L.J.; Deng, X.T.; Jiang, C.Y.; Pan, C.L.; Chen, L.; Han, Y.; Dai, W.L.; Hu, L.; Zhang, G.Q.; et al. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 2016, 157, 1711–1723. [Google Scholar] [CrossRef]
- Birmann, P.T.; Sousa, F.S.S.; Domingues, M.; Bruning, C.A.; Vieira, B.M.; Lenardao, E.J.; Savegnago, L. 3-(4-Chlorophenylselanyl)-1-methyl-1H-indole promotes recovery of neuropathic pain and depressive-like behavior induced by partial constriction of the sciatic nerve in mice. J. Trace Elem. Med. Biol. 2019, 54, 126–133. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, A.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Dong, F.; Hao, F.; Murray, I.A.; Smith, P.B.; Koo, I.; Tindall, A.M.; Kris-Etherton, P.M.; Gowda, K.; Amin, S.G.; Patterson, A.D.; et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 2020, 12, 1788899. [Google Scholar] [CrossRef]
- Rémond, D.; Buffière, C.; Godin, J.P.; Mirand, P.P.; Obled, C.; Papet, I.; Dardevet, D.; Williamson, G.; Breuillé, D.; Faure, M. Intestinal Inflammation Increases Gastrointestinal Threonine Uptake and Mucin Synthesis in Enterally Fed Minipigs. J. Nutr. 2009, 139, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Tan, P.; Ma, N.; Ma, X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients 2021, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Doolittle, T.H.; Lopezbresnahan, M.; Shahani, B.; Schoenfeld, D.; Shih, V.E.; Growdon, J.; Lehrich, J.R. An Antispasticity Effect of Threonine in Multiple-Sclerosis. Arch. Neurol. 1992, 49, 923–926. [Google Scholar] [CrossRef]
- Ye, X.W.; Li, H.Y.; Anjum, K.; Zhong, X.Y.; Miao, S.P.; Zheng, G.W.; Liu, W.; Li, L.J. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D'Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Z.; Zhao, D.Y.; Zhou, Y.C.; Wang, Q.Q.; Qin, G.; Yao, S.K. Alteration of fecal tryptophan metabolism correlates with shifted microbiota and may be involved in pathogenesis of colorectal cancer. World J. Gastroenterol. 2020, 26, 7173–7190. [Google Scholar] [CrossRef]
- Takahashi, K.; Mizukami, H.; Osonoi, S.; Ogasawara, S.; Hara, Y.; Kudoh, K.; Takeuchi, Y.; Sasaki, T.; Daimon, M.; Yagihashi, S. Inhibitory effects of xanthine oxidase inhibitor, topiroxostat, on development of neuropathy in db/db mice. Neurobiol. Dis. 2021, 155, 105392. [Google Scholar] [CrossRef]
- Cheng, S.; Yu, J.; Cui, M.L.; Su, H.M.; Cao, Y. Changes in the composition of the fecal metabolome and gut microbiota contribute to intervertebral disk degeneration in a rabbit model. J. Orthop. Surg. Res. 2024, 19, 6. [Google Scholar] [CrossRef]
- Novotná, K.; Tenora, L.; Slusher, B.; Rais, R. Therapeutic resurgence of 6-diazo-5-oxo-l-norleucine (DON) through tissue-targeted prodrugs. Adv. Pharmacol. 2024, 100, 157–180. [Google Scholar]
- Lemberg, K.M.; Vornov, J.J.; Rais, R.; Slusher, B.S. We’re Not “DON” Yet: Optimal Dosing and Prodrug Delivery of 6-Diazo-5-oxo-L-norleucine. Mol. Cancer Ther. 2018, 17, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Kiritoshi, T.; Yakhnitsa, V.; Singh, S.; Wilson, T.D.; Chaudhry, S.; Neugebauer, B.; Torres-Rodriguez, J.M.; Lin, J.L.; Carrasquillo, Y.; Neugebauer, V. Cells and circuits for amygdala neuroplasticity in the transition to chronic pain. Cell Rep. 2024, 43, 114669. [Google Scholar] [CrossRef]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Quindlen-Hotek, J.C.; Kartha, S.; Winkelstein, B.A. Immediate inhibition of spinal secretory phospholipase A prevents the pain and elevated spinal neuronal hyperexcitability and neuroimmune regulatory genes that develop with nerve root compression. Neuroreport 2020, 31, 1084–1089. [Google Scholar] [CrossRef]
- Kartha, S.; Ghimire, P.; Winkelstein, B.A. Inhibiting spinal secretory phospholipase A(2) after painful nerve root injury attenuates established pain and spinal neuronal hyperexcitability by altering spinal glutamatergic signaling. Mol. Pain 2021, 17, 17448069211066221. [Google Scholar] [CrossRef]
- Pan, B.; Jing, L.; Cao, M.H.; Hu, Y.Z.; Gao, X.; Bu, X.B.; Li, Z.; Feng, H.; Guo, K.J. Melatonin promotes Schwann cell proliferation and migration via the shh signalling pathway after peripheral nerve injury. Eur. J. Neurosci. 2021, 53, 720–731. [Google Scholar] [CrossRef]
- Moreau, N.; Boucher, Y. Hedging against Neuropathic Pain: Role of Hedgehog Signaling in Pathological Nerve Healing. Int. J. Mol. Sci. 2020, 21, 9115. [Google Scholar] [CrossRef]
- Viñuela-Berni, V.; Gómez-González, B.; Quintanar-Stephano, A. Blockade of Arginine Vasopressin receptors prevents blood-brain barrier breakdown in Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2020, 10, 467. [Google Scholar] [CrossRef]
- Calvillo-Robledo, A.; Ramirez-Farias, C.; Valdez-Urias, F.; Huerta-Carreon, E.P.; Quintanar-Stephano, A. Arginine vasopressin hormone receptor antagonists in experimental autoimmune encephalomyelitis rodent models: A new approach for human multiple sclerosis treatment. Front. Neurosci. 2023, 17, 1138627. [Google Scholar] [CrossRef]
- Cragg, B.; Ji, G.; Neugebauer, V. Differential contributions of vasopressin V1A and oxytocin receptors in the amygdala to pain-related behaviors in rats. Mol. Pain 2016, 12, 1744806916676491. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.X.; Yue, J.R.; Hu, X.L.; Liu, Y.X.; Chen, S.; Lin, X.F.; Zhang, G.C.; Xiao, H.Y.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Druart, M.; Nosten-Bertrand, M.; Poll, S.; Crux, S.; Nebeling, F.; Delhaye, C.; Dubois, Y.; Mittag, M.; Leboyer, M.; Tamouza, R.; et al. Elevated expression of complement C4 in the mouse prefrontal cortex causes schizophrenia-associated phenotypes. Mol. Psychiatry 2021, 26, 3489–3501. [Google Scholar] [CrossRef]
- Vicente-Rodríguez, M.; Mancuso, R.; Peris-Yague, A.; Simmons, C.; Consortium, N.; Gómez-Nicola, D.; Perry, V.H.; Turkheimer, F.; Lovestone, S.; Parker, C.A.; et al. Pharmacological modulation of TSPO in microglia/macrophages and neurons in a chronic neurodegenerative model of prion disease. J. Neuroinflamm. 2023, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.E.; Lucas, C.D.; Marwick, J.A.; Duffin, R.; Haslett, C.; Rossi, A.G. Cyclin-dependent kinases 7 and 9 specifically regulate neutrophil transcription and their inhibition drives apoptosis to promote resolution of inflammation. Cell Death Differ. 2012, 19, 1950–1961. [Google Scholar] [CrossRef]
- Rigter, P.M.F.; de Konink, C.; Dunn, M.J.; Onori, M.P.; Humberson, J.B.; Thomas, M.; Barnes, C.; Prada, C.E.; Weaver, K.N.; Ryan, T.D.; et al. Role of CAMK2D in neurodevelopment and associated conditions. Am. J. Hum. Genet. 2024, 111, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Wong, L.M.; Ellis, K.; Bodnar, B.; Saribas, S.; Ting, J.; Wei, Z.Y.; Tang, Y.Y.; Wang, X.W.; Wang, H.; et al. Microglia-Specific Promoter Activities of HEXB Gene. Front. Cell. Neurosci. 2022, 16, 808598. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sasanuma, Y.; Shitara, S.; Koshizuka, A.; Okada, R.; Sakuraba, H.; Oishi, K. Abnormal organization during neurodevelopment in a mouse model of Sandhoff disease. Neurosci. Res. 2020, 155, 12–19. [Google Scholar] [CrossRef]
- Rhinn, H.; Tatton, N.; McCaughey, S.; Kurnellas, M.; Rosenthal, A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol. Sci. 2022, 43, 641–652. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Yu, H.M.; Tian, Y.; Chen, X.Y.; Chen, C.; Ren, Y.K.; Chen, Z.; Ren, Y.; Gong, X.; et al. Protective effect of Nr4a2 (Nurr1) against LPS-induced depressive-like behaviors via regulating activity of microglia and CamkII neurons in anterior cingulate cortex. Pharmacol. Res. 2023, 191, 106717. [Google Scholar] [CrossRef]
- Vallés, A.S.; Barrantes, F.J. Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment. Biomolecules 2021, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Wu, S.; Su, C. Neuronal compartmentalization: A means to integrate sensory input at the earliest stage of information processing? BioEssays 2020, 42, 2000026. [Google Scholar] [CrossRef]
- Yokota, Y.; Kim, W.Y.; Chen, Y.J.; Wang, X.S.; Stanco, A.; Komuro, Y.; Snider, W.; Anton, E.S. The Adenomatous Polyposis Coli Protein Is an Essential Regulator of Radial Glial Polarity and Construction of the Cerebral Cortex. Neuron 2009, 61, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, G.; Kästner, B.; Weth, F.; Bolz, J. Multiple effects of ephrin-A5 on cortical neurons are mediated by Src family kinases. J. Neurosci. 2007, 27, 5643–5653. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, J.P.; Schnekenberg, R.P.; McGowan, S.; Sims, D.; McEntagart, M.; Elmslie, F.; Shears, D.; Stewart, H.; Tofaris, G.K.; Dabir, T.; et al. Detailed Analysis of Missense Variants Guides Diagnostics and Therapeutic Design. Mov. Disord. 2024, 39, 141–151. [Google Scholar] [CrossRef]
- Herrera, F.J.; Yamaguchi, T.; Roelink, H.; Tjian, R. Core promoter factor TAF9B regulates neuronal gene expression. Elife 2014, 3, e02559. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Qiao, H.; Yang, L.; Zhang, P.; Wang, J.; Zhang, C.; Jiang, W.; Xu, R.; Zhang, X. Proprotein convertase subtilisin/kexin type 9 inhibitor ameliorates cerebral ischemia in mice by inhibiting inflammation. J. Stroke Cerebrovasc. Dis. 2024, 33, 107517. [Google Scholar] [CrossRef]
- Li, L.; Venkataraman, L.; Chen, S.; Fu, H. Function of WFS1 and WFS2 in the Central Nervous System: Implications for Wolfram Syndrome and Alzheimer’s disease. Neurosci. Biobehav. Rev. 2020, 118, 775–783. [Google Scholar] [CrossRef]
- Takei, D.; Ishihara, H.; Yamaguchi, S.; Yamada, T.; Tamura, A.; Katagiri, H.; Maruyama, Y.; Oka, Y. WFS1 protein modulates the free Ca2+ concentration in the endoplasmic reticulum. FEBS Lett. 2006, 580, 5635–5640. [Google Scholar] [CrossRef]
- Hao, H.; Song, L.; Zhang, L. Wolfram syndrome 1 regulates sleep in dopamine receptor neurons by modulating calcium homeostasis. PLoS Genet. 2023, 19, e1010827. [Google Scholar] [CrossRef] [PubMed]
- Caruso, V.; Rigoli, L. Beyond Wolfram Syndrome 1: The WFS1 Gene's Role in Alzheimer's Disease and Sleep Disorders. Biomolecules 2024, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Samara, A.; Rahn, R.; Neyman, O.; Park, K.Y.; Samara, A.; Marshall, B.; Dougherty, J.; Hershey, T. Developmental hypomyelination in Wolfram syndrome: New insights from neuroimaging and gene expression analyses. Orphanet J. Rare Dis. 2019, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Lutfy, K.; Parikh, D.; Lee, D.L.; Liu, Y.; Ferrini, M.G.; Hamid, A.; Friedman, T.C. Prohormone Convertase 2 (Pc2) Null Mice Have Increased Mu Opioid Receptor Levels Accompanied by Altered Morphine-Induced Antinociception, Tolerance and Dependence. Neuroscience 2016, 329, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Yap, E.L.; Greenberg, M.E. Activity-Regulated Transcription: Bridging the Gap between Neural Activity and Behavior. Neuron 2018, 100, 330–348. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| APC | 5′-AGC AAG TTG AGG CCC TGA AGA-3′ | 5′-ATA CTT CCC TGC AGC TGC TTA-3′ |
| CCNH | 5′-AGC CAC CCA GAT CTG AAG AAG TT-3′ | 5′-GTC ATC GTC CGT CCA CTC CT-3′ |
| EFNA5 | 5′-GGA CCG CTG AAG TTC TCG GA-3′ | 5′-CGA AAA CAC GAT CAC GAA CAC CT-3′ |
| GRN | 5′-CTG GAG CTG ACC GCC AGA TG-3′ | 5′-GTC AAG GCA GCA GGC AAC AG-3′ |
| ITPR1 | 5′-TTG GAA AAT GCC GAG CTG CC-3′ | 5′-GGG GTG GAC TTG GTT CAA GC-3′ |
| PCSK2 | 5′-ACG TTC AGC AAC GGG AGG AA-3′ | 5′-AAG CCA ATG CAA ACA CGC CA-3′ |
| TAF9 | 5′-TTC CGA CA TCC TGC TCA CCG-3′ | 5′-CATBCTG TGC TC TTT CGG CA-3′ |
| WFS1 | TGC TGG AGC GTC TAG TGA GC-3′ | 5′-CTT CTG GCG TAG TGG CAG GT-3′ |
| β-actin | 5′-ACA ACC TTC TTG CAG CTC CTC C-3′ | 5′-TGA CCC ATA CCC ACC ATC ACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-L.; Santos, J.M.; Elmassry, M.M.; Chen, F.; Ji, G.; Presto, P.; Kiritoshi, T.; Liu, X.; Neugebauer, V. Crosstalk Among Gut Microbiota, Fecal Metabolites, and Amygdala Neuropathology Genes After Ginger Polyphenol Administration in Female Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Connection. Nutrients 2025, 17, 1444. https://doi.org/10.3390/nu17091444
Shen C-L, Santos JM, Elmassry MM, Chen F, Ji G, Presto P, Kiritoshi T, Liu X, Neugebauer V. Crosstalk Among Gut Microbiota, Fecal Metabolites, and Amygdala Neuropathology Genes After Ginger Polyphenol Administration in Female Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Connection. Nutrients. 2025; 17(9):1444. https://doi.org/10.3390/nu17091444
Chicago/Turabian StyleShen, Chwan-Li, Julianna Maria Santos, Moamen M. Elmassry, Fang Chen, Guangchen Ji, Peyton Presto, Takaki Kiritoshi, Xiaobo Liu, and Volker Neugebauer. 2025. "Crosstalk Among Gut Microbiota, Fecal Metabolites, and Amygdala Neuropathology Genes After Ginger Polyphenol Administration in Female Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Connection" Nutrients 17, no. 9: 1444. https://doi.org/10.3390/nu17091444
APA StyleShen, C.-L., Santos, J. M., Elmassry, M. M., Chen, F., Ji, G., Presto, P., Kiritoshi, T., Liu, X., & Neugebauer, V. (2025). Crosstalk Among Gut Microbiota, Fecal Metabolites, and Amygdala Neuropathology Genes After Ginger Polyphenol Administration in Female Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Connection. Nutrients, 17(9), 1444. https://doi.org/10.3390/nu17091444







