Associations Between B Vitamin Interactions with Polyunsaturated Fatty Acids and Cognitive Function Among Cognitively Healthy Older People as Modified by Amyloid Status and Sex
Abstract
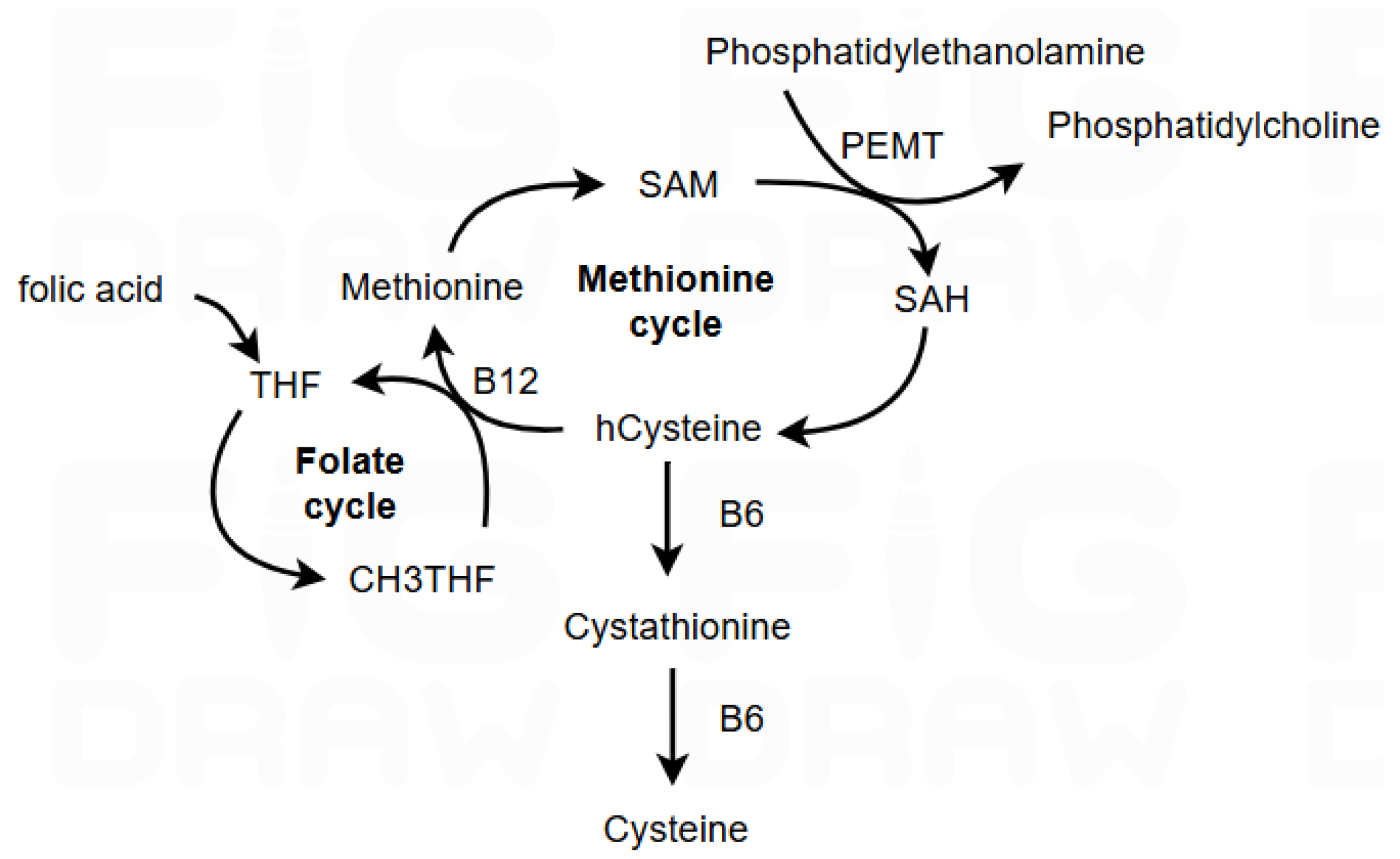
1. Introduction
2. Materials and Methods
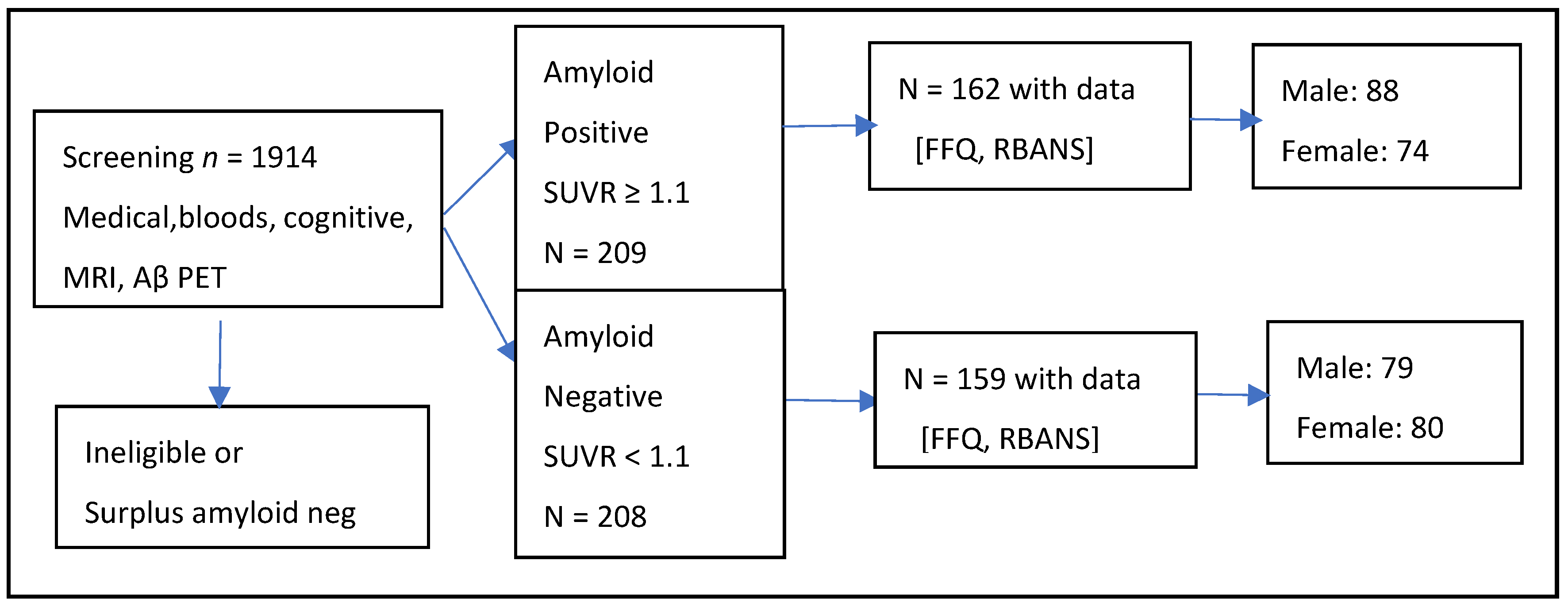
2.1. Study Population
2.2. Amyloid-Beta Measurement
2.3. Nutritional Assessment
2.4. Cognitive Tests
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
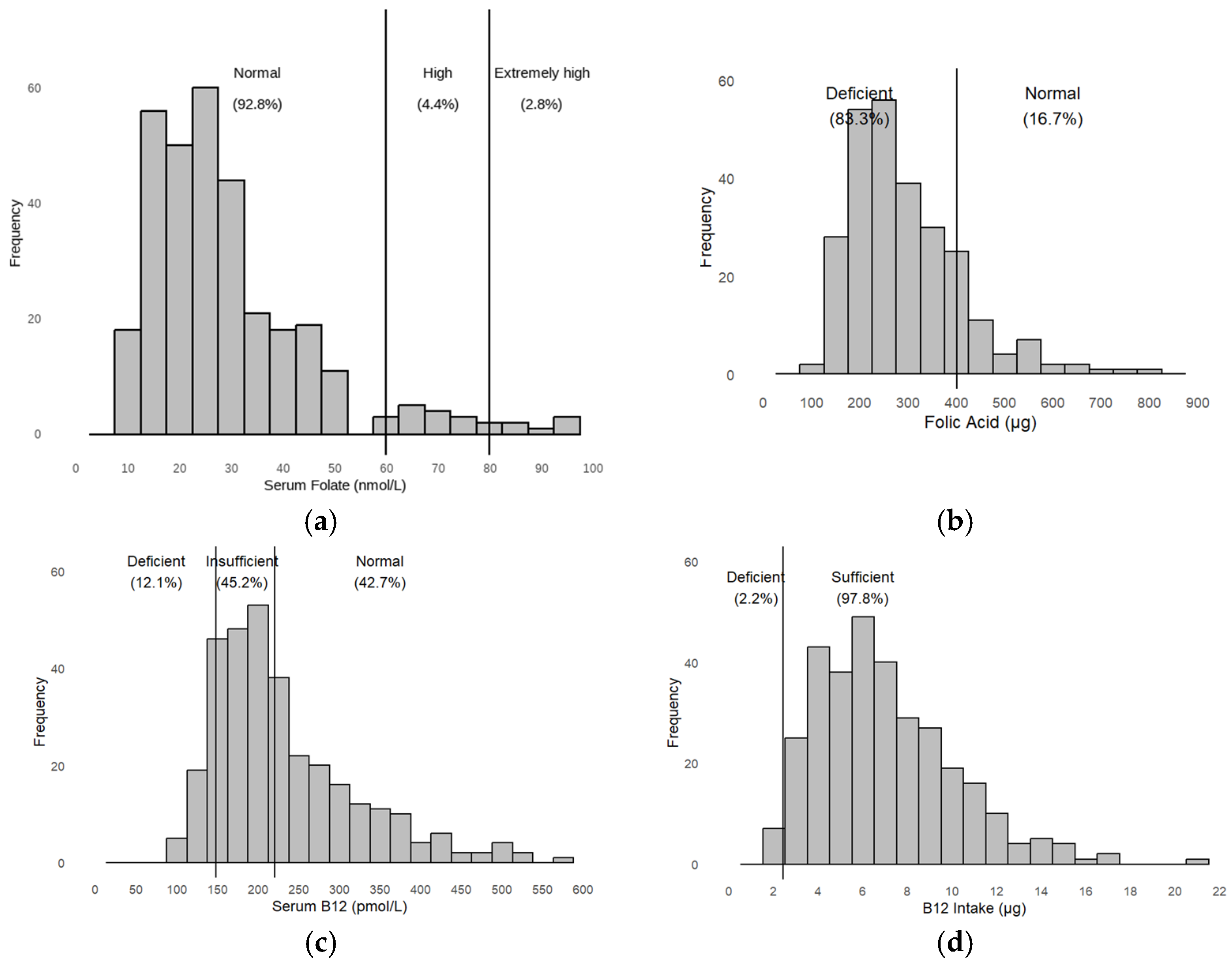
3.1. Descriptive Results
3.2. Multivariate Model Results
- For all participants
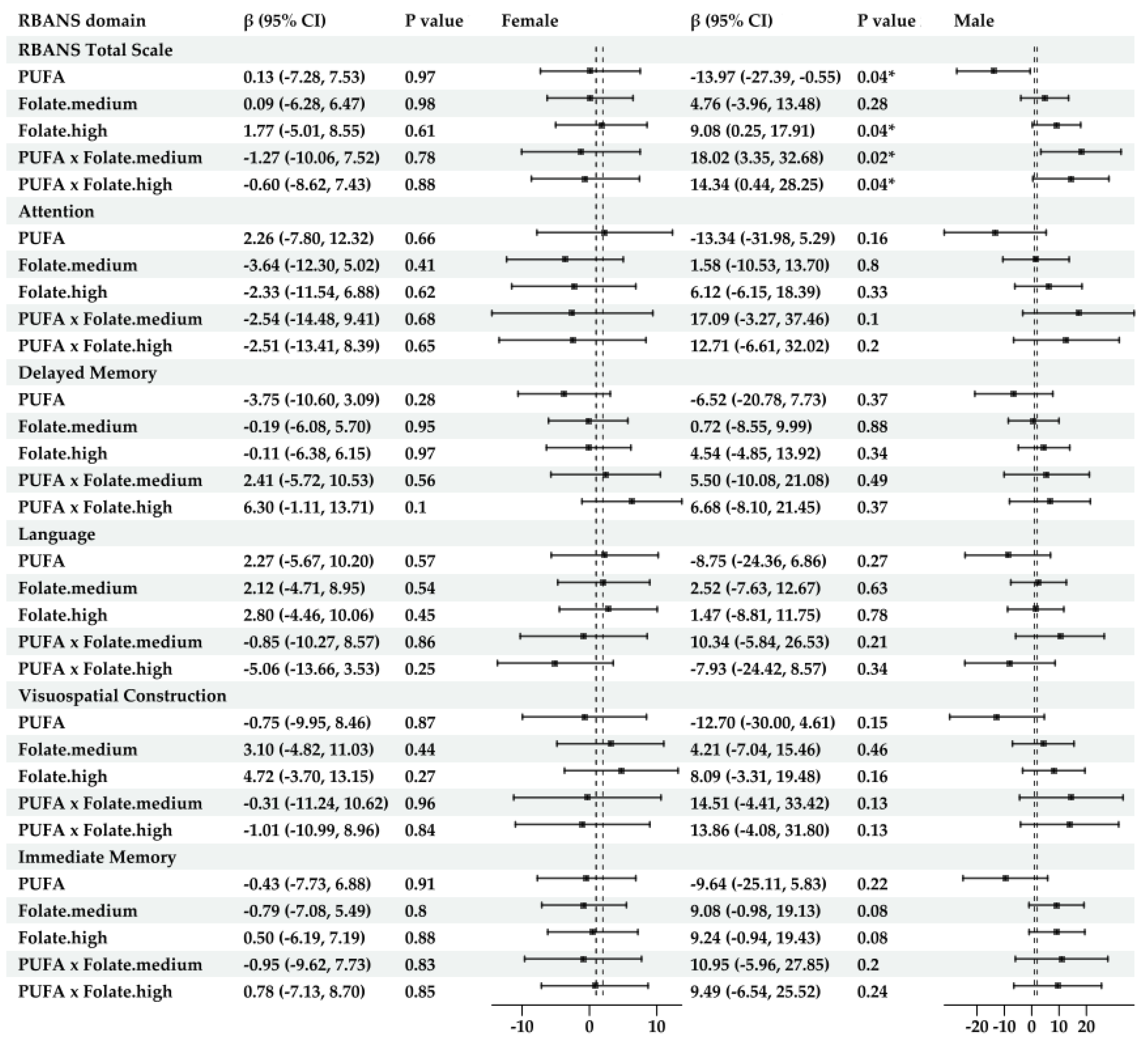
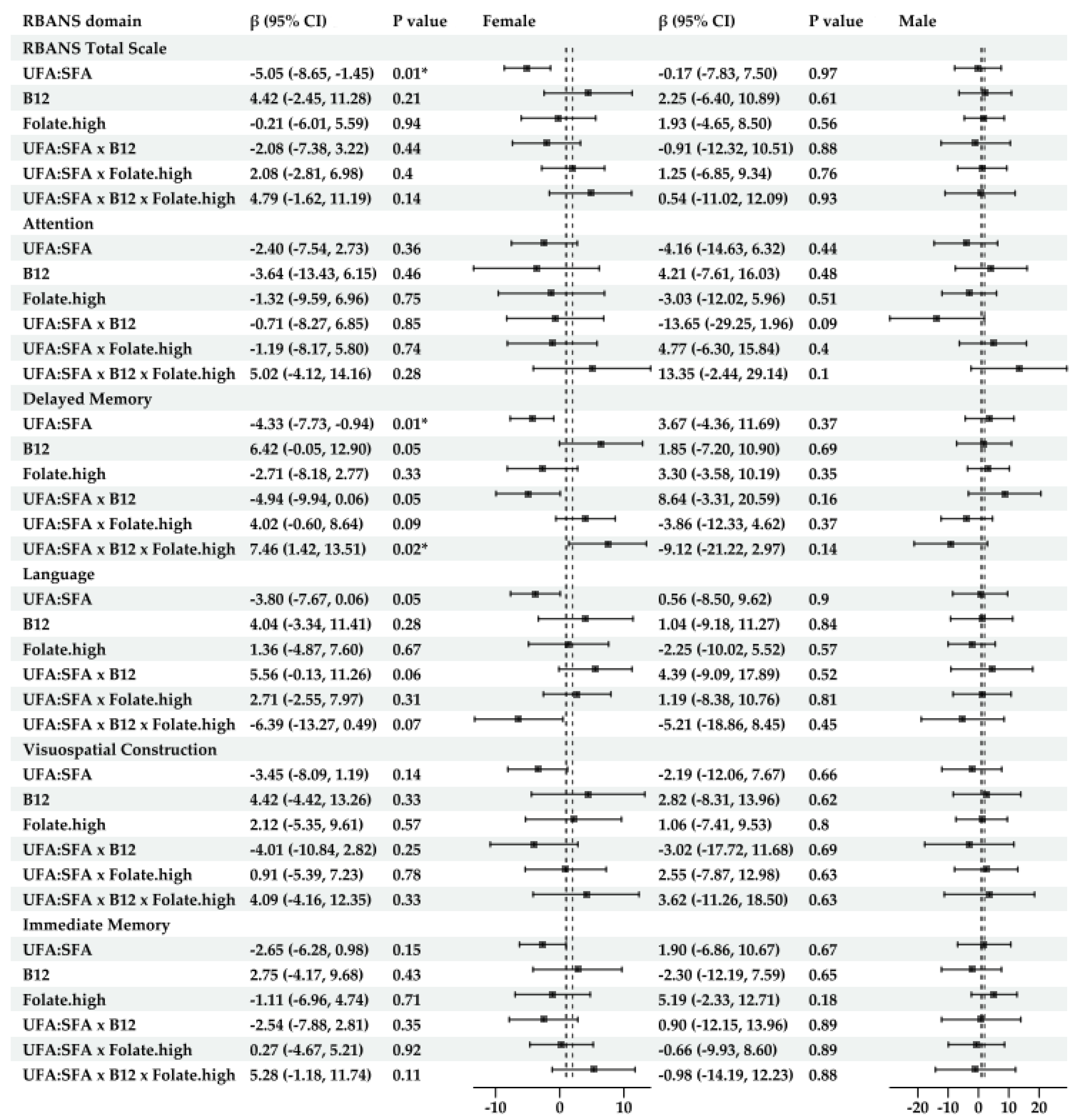
- For sex-specific models (Figure 4)
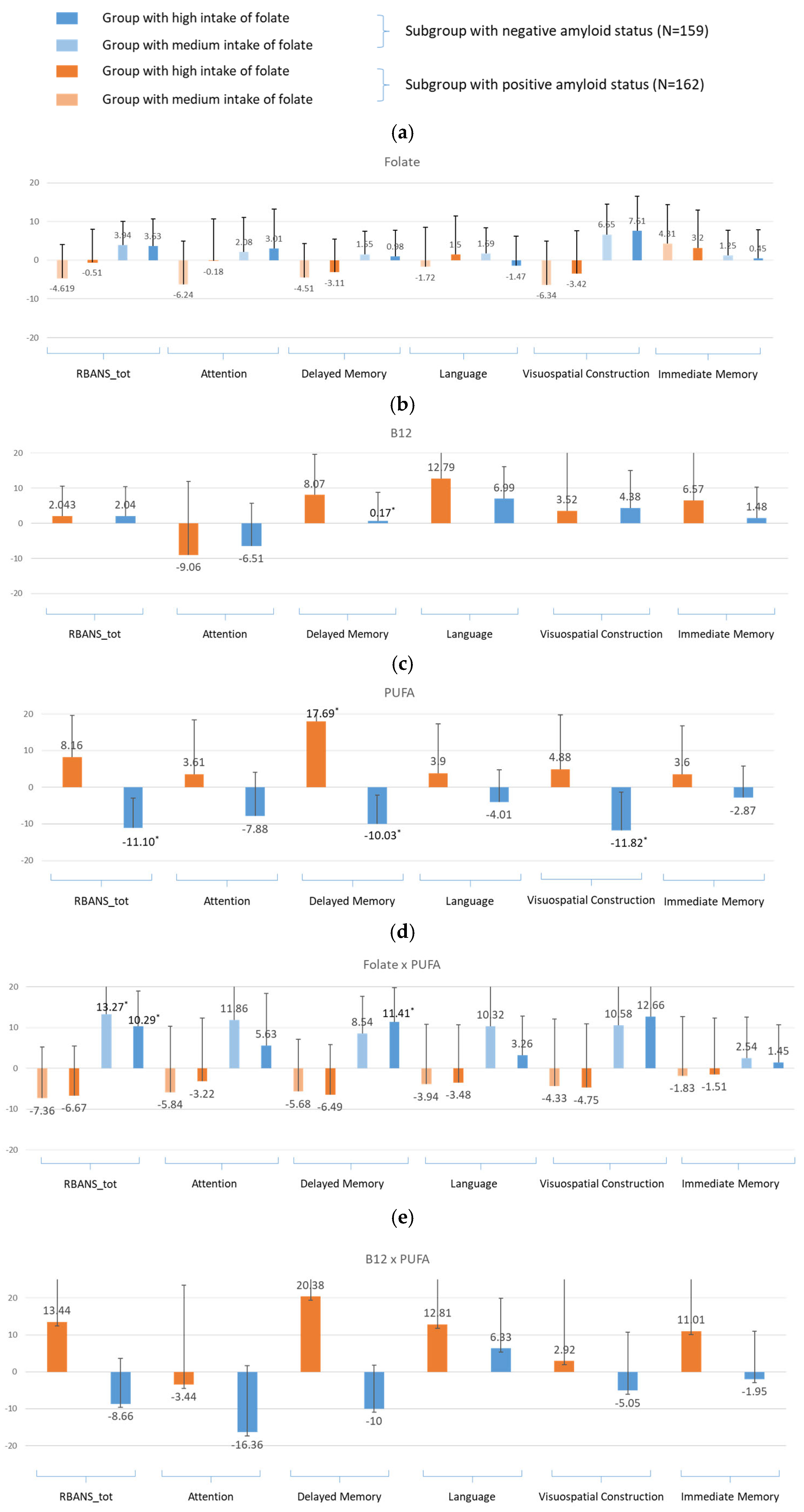
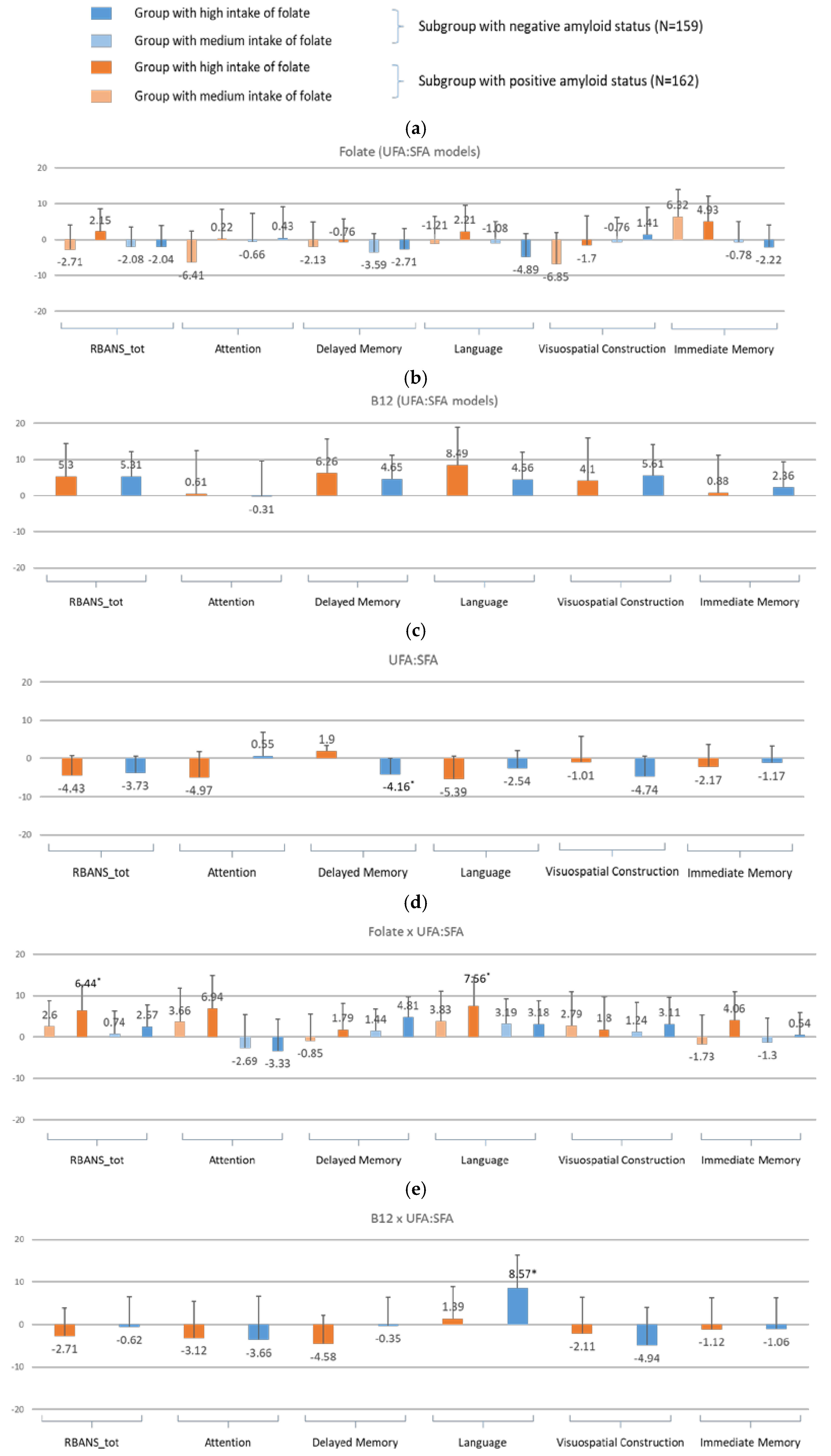
- For amyloid-specific models (Figure 5)
- For all participants
- For sex-specific models (Figure 6)
- For amyloid-specific models (Figure 7)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFA | Polyunsaturated fatty acid |
| SCG FFQ | Scottish Collaborative Group—Food Frequency Questionnaire |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| SFA | Saturated fatty acid |
| UFA | Unsaturated fatty acid |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| CVD | Cardiovascular disease |
| EM | Endocrine and metabolic disease |
| APOE | Apolipoprotein E |
| PET | Positron emission tomography |
| CSF | Cerebrospinal fluid |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| AD | Alzheimer’s disease |
| Hcy | Homocysteine |
| MCI | Mild cognitive impairment |
| n-3 FA | Omega-3 fatty acid |
| PE | Phosphatidylethanolamine |
| PC | Phosphatidylcholine |
| PEMT | Phosphatidylethalonamine N-methyltransferase |
| Aβ | Amyloid beta |
References
- World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019.
- Wittenberg, R.; Hu, B.; Jagger, C.; Kingston, A.; Knapp, M.; Comas-Herrera, A.; King, D.; Rehill, A.; Banerjee, S. Projections of care for older people with dementia in England: 2015 to 2040. Age Ageing 2020, 49, 264–269. [Google Scholar] [CrossRef]
- Halsall, B.; Riley, M.; Hogervorst, E. Design for Dementia: Living Well at Home; Taylor & Francis: Oxford, UK, 2023. [Google Scholar]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef]
- Yu, J.-T.; Xu, W.; Tan, C.-C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Epelbaum, S.; Genthon, R.; Cavedo, E.; Habert, M.O.; Lamari, F.; Gagliardi, G.; Lista, S.; Teichmann, M.; Bakardjian, H.; Hampel, H. Preclinical Alzheimer’s disease: A systematic review of the cohorts underlying the concept. Alzheimer’s Dement. 2017, 13, 454–467. [Google Scholar] [CrossRef]
- van Soest, A.P.; de Groot, L.C.; Witkamp, R.F.; van Lent, D.M.; Seshadri, S.; van de Rest, O. Concurrent nutrient deficiencies are associated with dementia incidence. Alzheimer’s Dement. 2024, 20, 4594–4601. [Google Scholar] [CrossRef]
- Nalder, L.; Zheng, B.; Chiandet, G.; Middleton, L.; De Jager, C.A. Vitamin B12 and folate status in cognitively healthy older adults and associations with cognitive performance. J. Nutr. Health Aging 2021, 25, 287–294. [Google Scholar] [CrossRef]
- van Soest, A.P.; Beers, S.; van de Rest, O.; de Groot, L.C. The MIND diet for the ageing brain: A systematic review. Adv. Nutr. 2024, 15, 100184. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; He, X.; Fang, J.; Zhou, L.; Qi, Z.; Tao, M.; Yuan, H.; Zhou, Y. Synergistically effects of n-3 PUFA and B vitamins prevent diabetic cognitive dysfunction through promoting TET2-mediated active DNA demethylation. Clin. Nutr. 2025, 45, 111–123. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, X.; Li, Q.; Zhao, J.; Song, A.; An, P.; Du, Y.; Xu, W.; Huang, G. Effects of folic acid and vitamin B12, alone and in combination on cognitive function and inflammatory factors in the elderly with mild cognitive impairment: A single-blind experimental design. Curr. Alzheimer Res. 2019, 16, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Olaso-Gonzalez, G.; Inzitari, M.; Bellelli, G.; Morandi, A.; Barcons, N.; Viña, J. Impact of supplementation with vitamins B6, B12, and/or folic acid on the reduction of homocysteine levels in patients with mild cognitive impairment: A systematic review. IUBMB Life 2022, 74, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; Van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, M.; Beck, W.; Colonetti, T.; Budni, J.; Falchetti, A.; Colonetti, L.; Coral, A.; Meller, F. Association of vitamin D and vitamin B12 with cognitive impairment in elderly aged 80 years or older: A cross-sectional study. J. Hum. Nutr. Diet. 2019, 32, 518–524. [Google Scholar] [CrossRef]
- Sawaengsri, H.; Bergethon, P.R.; Qiu, W.Q.; Scott, T.M.; Jacques, P.F.; Selhub, J.; Paul, L. Transcobalamin 776C→G polymorphism is associated with peripheral neuropathy in elderly individuals with high folate intake. Am. J. Clin. Nutr. 2016, 104, 1665–1670. [Google Scholar] [CrossRef]
- Carney, E.; Canada, T. Dietary Folate and Vitamin B12 Intake and Cognitive Decline Among Community-Dwelling Older Persons. Nutr. Clin. Pract. 2006, 21, 188–189. [Google Scholar] [CrossRef] [PubMed]
- van Soest, A.P.; Van de Rest, O.; Witkamp, R.F.; De Groot, L.C. Positive effects of folic acid supplementation on cognitive aging are dependent on ω-3 fatty acid status: A post hoc analysis of the FACIT trial. Am. J. Clin. Nutr. 2021, 113, 801–809. [Google Scholar] [CrossRef]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; De Jager, C.A. Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef]
- Van Soest, A.P.; Van De Rest, O.; Witkamp, R.F.; Cederholm, T.; De Groot, L.C. DHA status influences effects of B-vitamin supplementation on cognitive ageing: A post-hoc analysis of the B-proof trial. Eur. J. Nutr. 2022, 61, 3731–3739. [Google Scholar] [CrossRef]
- Jernerén, F.; Elshorbagy, A.K.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith, A.D. Brain atrophy in cognitively impaired elderly: The importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Y.; Men, J.; Fu, H.; Xu, T. The preventive efficacy of vitamin B supplements on the cognitive decline of elderly adults: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 367. [Google Scholar] [CrossRef]
- Smith, A.D.; Jernerén, F.; Refsum, H. ω-3 fatty acids and their interactions. Am. J. Clin. Nutr. 2021, 113, 775–778. [Google Scholar] [CrossRef]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R., Jr.; Vergallo, A. Developing the ATX (N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Chételat, G.; Villemagne, V.L.; Bourgeat, P.; Pike, K.E.; Jones, G.; Ames, D.; Ellis, K.A.; Szoeke, C.; Martins, R.N.; O’Keefe, G.J. Relationship between atrophy and β-amyloid deposition in Alzheimer disease. Ann. Neurol. 2010, 67, 317–324. [Google Scholar] [CrossRef]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: A systematic review. Int. J. Neurosci. 2017, 127, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Goodwill, A.M.; Gorelik, A.; Szoeke, C. Diet and biomarkers of Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol. Aging 2019, 76, 45–52. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35, S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Refsum, H.; Johnston, C.; Smith, S.; Bradley, K.; De Jager, C.; Budge, M.; Smith, A. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008, 71, 826–832. [Google Scholar] [CrossRef]
- Oikonomidi, A.; Lewczuk, P.; Kornhuber, J.; Smulders, Y.; Linnebank, M.; Semmler, A.; Popp, J. Homocysteine metabolism is associated with cerebrospinal fluid levels of soluble amyloid precursor protein and amyloid beta. J. Neurochem. 2016, 139, 324–332. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Zhao, S.; Zhang, X.; Zhang, M.; Xiao, Y.; Wilson, J.X.; Huang, G. Folic acid attenuates the effects of amyloid β oligomers on DNA methylation in neuronal cells. Eur. J. Nutr. 2016, 55, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.P.M.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef]
- Udeh-Momoh, C.T.; Watermeyer, T.; Price, G.; de Jager Loots, C.A.; Reglinska-Matveyev, N.; Ropacki, M.; Ketter, N.; Fogle, M.; Raghavan, N.; Arrighi, M. Protocol of the cognitive health in ageing register: Investigational, observational and trial studies in dementia research (CHARIOT): Prospective readiness cohort (PRO) substudy. BMJ Open 2021, 11, e043114. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Wiste, H.J.; Therneau, T.M.; Weigand, S.D.; Knopman, D.S.; Mielke, M.M.; Lowe, V.J.; Vemuri, P.; Machulda, M.M.; Schwarz, C.G. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 2019, 321, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.L.; Craig, L.C.; Whybrow, S.; Clark, H.; Kyle, J.A.; McNeill, G. Assessing the relative validity of the Scottish Collaborative Group FFQ for measuring dietary intake in adults. Public Health Nutr. 2017, 20, 449–455. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef]
- Buuren, S.v.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Watson, J.; Lee, M.; Garcia-Casal, M.N. Consequences of inadequate intakes of vitamin a, vitamin B 12, vitamin D, calcium, iron, and folate in older persons. Curr. Geriatr. Rep. 2018, 7, 103–113. [Google Scholar] [CrossRef]
- Slimani, N.; Ferrari, P.; Stripp, C.; Engeset, D.; Charrondiere, R.; Buzzard, M.; Van Staveren, W.; Riboli, E.; Ocke, M.; Welch, A.; et al. Standardization of the 24-hour diet recall calibration method used in the European Prospective Investigation into Cancer and Nutrition (EPIC): General concepts and preliminary results. Eur. J. Clin. Nutr. 2000, 54, 900–917. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Oulhaj, A.; Konstantinova, S.; Nurk, E.; Ueland, P.M.; Tell, G.S.; Nygård, O.; Vollset, S.E.; Refsum, H. Plasma creatinine as a determinant of plasma total homocysteine concentrations in the Hordaland Homocysteine Study: Use of statistical modeling to determine reference limits. Clin. Biochem. 2007, 40, 1209–1218. [Google Scholar] [CrossRef]
- Dong, X.; Li, S.; Chen, J.; Li, Y.; Wu, Y.; Zhang, D. Association of dietary ω-3 and ω-6 fatty acids intake with cognitive performance in older adults: National Health and nutrition examination Survey (NHANES) 2011–2014. Nutr. J. 2020, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Liu, M.; Liu, C.; Zhou, S.; Jiao, Y.; Sun, H.; Ji, Y. Effects of vitamins and polyunsaturated fatty acids on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2024, 63, 1003–1022. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M.; Investigators, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and folate status in relation to decline in scores on the Mini-Mental State Examination in the Framingham Heart Study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Li, L.; Cao, D.; Desmond, R.; Rahman, A.; Lah, J.J.; Levey, A.I.; Zamrini, E. Cognitive performance and plasma levels of homocysteine, vitamin B12, folate and lipids in patients with Alzheimer disease. Dement. Geriatr. Cogn. Disord. 2008, 26, 384–390. [Google Scholar] [CrossRef]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014, 72, 22–36. [Google Scholar] [CrossRef]
- Hussain, S.M.; Robb, C.; Tonkin, A.M.; Lacaze, P.; Chong, T.T.-J.; Beilin, L.J.; Yu, C.; Watts, G.F.; Ryan, J.; Ernst, M.E. Association of plasma high-density lipoprotein cholesterol level with risk of incident dementia: A cohort study of healthy older adults. Lancet Reg. Health-West. Pac. 2024, 43, 100963. [Google Scholar] [CrossRef]
- Kechko, O.I.; Petrushanko, I.Y.; Brower, C.S.; Adzhubei, A.A.; Moskalev, A.A.; Piatkov, K.I.; Mitkevich, V.A.; Makarov, A.A. Beta-amyloid induces apoptosis of neuronal cells by inhibition of the Arg/N-end rule pathway proteolytic activity. Aging 2019, 11, 6134. [Google Scholar] [CrossRef]
- Misiti, F.; Clementi, M.E.; Giardina, B. Oxidation of methionine 35 reduces toxicity of the amyloid beta-peptide (1–42) in neuroblastoma cells (IMR-32) via enzyme methionine sulfoxide reductase A expression and function. Neurochem. Int. 2010, 56, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Matura, S.; Prvulovic, D.; Mohadjer, N.; Fusser, F.; Oertel, V.; Reif, A.; Pantel, J.; Karakaya, T. Association of dietary fat composition with cognitive performance and brain morphology in cognitively healthy individuals. Acta Neuropsychiatr. 2021, 33, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.Z.; Harty, B.; Mukamal, K.J.; Stoddard, A.M.; Vitolins, M.; Dunn, J.E. Monounsaturated, trans, and saturated fatty acids and cognitive decline in women. J. Am. Geriatr. Soc. 2011, 59, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Rushworth, J.V.; Hooper, N.M. Lipid Rafts: Linking Alzheimer’s Amyloid-β Production, Aggregation, and Toxicity at Neuronal Membranes. Int. J. Alzheimer’s Dis. 2011, 2011, 603052. [Google Scholar] [CrossRef]
- Creighton, S.D.; Stefanelli, G.; Reda, A.; Zovkic, I.B. Epigenetic mechanisms of learning and memory: Implications for aging. Int. J. Mol. Sci. 2020, 21, 6918. [Google Scholar] [CrossRef]
- Lowndes, G.; Savage, G. Early detection of memory impairment in Alzheimer’s disease: A neurocognitive perspective on assessment. Neuropsychol. Rev. 2007, 17, 193–202. [Google Scholar] [CrossRef]
- Hughes, C.F.; Ward, M.; Tracey, F.; Hoey, L.; Molloy, A.M.; Pentieva, K.; McNulty, H. B-vitamin intake and biomarker status in relation to cognitive decline in healthy older adults in a 4-year follow-up study. Nutrients 2017, 9, 53. [Google Scholar] [CrossRef]
- Tangney, C.; Aggarwal, N.; Li, H.; Wilson, R.; Decarli, C.; Evans, D.; Morris, M. Vitamin B12, cognition, and brain MRI measures: A cross-sectional examination. Neurology 2011, 77, 1276–1282. [Google Scholar] [CrossRef]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522–527.e2. [Google Scholar] [CrossRef]
- Suhrie, K.R.; Hammers, D.B.; Porter, S.M.; Dixon, A.M.; King, J.B.; Anderson, J.S.; Duff, K.; Hoffman, J.M. Predicting biomarkers in intact older adults and those with amnestic mild cognitive impairment, and mild Alzheimer’s disease using the Repeatable Battery for the Assessment of Neuropsychological Status. J. Clin. Exp. Neuropsychol. 2021, 43, 861–878. [Google Scholar] [CrossRef] [PubMed]
| Variable | Amyloid Positive | Amyloid Negative | p Value 1 |
|---|---|---|---|
| n = 162 (50.57%) | n = 159 (49.53%) | ||
| Age (SD 2) | 72.44 (5.48) | 71.09 (5.43) | 0.03 * |
| Sex | |||
| Female | 74 (45.70) | 80 (50.31) | 0.47 |
| Male | 88 (54.30) | 79 (49.75) | |
| BMI (SD) | 25.99 (4.10) | 26.00 (4.05) | 0.98 |
| Creatinine (SD) | 77.12 (15.19) | 75.29 (14.63) | 0.27 |
| Triglycerides (IQR 3) | 1.30 (0.63) | 1.36 (0.79) | 0.44 |
| Vitamin B12 (blood level) (IQR) | 231.60 (96.25) | 233.30 (117.50) | 0.83 |
| Folate (blood level) (IQR) | 31.07 (18.95) | 28.29 (14.50) | 0.02 * |
| HDL/mmol/L (IQR) | 1.73 (0.58) | 1.78 (0.66) | 0.37 |
| LDL/mmol/L (IQR) | 2.98 (1.35) | 3.03 (1.22) | 0.63 |
| Education level (%) | 0.83 | ||
| Bachelor | 53 (32.72) | 50 (31.45) | |
| Master or higher | 47 (29.01) | 43 (27.04) | |
| Secondary school or lower | 62 (38.27) | 66 (41.51) | |
| Smoking status (%) | 0.13 | ||
| No | 63 (38.89) | 70 (44.03) | |
| Stopped | 94 (58.02) | 78 (49.05) | |
| Yes | 5 (3.09) | 11 (6.92) | |
| CVD (%) | 0.79 | ||
| Yes | 87 (53.70) | 82 (51.57) | |
| No | 75 (46.30) | 77 (48.43) | |
| EM (%) | 0.45 | ||
| Yes | 34 (20.99) | 29 (18.24) | |
| No | 128 (79.01) | 130 (81.76) | |
| APOEε4 carrier (%) | 0.00 * | ||
| Yes | 85 (52.47) | 36 (22.64) | |
| No | 77 (47.53) | 123 (77.36) | |
| PUFA/g (IQR) | 12.18 (6.35) | 12.62 (6.40) | 0.50 |
| UFA:SFA ratio (IQR) | 1.44 (0.37) | 1.45 (0.34) | 0.56 |
| Vitamin B12/µg (IQR) | 7.14 (3.43) | 7.05 (4.60) | 0.82 |
| Folate/ug (IQR) | 292.20 (159.99) | 282.60 (137.26) | 0.16 |
| RBANS (SD) | 105.50 (12.26) | 105.8 (12.26) | 0.82 |
| Attention (SD) | 109.00 (15.13) | 111.10 (16.90) | 0.25 |
| Language (SD) | 105.00 (14.17) | 104.70 (13.00) | 0.84 |
| Immediate memory (SD) | 108.17 (13.68) | 108.50 (12.64) | 0.70 |
| Delayed memory (SD) | 101.90 (11.91) | 102.8 (11.46) | 0.45 |
| Visuospatial construction (SD) | 96.86 (14.85) | 94.74 (15.06) | 0.20 |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Nutrients | Model 1 1 | Model 2 2 | Model 3 3 | |||
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| PUFA | −1.69 (−7.80, 4.42) | 0.59 | −2.32 (−8.52, 3.87) | 0.46 | −2.21 (−8.47, 4.05) | 0.49 |
| B12 | 3.57 (−3.33, 10.48) | 0.31 | 2.09 (−5.07, 9.25) | 0.57 | 2.06 (−5.20, 9.31) | 0.58 |
| Folate, medium | 0.15 (−4.46, 4.76) | 0.95 | 0.32 (−4.36, 5.01) | 0.89 | 0.10 (−4.66, 4.87) | 0.97 |
| Folate, highest | 3.04 (−1.76, 7.84) | 0.21 | 3.66 (−1.24, 8.56) | 0.14 | 3.57 (−1.43, 8.57) | 0.16 |
| PUFA: B12 | 0.77 (−9.04, 10.58) | 0.878 | −0.55 (−10.48, 9.37) | 0.91 | −0.84 (−10.87, 9.19) | 0.87 |
| PUFA: Folate.medium | 2.76 (−4.23, 9.74) | 0.44 | 3.40 (−3.67, 10.47) | 0.34 | 3.18 (−3.97, 10.33) | 0.38 |
| PUFA: Folate.high | 1.29 (−5.21, 7.79) | 0.70 | 1.72 (−4.84, 8.29) | 0.61 | 1.51 (−5.15, 8.16) | 0.66 |
| B12: Folate.medium | −6.24 (−13.91, 1.44) | 0.11 | −4.76 (−12.75, 3.23) | 0.24 | −4.89 (−12.96, 3.19) | 0.23 |
| B12: Folate.high | −3.54 (−10.66, 3.58) | 0.33 | −2.25 (−9.59, 5.09) | 0.55 | −2.19 (−9.62, 5.24) | 0.56 |
| PUFA: B12: Folate.medium | −0.77 (−11.17, 9.63) | 0.89 | 0.80 (−9.77, 11.36) | 0.88 | 1.15 (−9.51, 11.82) | 0.83 |
| PUFA: B12: Folate.high | −0.36 (−10.31, 9.60) | 0.94 | 1.19 (−8.94, 11.32) | 0.82 | 1.50 (−8.73, 11.74) | 0.77 |
| Sex.male | −2.89 (−5.53, −0.25) | 0.03 * | −2.41 (−5.89, 1.08) | 0.18 | −2.18 (−5.78, 1.42) | 0.24 |
| BMI | 0.24 (−0.09, 0.56) | 0.15 | 0.23 (−0.12, 0.59) | 0.20 | 0.24 (−0.12, 0.61) | 0.18 |
| Education.master/higher | 0.52 (−2.88, 3.93) | 0.76 | 1.09 (−2.37, 4.54) | 0.54 | 1.24 (−2.26, 4.74) | 0.49 |
| Education.secondary | −7.30 (−10.39, −4.21) | 0.00 * | −6.72 (−9.88, −3.57) | 0.00 * | −6.64 (−9.85, −3.44) | 0.00 * |
| Creatinine | 0.01 (−0.10, 0.13) | 0.84 | 0.01 (−0.10, 0.13) | 0.85 | ||
| HDL | 1.95 (−1.60, 5.51) | 0.28 | 1.93 (−1.74, 5.59) | 0.30 | ||
| LDL | 0.69 (−0.87, 2.24) | 0.39 | 0.77 (−0.80, 2.34) | 0.34 | ||
| Triglycerides | 1.44 (−1.05, 3.94) | 0.26 | 1.34 (−1.20, 3.89) | 0.30 | ||
| CVD.yes | 1.70 (−1.12, 4.53) | 0.24 | 1.91 (−0.96, 4.78) | 0.19 | ||
| EM.yes | −1.97 (−5.38, 1.43) | 0.26 | −1.93 (−5.35, 1.49) | 0.27 | ||
| Smokingstatus.yes | 0.25 (−6.20, 6.70) | 0.94 | ||||
| Smokingstatus.stopped | −1.20 (−4.06, 1.67) | 0.41 | ||||
| APOEε4 gene carrier.no | 0.55 (−2.26, 3.37) | 0.70 | ||||
| (b) | ||||||
| Nutrients | Model 1 1 | Model 2 2 | Model 3 3 | |||
| β (95% CI) | pValue | β (95% CI) | pValue | β (95% CI) | pValue | |
| PUFA | −0.06 (−6.87, 6.76) | 0.99 | −0.43 (−7.36, 6.51) | 0.90 | −1.12 (−8.10, 5.87) | 0.75 |
| B12 | 8.86 (1.16, 16.55) | 0.02 * | 7.92 (−0.11, 15.94) | 0.05 | 7.28 (−0.82, 15.38) | 0.08 |
| Folate.medium | −0.52 (−5.66, 4.62) | 0.84 | −0.02 (−5.27, 5.23) | 0.99 | 0.66 (−4.66, 5.98) | 0.81 |
| Folate.high | 0.64 (−4.71, 5.99) | 0.81 | 1.29 (−4.19, 6.78) | 0.64 | 1.94 (−3.64, 7.52) | 0.49 |
| PUFA × B12 | 8.98 (−1.96, 19.92) | 0.11 | 8.31 (−2.81, 19.43) | 0.14 | 7.96 (−3.24, 19.15) | 0.16 |
| PUFA × Folate.medium | 3.32 (−4.47, 11.12) | 0.40 | 3.83 (−4.08, 11.75) | 0.34 | 4.71 (−3.26, 12.69) | 0.25 |
| PUFA × Folate.high | −0.89 (−8.14, 6.36) | 0.81 | −0.65 (−8.00, 6.70) | 0.86 | 0.25 (−7.18, 7.67) | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Abbott, K.A.; Udeh-Momoh, C.; Price, G.; Robinson, O.J.K.; Kang, S.; de Jager Loots, C.A. Associations Between B Vitamin Interactions with Polyunsaturated Fatty Acids and Cognitive Function Among Cognitively Healthy Older People as Modified by Amyloid Status and Sex. Nutrients 2025, 17, 1407. https://doi.org/10.3390/nu17091407
Zhao C, Abbott KA, Udeh-Momoh C, Price G, Robinson OJK, Kang S, de Jager Loots CA. Associations Between B Vitamin Interactions with Polyunsaturated Fatty Acids and Cognitive Function Among Cognitively Healthy Older People as Modified by Amyloid Status and Sex. Nutrients. 2025; 17(9):1407. https://doi.org/10.3390/nu17091407
Chicago/Turabian StyleZhao, Chuliang, Karen A. Abbott, Chinedu Udeh-Momoh, Geraint Price, Oliver J. K. Robinson, Sujin Kang, and Celeste A. de Jager Loots. 2025. "Associations Between B Vitamin Interactions with Polyunsaturated Fatty Acids and Cognitive Function Among Cognitively Healthy Older People as Modified by Amyloid Status and Sex" Nutrients 17, no. 9: 1407. https://doi.org/10.3390/nu17091407
APA StyleZhao, C., Abbott, K. A., Udeh-Momoh, C., Price, G., Robinson, O. J. K., Kang, S., & de Jager Loots, C. A. (2025). Associations Between B Vitamin Interactions with Polyunsaturated Fatty Acids and Cognitive Function Among Cognitively Healthy Older People as Modified by Amyloid Status and Sex. Nutrients, 17(9), 1407. https://doi.org/10.3390/nu17091407





