The Allergy Crossroads of Subtropical Regions: Mites, Crustaceans, and the Rise of Edible Insects
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Serological Analysis
2.3. Statistical Analysis
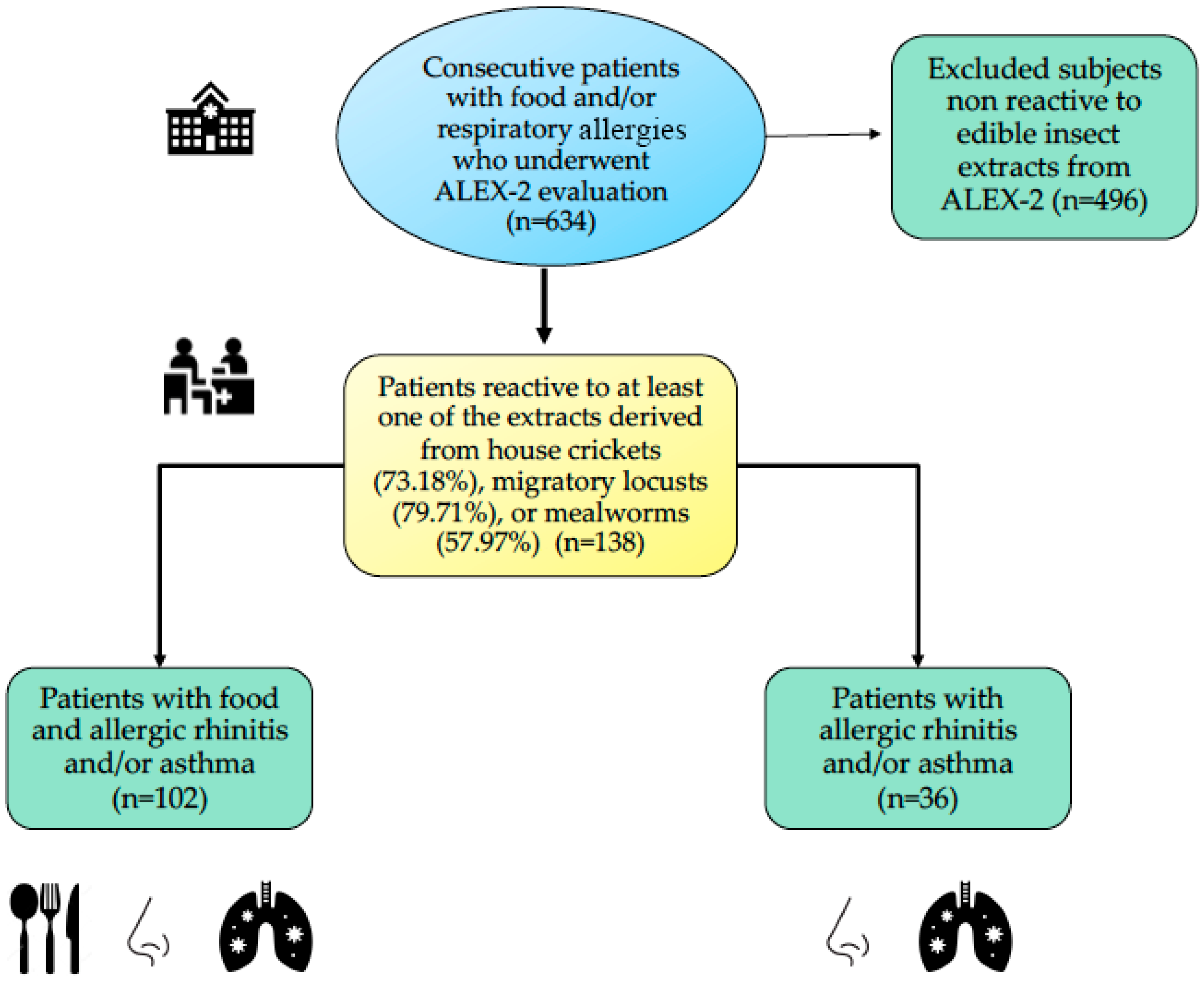
3. Results
3.1. Study Population
3.2. Specific IgE Profile in Patients with a Sensitization to EIs
3.3. Multiplex IgE Reactivity Profiles in Patients with Sensitization to EIs and Exclusively Affected by Respiratory Allergies
3.4. Allergen-Specific IgE Levels to TMs, AKs, and Different EI Extracts Were Significantly Correlated
4. Discussion
4.1. Molecular Sensitization Patterns in the Investigated Cohort
4.2. Cross-Reactivity Among EIs and Other Allergens
4.3. Insect-Specific Proteins and Sensitization Mechanisms
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Tm | Tenebrio molitor |
| EIs | Edible Insects |
| EFSA | European Food Safety Authority |
| HDMs | House Dust Mites |
| TM | Tropomyosin |
| Lm | Locusta migratoria |
| Ad | Acheta domesticus |
| AK | Arginine Kinase |
Appendix A
| Mite Allergen | Acheta domesticus r (p Value) | Locusta migratoria r (p Value) | Tenebrio mollitor r (p Value) |
|---|---|---|---|
| Der f 2 | 0.13 (0.1) | 0.1 (0.21) | 0.02 (0.79) |
| Der p 2 | −0.28 (0.06) | −0.05 (0.34) | −0.15 (0.3) |
| Der p 1 | 0.32 (0.05) | 0.29 (0.08) | 0.14 (0.09) |
| Der p 23 | 0.28 (0.09) | 0.25 (0.13) | 0.24 (0.1) |
| Der f 1 | 0.18 (0.27) | 0.13 (0.43) | 0.03 (0.8) |
| Der p 5 | 0.25 (0.12) | 0.29 (0.08) | 0.17 (0.31) |
| Der p 7 | 0.24 (0.1) | 0.21 (0.12) | 0.22 (0.2) |
| Blo t 21 | 0.22 (0.18) | 0.14 (0.39) | 0.06 (0.69) |
| Gly d 2 | 0.06 (0.68) | −0.05 (0.72) | 0.003 (0.98) |
| Tyr p 2 | 0.12 (0.09) | 0.15 (0.1) | 0.23 (0.16) |
| Der p 21 | −0.07 (0.66) | −0.11 (0.51) | −0.15 (0.37) |
| Blo t 5 | 0.16 (0.33) | 0.09 (0.58) | 0.02 (0.88) |
| Lep d 2 | 0.23 (0.15) | 0.09 (0.56) | 0.18 (0.28) |
| Der p 20 | 0.29 (0.001) | 0.31 (0.0019) | 0.26 (0.0017) |
| Der p 10 | 0.71 (<0.0001) | 0.77 (<0.0001) | 0.82 (<0.0001) |
| Blo t 10 | 0.7 (<0.0001) | 0.71 (<0.0001) | 0.78 (<0.0001) |
| Der p 11 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
References
- Commission Implementing Regulation (Eu) 2025/89 of 20 January 2025. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202500089 (accessed on 3 February 2025).
- Edible Insects: The Science of Novel Food Evaluations. Available online: https://www.efsa.europa.eu/en/news/edible-insects-science-novel-food-evaluations (accessed on 7 February 2025).
- Francis, F.; Doyen, V.; Debaugnies, F.; Mazzucchelli, G.; Caparros, R.; Alabi, T.; Blecker, C.; Haubruge, E.; Corazza, F. Limited cross reactivity among arginine kinase allergens from mealworm and cricket edible insects. Food Chem. 2019, 276, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Pichereaux, C.; Simplicien, M.; Burlet-Schiltz, O.; Benoist, H.; Rougé, P. A Proteomic- and Bioinformatic-Based Identification of Specific Allergens from Edible Insects: Probes for Future Detection as Food Ingredients. Foods 2021, 10, 280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, C.; Nebbia, S.; Cirrincione, S.; Brussino, L.; Giorgis, V.; Romito, A.; Marchese, C.; Manfredi, M.; Marengo, E.; Giuffrida, M.G.; et al. Thermal processing of insect allergens and IgE cross-recognition in Italian patients allergic to shrimp, house dust mite and mealworm. Food Res. Int. 2021, 148, 110567. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Park, J.W. Insect Allergens on the Dining Table. Curr. Protein Pept. Sci. 2020, 21, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barre, A.; Pichereaux, C.; Velazquez, E.; Maudouit, A.; Simplicien, M.; Garnier, L.; Bienvenu, F.; Bienvenu, J.; Burlet-Schiltz, O.; Auriol, C.; et al. Insights into the Allergenic Potential of the Edible Yellow Mealworm (Tenebrio molitor). Foods 2019, 8, 515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Broekhoven, S.; Bastiaan-Net, S.; de Jong, N.W.; Wichers, H.J. Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species. Food Chem. 2016, 196, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Sokol, W.N. Grasshopper sensitization in patients allergic to crustaceans, mites, and cockroaches: Should grasshopper-containing products carry a warning? Ann. Allergy Asthma Immunol. 2020, 124, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Shao, J.; Degreef, J.M.; van Leeuwen, A.; van Ree, R.; Pauli, G.; de Blay, F. Role of tropomyosin as a cross-reacting allergen in sensitization to cockroach in patients from Martinique (French Caribbean island) with a respiratory allergy to mite and a food allergy to crab and shrimp. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 85–88. [Google Scholar] [PubMed]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2017, 62, 1700030. [Google Scholar] [CrossRef] [PubMed]
- Sozener, Z.C.; Ozturk, B.O.; Cerci, P.; Turk, M.; Akin, B.G.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caraballo, L.; Zakzuk, J.; Lee, B.W.; Acevedo, N.; Soh, J.Y.; Sánchez-Borges, M.; Hossny, E.; García, E.; Rosario, N.; Ansotegui, I.; et al. Particularities of allergy in the Tropics. World Allergy Organ. J. 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muddaluru, V.; Valenta, R.; Vrtala, S.; Schlederer, T.; Hindley, J.; Hickey, P.; Larché, M.; Tonti, E. Comparison of house dust mite sensitization profiles in allergic adults from Canada, Europe, South Africa and USA. Allergy 2021, 76, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, R.; Galván-Calle, C.A.; Galán, T.; Poza-Guedes, P.; Sánchez-Machín, I.; Enrique-Calderón, O.M.; Pineda, F. Molecular Signatures of Aeroallergen Sensitization in Respiratory Allergy: A Comparative Study Across Climate-Matched Populations. Int. J. Mol. Sci. 2024, 26, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solmon, F.; Storkey, J.; Vautard, R.; Epstein, M.M. Climate Change and Future Pollen Allergy in Europe. Environ. Heal. Perspect. 2017, 125, 385–391. [Google Scholar]
- Beggs, P.J.; Clot, B.; Sofiev, M.; Johnston, F.H. Climate change, airborne allergens, and three translational mitigation approaches. eBioMedicine 2023, 93, 104478. [Google Scholar] [CrossRef] [PubMed]
- Plume Labs. Available online: https://plumelabs.com/en/air/ (accessed on 16 April 2025).
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Alvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K.; et al. EAACI guidelines on the diagnosis of IgE-mediated food allergy. Allergy 2023, 78, 3057–3076. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Tebbe, J.; Jakob, T. Molecular allergy diagnostics using IgE singleplex determinations: Methodological and practical considerations for use in clinical routine: Part 18 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 185–197. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Jappe, U. Molecular allergy diagnostic tests: Development and relevance in clinical practice. Allergologie 2017, 1, 169–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Platteel, A.C.; van der Pol, P.; Murk, J.-L.; Verbrugge-Bakker, I.; Hack-Steemers, M.; Roovers, T.H.; Heron, M. A comprehensive comparison between ISAC and ALEX2 multiplex test systems. Clin. Chem. Lab. Med. 2022, 60, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, R.; Poza-Guedes, P.; Pineda, F.; Galán, T.; Mederos-Luis, E.; Abel-Fernández, E.; Martínez, M.J.; Sánchez-Machín, I. Molecular Mapping of Allergen Exposome among Different Atopic Phenotypes. Int. J. Mol. Sci. 2023, 24, 10467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soil-Transmitted Helminthiases: Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/44804/9789241503129_eng.pdf (accessed on 13 February 2025).
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Allergic Rhinitis and Its Impact on Asthma Working Group. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar]
- 2022 GINA Main Report. Available online: https://ginasthma.org/gina-reports/ (accessed on 15 March 2025).
- Bojcukova, J.; Vlas, T.; Forstenlechner, P.; Panzner, P. Comparison of two multiplex arrays in the diagnostics of allergy. Clin. Transl. Allergy 2019, 9, 31. [Google Scholar] [CrossRef]
- Lis, K.; Bartuzi, Z. Selected Technical Aspects of Molecular Allergy Diagnostics. Curr. Issues Mol. Biol. 2023, 45, 5481–5493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nösslinger, H.; Mair, E.; Oostingh, G.J.; Ahlgrimm-Siess, V.; Ringauf, A.; Lang, R. Multiplex Assays in Allergy Diagnosis: Allergy Explorer 2 versus ImmunoCAP ISAC E112i. Diagnostics 2024, 14, 976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altmann, F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J. Int. 2016, 25, 98–105. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Q.; Yang, Y.; Zhang, W.; Yang, L.; Zhu, R. Cross-Reacting Carbohydrate Determinants Inhibitor Can Improve the Diagnostic Accuracy in Pollen and Food Allergy. J. Asthma Allergy 2022, 15, 713–725. [Google Scholar] [CrossRef]
- Omuse, E.R.; Tonnang, H.E.Z.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J.; et al. The global atlas of edible insects: Analysis of diversity and commonality contributing to food systems and sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siddiqui, S.A.; Tettey, E.; Yunusa, B.M.; Ngah, N.; Debrah, S.K.; Yang, X.; Fernando, I.; Povetkin, S.N.; Shah, M.A. Legal situation and consumer acceptance of insects being eaten as human food in different nations across the world-A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4786–4830. [Google Scholar] [CrossRef] [PubMed]
- Sokol, W.N.; Wünschmann, S.; Agah, S. Grasshopper anaphylaxis in patients allergic to dust mite, cockroach, and crustaceans: Is tropomyosin the cause? Ann. Allergy Asthma Immunol. 2017, 119, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int. Arch. Allergy Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, B.; Stoenchev, K.V.; Skypala, I.J. Anaphylaxis across Europe: Are pollen food syndrome and lipid transfer protein allergy so far apart? Curr. Opin. Allergy Clin. Immunol. 2022, 22, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Betancor, D.; Gomez-Lopez, A.; Villalobos-Vilda, C.; Nuñez-Borque, E.; Fernández-Bravo, S.; De Las Heras Gozalo, M.; Pastor-Vargas, C.; Esteban, V.; Cuesta-Herranz, J. LTP Allergy Follow-Up Study: Development of Allergy to New Plant Foods 10 Years Later. Nutrients 2021, 13, 2165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahammed, L.L.; Belaid, B.; Berkani, L.M.; Merah, F.; Rahali, S.Y.; Kaci, A.A.; Berkane, I.; Sayah, W.; Allam, I.; Djidjik, R. Shrimp sensitization in house dust mite algerian allergic patients: A single center experience. World Allergy Organ. J. 2022, 15, 100642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farioli, L.; Losappio, L.M.; Giuffrida, M.G.; Pravettoni, V.; Micarelli, G.; Nichelatti, M.; Scibilia, J.; Mirone, C.; Cavallarin, L.; Lamberti, C.; et al. Mite-Induced Asthma and IgE Levels to Shrimp, Mite, Tropomyosin, Arginine Kinase, and Der p 10 Are the Most Relevant Risk Factors for Challenge-Proven Shrimp Allergy. Int. Arch. Allergy Immunol. 2017, 174, 133–143. [Google Scholar] [CrossRef] [PubMed]
- del Giudice, M.M.; Dinardo, G.; Klain, A.; D’addio, E.; Bencivenga, C.L.; Decimo, F.; Indolfi, C. Anaphylaxis after Shrimp Intake in a European Pediatric Population: Role of Molecular Diagnostics and Implications for Novel Foods. Children 2023, 10, 1583. [Google Scholar] [CrossRef]
- Giusti, D.; Guemari, A.; Perotin, J.-M.; Fontaine, J.-F.; Libyh, M.T.; Gatouillat, G.; Tabary, T.; Pham, B.-N.; Vitte, J. Molecular allergology: A clinical laboratory tool for precision diagnosis, stratification and follow-up of allergic patients. Clin. Chem. Lab. Med. 2024, 62, 2339–2355. [Google Scholar] [CrossRef] [PubMed]
- Riggioni, C.; Leung, A.S.; Wai, C.Y.; Davies, J.M.; Sompornrattanaphan, M.; Pacharn, P.; Chamani, S.; Brettig, T.; Peters, R.L. Exploring geographical variances in component-resolved diagnosis within the Asia-Pacific region. Pediatr. Allergy Immunol. 2025, 36, e70054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mittermann, I.; Lupinek, C.; Wieser, S.; Aumayr, M.; Kuchler, W.W.; Chan, A.W.; Lee, T.H.; Zieglmayer, P. IgE reactivity patterns in Asian and central European cockroach-sensitized patients reveal differences in primary sensitizing allergen sources. J. Allergy Clin. Immunol. Glob. 2022, 1, 145–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wangorsch, A.; Jamin, A.; Spiric, J.; Vieths, S.; Scheurer, S.; Mahler, V.; Hofmann, S.C. Allergic Reaction to a Commercially Available Insect Snack Caused by House Cricket (Acheta domesticus) Tropomyosin. Mol. Nutr. Food Res. 2024, 68, e2300420. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Rotter, N.S.; Stieb, E.S.; Stockbridge, J.L.; Theodorakakis, M.D.; Shreffler, W.G. Utility of food allergy thresholds. Ann. Allergy Asthma Immunol. 2023, 132, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Gattinger, P.; van Hage, M.; Flicker, S.; Linhart, B.; Campana, R.; Focke-Tejkl, M.; Curin, M.; et al. Molecular Aspects of Allergens and Allergy. Adv. Immunol. 2018, 138, 195–256. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Vitte, J. A systematic review of allergen cross-reactivity: Translating basic concepts into clinical relevance. J. Allergy Clin. Immunol. Glob. 2024, 3, 100230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dramburg, S.; Hilger, C.; Santos, A.F.; Vecillas, L.d.L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, e13854. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scala, E.; Abeni, D.; Villella, V.; ViIlalta, D.; Cecchi, L.; Caprini, E.; Asero, R. Investigating Novel Food Sensitization: A Real-Life Prevalence Study of Cricket, Locust, and Mealworm IgE-Reactivity in Naïve allergic Individuals. J. Investig. Allergol. Clin. Immunol. 2024, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Huang, C.H.; Lee, B.W. Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin? Allergy Asthma Immunol. Res. 2016, 8, 101–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emilia, M.; Magdalena, C.; Weronika, G.; Julia, W.; Danuta, K.; Jakub, S.; Bożena, C.; Krzysztof, K. IgE-based analysis of sensitization and cross-reactivity to yellow mealworm and edible insect allergens before their widespread dietary introduction. Sci. Rep. 2025, 15, 1466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Linacero, R.; Cuadrado, C. New Research in Food Allergen Detection. Foods 2022, 11, 1520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Retzinger, A.C.; Retzinger, G.S. The Acari Hypothesis, II: Interspecies Operability of Pattern Recognition Receptors. Pathogens 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-Karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009, 457, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Edwards, L.L.; Aloor, J.J.; Fessler, M.B.; Glesner, J.; Pomés, A.; Chapman, M.D.; London, R.E.; Pedersen, L.C. The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J. Allergy Clin. Immunol. 2010, 125, 909–917.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible insects: Cross-recognition of IgE from crustacean- and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shroba, J.; Rath, N.; Barnes, C. Possible Role of Environmental Factors in the Development of Food Allergies. Clin. Rev. Allergy Immunol. 2018, 57, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a Food Safety and Nutritional Perspective: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Conway, A.; Jaiswal, S.; Jaiswal, A.K. The Potential of Edible Insects as a Safe, Palatable, and Sustainable Food Source in the European Union. Foods 2024, 13, 387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.; Zhang, Y.; Zhang, S.; Zhang, L.; Lan, F. The effect of immunotherapy on cross-reactivity between house dust mite and other allergens in house dust mite -sensitized patients with allergic rhinitis. Expert Rev. Clin. Immunol. 2021, 17, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.; van Broekhoven, S.; den Hartog-Jager, C.F.; Gaspari, M.; de Jong, G.A.; Wichers, H.J.; van Hoffen, E.; Houben, G.F.; Knulst, A.C. House dust mite (Der p 10) and crustacean allergic patients may react to food containing Yellow mealworm proteins. Food Chem. Toxicol. 2014, 65, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.C.H.P.; Knulst, A.C.; de Jong, G.; Gaspari, M.; Jager, C.F.D.H.; Houben, G.F.; Verhoeckx, K.C.M. Is mealworm or shrimp allergy indicative for food allergy to insects? Mol. Nutr. Food Res. 2017, 61, 1601061. [Google Scholar] [CrossRef] [PubMed]
- Gałęcki, R.; Bakuła, T.; Gołaszewski, J. Foodborne Diseases in the Edible Insect Industry in Europe-New Challenges and Old Problems. Foods 2023, 12, 770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abro, Z.; Sibhatu, K.T.; Fetene, G.M.; Alemu, M.H.; Tanga, C.M.; Sevgan, S.; Kassie, M. Global review of consumer preferences and willingness to pay for edible insects and derived products. Glob. Food Secur. 2025, 44, 100834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quintieri, L.; Nitride, C.; De Angelis, E.; Lamonaca, A.; Pilolli, R.; Russo, F.; Monaci, L. Alternative Protein Sources and Novel Foods: Benefits, Food Applications and Safety Issues. Nutrients 2023, 15, 1509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sicherer, S.H.; Sampson, H.A. Food allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [Google Scholar] [CrossRef] [PubMed]
- Schussler, E.; Kattan, J. Allergen Component Testing in the Diagnosis of Food Allergy. Curr. Allergy Asthma Rep. 2015, 15, 55. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | (n = 138) |
|---|---|
| Age (y.o.) median (range) | 17 (3–75) |
| Sex (F/M) | 43/95 |
| Food and respiratory allergy | 80 (58%) |
| Food allergy | 22 (15.9%) |
| Seafood allergy | 40 (50%) |
| Respiratory allergy | 36 (26%) |
| Allergic rhinitis | 21 (58.3%) |
| Allergic rhinitis and asthma | 15 (41.6% |
| Total IgE (IU/mL) median (range) | 532 (54–2500) |
| Family history of atopy (%) | 105 (77.08) |
| Pan-Allergens in 138 Subjects Sensitized to at Least One Edible Insect | Acheta domesticus (n = 101) | Locusta migratoria (n = 110) | Tenebrio molitor (n = 80) |
|---|---|---|---|
| Tropomyosin (any) molecules in 88/138 subjects (63.76%) | |||
| Ani s 3 (n = 76) | 69 (68.31) | 67 (60.9) | 58 (72.5) |
| Blo t 10 (n = 79) | 71 (70.29) | 68 (61.81) | 62 (77.5) |
| Der p 10 (n = 64) | 62 (61.38) | 58 (52.72) | 57 (71.25) |
| Per a 7 (n = 68) | 64 (63.36) | 60 (54.54) | 58 (72.5) |
| Pen m 1 (n = 63) | 62 (61.38) | 58 (52.72) | 54 (67.5) |
| Arginine kinase (any) molecules in 46/138 subjects (33.33%) | |||
| Bla g 9 (n = 36) | 27 (26.73) | 33 (30) | 22 (27.5) |
| Der p 20 (n = 39) | 32 (31.68) | 35 (31.81) | 27 (33.75) |
| Pen m 2 (n = 26) | 23 (22.77) | 25 (22.72) | 23 (28.75) |
| Paramyosin Der p 11 in 1/138 subjects (0.72%) | 1 (0.99) | 1 (0.9) | 1 (1.25) |
| Troponin-C Cra c 6 in 40/138 subjects (28.98%) | 34 (33.66) | 37 (33.63) | 31 (76.25) |
| Myosin light chain Pen m 3 in 9/138 subjects (6.52%) | 8 (7.92) | 8 (7.27) | 8 (10) |
| Sarcoplasmic calcium-binding protein Pen m 4 in 12/138 subjects (8.69%) | 8 (7.92) | 10 (9.09) | 8 (10) |
| None (32/138 (23.18%) subjects) | 24 (23.76) | 22 (20) | 10 (10) |
| Mite Allergen | Median sIgE M (IQR) | No. of Sensitized Patients (%) |
|---|---|---|
| Der f 2 | 17.41 (37.21) | 32 (88.88) |
| Der p 2 | 24.19 (41.42) | 31 (86.11) |
| Der p 1 | 9.46 (27.31) | 30 (83.33) |
| Der p 23 | 7.44 (24.89) | 28 (77.77) |
| Der f 1 | 2.47 (9.95) | 25 (69.44) |
| Der p 5 | 3.45 (27.02) | 22 (61.11) |
| Der p 7 | 2.53 (25.03) | 21 (58.33) |
| Blo t 21 | 0.2 (10.64) | 19 (52.77) |
| Gly d 2 | 0.11 (3.71) | 17 (47.22) |
| Tyr p 2 | 0.1 (1.75) | 15 (41.66) |
| Der p 21 | 0.1 (9.83) | 14 (38.88) |
| Blo t 5 | 0.1 (6.84) | 14 (38.88) |
| Lep d 2 | 0.1 (2.19) | 13 (36.11) |
| Der p 20 | 0.1 (0.0) | 3 (8.33) |
| Der p 10 | 0.1 (0.0) | 1 (2.77) |
| Blo t 10 | 0.1 (0.0) | 2 (5.55) |
| Der p 11 | 0.1 (0.0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Pérez, R.; Poza-Guedes, P.; Figueiras-Rincón, M.A.; Colque-Bayona, M.; Sánchez-Machín, I. The Allergy Crossroads of Subtropical Regions: Mites, Crustaceans, and the Rise of Edible Insects. Nutrients 2025, 17, 1405. https://doi.org/10.3390/nu17091405
González-Pérez R, Poza-Guedes P, Figueiras-Rincón MA, Colque-Bayona M, Sánchez-Machín I. The Allergy Crossroads of Subtropical Regions: Mites, Crustaceans, and the Rise of Edible Insects. Nutrients. 2025; 17(9):1405. https://doi.org/10.3390/nu17091405
Chicago/Turabian StyleGonzález-Pérez, Ruperto, Paloma Poza-Guedes, Manuel Alberto Figueiras-Rincón, Mónica Colque-Bayona, and Inmaculada Sánchez-Machín. 2025. "The Allergy Crossroads of Subtropical Regions: Mites, Crustaceans, and the Rise of Edible Insects" Nutrients 17, no. 9: 1405. https://doi.org/10.3390/nu17091405
APA StyleGonzález-Pérez, R., Poza-Guedes, P., Figueiras-Rincón, M. A., Colque-Bayona, M., & Sánchez-Machín, I. (2025). The Allergy Crossroads of Subtropical Regions: Mites, Crustaceans, and the Rise of Edible Insects. Nutrients, 17(9), 1405. https://doi.org/10.3390/nu17091405







