The Impact of Protein Feed on the Urea-to-Creatinine Ratio—A Retrospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Demographics and Group Characteristics
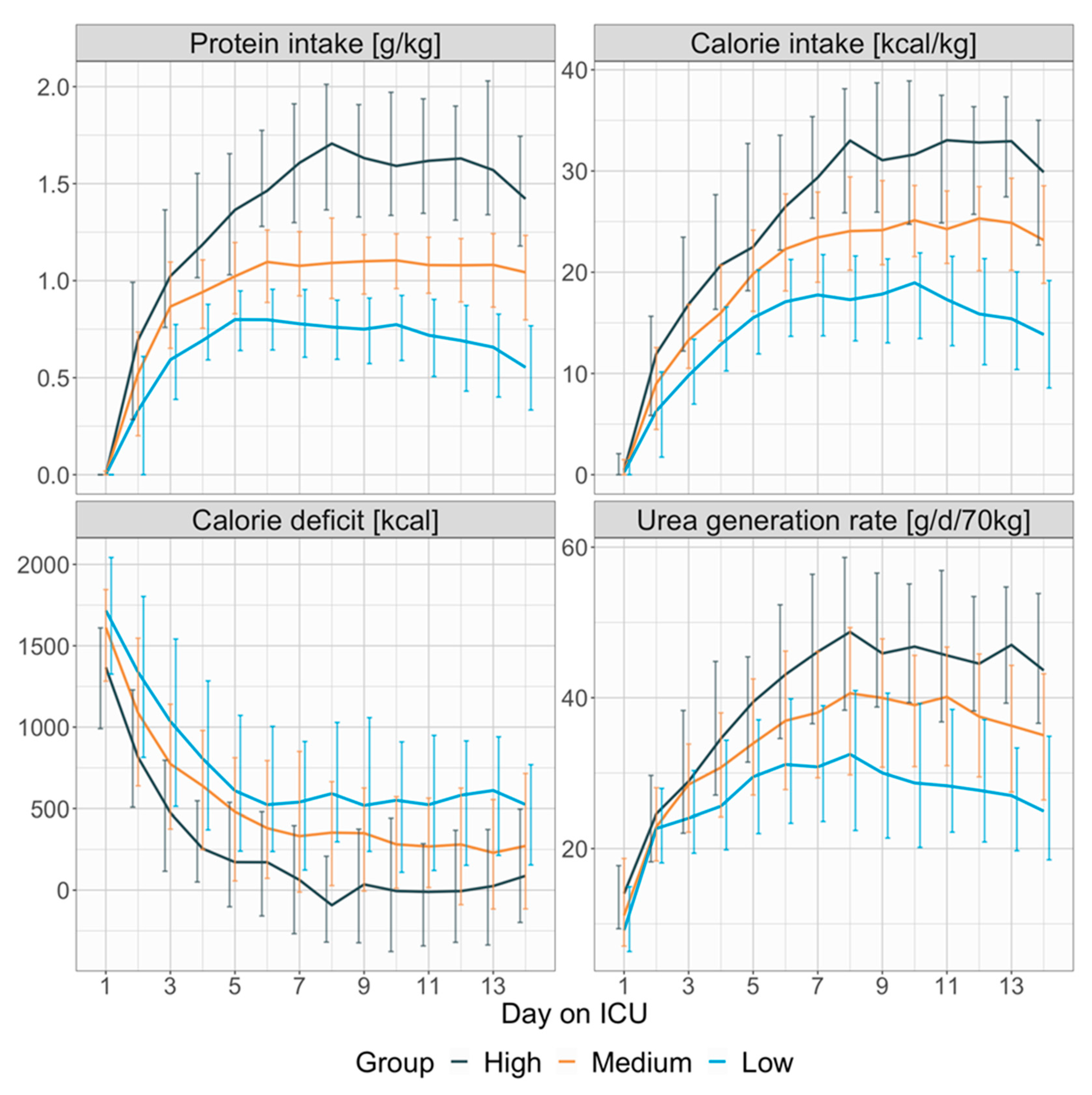
3.2. Protein- and Calorie-Intake
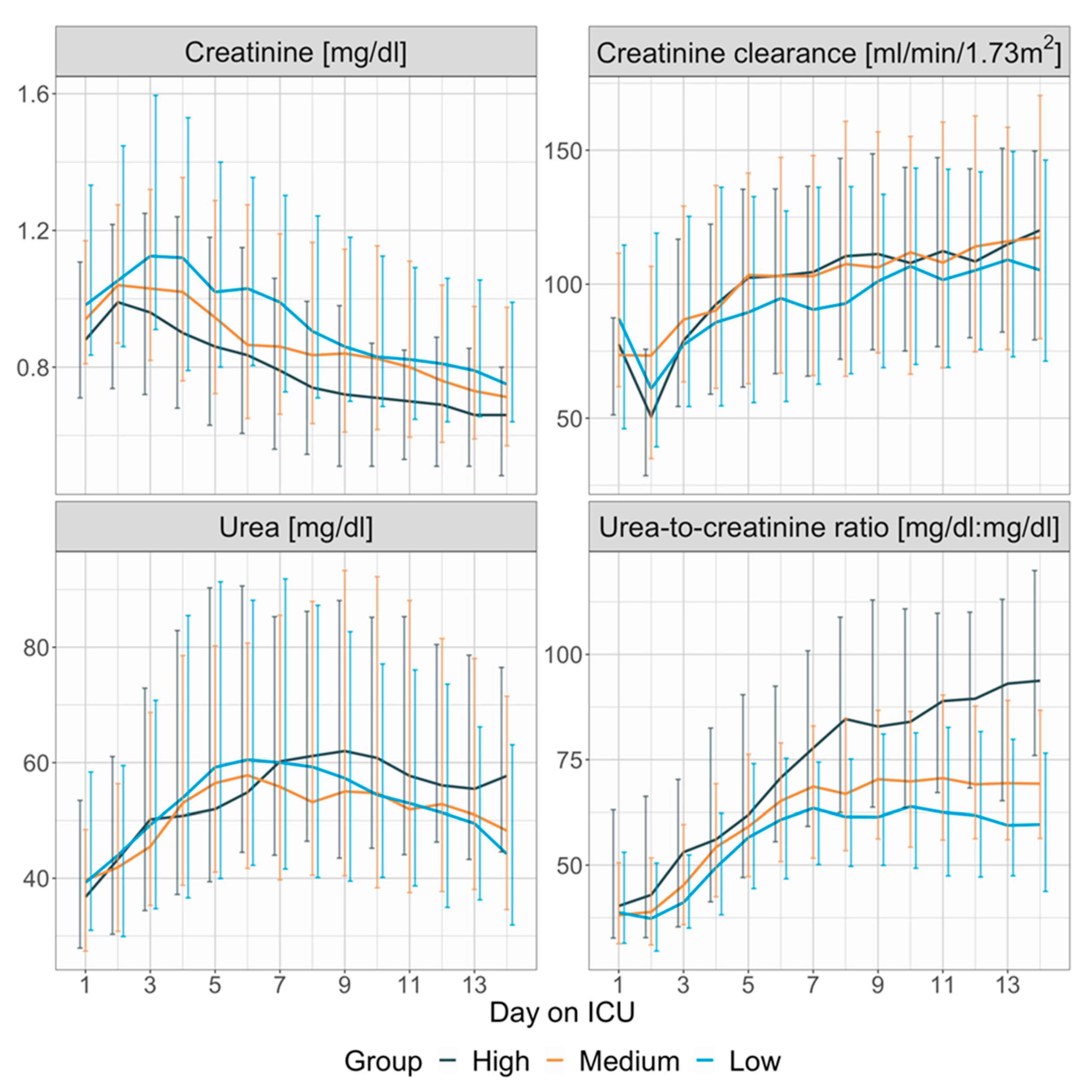
3.3. Renal Function and the Urea-to-Creatinine Ratio
3.4. Risk Factors for Developing a High Urea-to-Creatinine Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rosenthal, M.D.; Moore, F.A. Persistent Inflammatory, Immunosuppressed, Catabolic Syndrome (PICS): A New Phenotype of Multiple Organ Failure. J. Adv. Nutr. Hum. Metab. 2015, 1, e784. [Google Scholar] [CrossRef]
- Hesselink, L.; Hoepelman, R.J.; Spijkerman, R.; de Groot, M.C.H.; van Wessem, K.J.P.; Koenderman, L.; Leenen, L.P.H.; Hietbrink, F. Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) after Polytrauma: A Rare Syndrome with Major Consequences. J. Clin. Med. 2020, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The Rate and Assessment of Muscle Wasting during Critical Illness: A Systematic Review and Meta-Analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.; O’Brien, J.M.; Hoffmann, S.P.; Phillips, G.; Garland, A.; Finley, J.C.W.; Almoosa, K.; Hejal, R.; Wolf, K.M.; Lemeshow, S.; et al. Acquired Weakness, Handgrip Strength, and Mortality in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2008, 178, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, T.; Bastuji-Garin, S.; Stevens, R.D.; Durand, M.-C.; Malissin, I.; Rodriguez, P.; Cerf, C.; Outin, H.; Jonghe, B.D.; Bernard MD for the Groupe de Réflexion et d’Etude des Neuromyopathies En Réanimation (GRENER). Presence and Severity of Intensive Care Unit-Acquired Paresis at Time of Awakening Are Associated with Increased Intensive Care Unit and Hospital Mortality. Crit. Care Med. 2009, 37, 3047–3053. [Google Scholar] [CrossRef]
- Lee, Z.-Y.; Ong, S.P.; Ng, C.C.; Yap, C.S.L.; Engkasan, J.P.; Barakatun-Nisak, M.Y.; Heyland, D.K.; Hasan, M.S. Association between Ultrasound Quadriceps Muscle Status with Premorbid Functional Status and 60-Day Mortality in Mechanically Ventilated Critically Ill Patient: A Single-Center Prospective Observational Study. Clin. Nutr. 2021, 40, 1338–1347. [Google Scholar] [CrossRef]
- Gunst, J.; Kashani, K.B.; Hermans, G. The Urea-Creatinine Ratio as a Novel Biomarker of Critical Illness-Associated Catabolism. Intensive Care Med. 2019, 45, 1813–1815. [Google Scholar] [CrossRef]
- Haines, R.W.; Zolfaghari, P.; Wan, Y.; Pearse, R.M.; Puthucheary, Z.; Prowle, J.R. Elevated Urea-to-Creatinine Ratio Provides a Biochemical Signature of Muscle Catabolism and Persistent Critical Illness after Major Trauma. Intensive Care Med. 2019, 45, 1718–1731. [Google Scholar] [CrossRef]
- Snook, J. Value of a simple biochemical ratio in distinguishing upper and lower sites of gastrointestinal haemorrhage. Lancet 1986, 327, 1064–1065. [Google Scholar] [CrossRef]
- Chapple, L.S.; van Gassel, R.J.J.; Rooyackers, O. Protein Metabolism in Critical Illness. Curr. Opin. Crit. Care 2022, 28, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Sun, C.-Y.; Tsai, C.-Y.; Chen, M.-Y.; Wang, S.-Y.; Hsu, J.-T.; Yeh, C.-N.; Yeh, T.-S. Metabolism of Proteins and Amino Acids in Critical Illness: From Physiological Alterations to Relevant Clinical Practice. J. Multidiscip. Healthc. 2021, 14, 1107–1117. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. High Protein versus Medium Protein Delivery under Equal Total Energy Delivery in Critical Care: A Randomized Controlled Trial. Clin. Nutr. 2021, 40, 796–803. [Google Scholar] [CrossRef]
- Fetterplace, K.; Deane, A.M.; Tierney, A.; Beach, L.J.; Knight, L.D.; Presneill, J.; Rechnitzer, T.; Forsyth, A.; Gill, B.M.T.; Mourtzakis, M.; et al. Targeted Full Energy and Protein Delivery in Critically Ill Patients: A Pilot Randomized Controlled Trial (FEED Trial). J. Parenter. Enter. Nutr. 2018, 42, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Plank, L.D.; Sando, K.; Hill, G.L. Optimal Protein Requirements during the First 2 Weeks after the Onset of Critical Illness. Crit. Care Med. 1998, 26, 1529–1535. [Google Scholar] [CrossRef]
- Preiser, J.-C.; van Zanten, A.R.H.; Berger, M.M.; Biolo, G.; Casaer, M.P.; Doig, G.S.; Griffiths, R.D.; Heyland, D.K.; Hiesmayr, M.; Iapichino, G.; et al. Metabolic and Nutritional Support of Critically Ill Patients: Consensus and Controversies. Crit. Care 2015, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.; Casaer, M.P.; Preiser, J.-C.; Reignier, J.; Van den Berghe, G. Toward Nutrition Improving Outcome of Critically Ill Patients: How to Interpret Recent Feeding RCTs? Crit. Care 2023, 27, 43. [Google Scholar] [CrossRef]
- Moreno, R.P.; Metnitz, P.G.H.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.-R.; et al. SAPS 3--From Evaluation of the Patient to Evaluation of the Intensive Care Unit. Part 2: Development of a Prognostic Model for Hospital Mortality at ICU Admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Buckley, C.T. Impact of Propofol Sedation upon Caloric Overfeeding and Protein Inadequacy in Critically Ill Patients Receiving Nutrition Support. Pharmacy 2021, 9, 121. [Google Scholar] [CrossRef]
- Mehta, N.M.; Smallwood, C.D.; Joosten, K.F.M.; Hulst, J.M.; Tasker, R.C.; Duggan, C.P. Accuracy of a Simplified Equation for Energy Expenditure Based on Bedside Volumetric Carbon Dioxide Elimination Measurement–A Two-Center Study. Clin. Nutr. 2015, 34, 151–155. [Google Scholar] [CrossRef]
- Bois, D.D.; Bois, E.F.D. A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.-C.; et al. ESPEN Practical and Partially Revised Guideline: Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.; Wong, G.; Cheung, K.; Chua, A.; Chong, M.; Miller, M. Association between Malnutrition and 28-Day Mortality and Intensive Care Length-of-Stay in the Critically Ill: A Prospective Cohort Study. Nutrients 2017, 10, 10. [Google Scholar] [CrossRef]
- Villet, S.; Chiolero, R.L.; Bollmann, M.D.; Revelly, J.-P.; RN, M.-C.C.; Delarue, J.; Berger, M.M. Negative Impact of Hypocaloric Feeding and Energy Balance on Clinical Outcome in ICU Patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef] [PubMed]
- De Man, A.M.E.; Gunst, J.; Reintam Blaser, A. Nutrition in the Intensive Care Unit: From the Acute Phase to Beyond. Intensive Care Med. 2024, 50, 1035–1048. [Google Scholar] [CrossRef]
- Inker, L.A.; Shafi, T.; Okparavero, A.; Tighiouart, H.; Eckfeldt, J.H.; Katz, R.; Johnson, W.C.; Dermond, N.; Tariq, Z.; Benayache, I.; et al. Effects of Race and Sex on Measured GFR: The Multi-Ethnic Study of Atherosclerosis. Am. J. Kidney Dis. 2016, 68, 743–751. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef]
- Bels, J.L.M.; Abdelhamid, Y.A.; Van de Poll, M.C.G. Protein Supplementation in Critical Illness: Why, When and How? Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 146–153. [Google Scholar] [CrossRef]
- Rugg, C.; Ströhle, M.; Schmid, S.; Kreutziger, J. The Link between Hypermetabolism and Hypernatremia in Severely Burned Patients. Nutrients 2020, 12, 774. [Google Scholar] [CrossRef]
- Rugg, C.; Ströhle, M.; Treml, B.; Bachler, M.; Schmid, S.; Kreutziger, J. ICU-Acquired Hypernatremia Is Associated with Persistent Inflammation, Immunosuppression and Catabolism Syndrome. J. Clin. Med. 2020, 9, 3017. [Google Scholar] [CrossRef] [PubMed]
- Rugg, C.; Woyke, S.; Ronzani, M.; Levé, A.M.-L.; Spraider, P.; Loveys, S.; Schmid, S.; Kreutziger, J.; Ströhle, M. Catabolism Highly Influences ICU-Acquired Hypernatremia in a Mainly Trauma and Surgical Cohort. J. Crit. Care 2023, 76, 154282. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Stapleton, R.D.; Mourtzakis, M.; Hough, C.L.; Morris, P.; Deutz, N.E.; Colantuoni, E.; Day, A.; Prado, C.M.; Needham, D.M. Combining Nutrition and Exercise to Optimize Survival and Recovery from Critical Illness: Conceptual and Methodological Issues. Clin. Nutr. 2016, 35, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Day, A.; Clarke, G.J.; Hough, C.T.; Files, D.C.; Mourtzakis, M.; Deutz, N.; Needham, D.M.; Stapleton, R. Nutrition and Exercise in Critical Illness Trial (NEXIS Trial): A Protocol of a Multicentred, Randomised Controlled Trial of Combined Cycle Ergometry and Amino Acid Supplementation Commenced Early during Critical Illness. BMJ Open 2019, 9, e027893. [Google Scholar] [CrossRef]
| Low (n = 120) | Medium (n = 132) | High (n = 94) | p Value | |
|---|---|---|---|---|
| Sex | <0.001 | |||
| Female | 24 (20.0%) | 38 (28.8%) | 43 (45.7%) | |
| Male | 96 (80.0%) | 94 (71.2%) | 51 (54.3%) | |
| Age [years] | 0.662 | |||
| Median (Q1, Q3) | 56.0 (46.8, 69.0) | 57.0 (41.0, 68.3) | 59.5 (43.3, 69.0) | |
| Bodyweight [kg] | <0.001 | |||
| Median (Q1, Q3) | 88.9 (78.8, 100.0) | 76.0 (68.4, 84.0) | 63.1 (52.6, 75.0) | |
| Height [cm] | <0.001 | |||
| Median (Q1, Q3) | 175.333 (167.8, 181.0) | 173.0 (167.2, 179.0) | 170.0 (163.5, 177.0) | |
| Admission type | 0.563 | |||
| Elective | 42 (35.0%) | 38 (28.8%) | 29 (30.9%) | |
| Emergency | 78 (65.0%) | 94 (71.2%) | 65 (69.1%) | |
| Medical discipline | 0.730 | |||
| Cardiac surgery | 20 (17.7%) | 17 (14.2%) | 9 (10.1%) | |
| Orthopaedics/Traumatology | 28 (24.8%) | 35 (29.2%) | 19 (21.3%) | |
| Visceral surgery | 13 (11.5%) | 13 (10.8%) | 14 (15.7%) | |
| Internal medicine | 29 (25.7%) | 28 (23.3%) | 25 (28.1%) | |
| Other | 23 (20.4%) | 27 (22.5%) | 22 (24.7%) | |
| Missing | 7 | 12 | 5 | |
| SAPS III admission score | <0.001 | |||
| Median (Q1, Q3) | 58.0 (51.0, 70.0) | 61.0 (54.8, 71.0) | 67.0 (62.0, 77.0) | |
| ICU length of stay [days] | <0.001 | |||
| Median (Q1, Q3) | 19.0 (15.0, 24.3) | 21.0 (17.0, 31.0) | 21.0 (18.0, 27.0) |
| High vs. Low Urea-to-Creatinine Ratio | Crude OR (95% CI) | Adjusted OR (95% CI) | p |
|---|---|---|---|
| Calorie deficit per 100 kcal increase | 0.85 (0.80, 0.90) | 0.94 (0.88, 1.00) | 0.052 |
| Sex male vs. female | 0.30 (0.18, 0.50) | 0.45 (0.25, 0.83) | 0.010 |
| Age per year increase | 1.05 (1.03, 1.06) | 1.05 (1.03, 1.07) | <0.001 |
| Bodyweight per kg increase | 0.97 (0.96, 0.98) | 0.99 (0.97, 1.01) | 0.375 |
| SAPS III admission score per 1 point increase | 1.03 (1.01, 1.05) | 1.00 (0.98, 1.02) | 0.82 |
| Protein intake per 0.1 g/kg increase | 1.20 (1.12, 1.28) | 1.16 (1.05, 1.28) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillinger, P.; Markl-Le Levé, A.; Woyke, S.; Ronzani, M.; Kreutziger, J.; Schmid, S.; Rugg, C. The Impact of Protein Feed on the Urea-to-Creatinine Ratio—A Retrospective Single-Center Study. Nutrients 2025, 17, 1293. https://doi.org/10.3390/nu17081293
Hillinger P, Markl-Le Levé A, Woyke S, Ronzani M, Kreutziger J, Schmid S, Rugg C. The Impact of Protein Feed on the Urea-to-Creatinine Ratio—A Retrospective Single-Center Study. Nutrients. 2025; 17(8):1293. https://doi.org/10.3390/nu17081293
Chicago/Turabian StyleHillinger, Petra, Andreas Markl-Le Levé, Simon Woyke, Marco Ronzani, Janett Kreutziger, Stefan Schmid, and Christopher Rugg. 2025. "The Impact of Protein Feed on the Urea-to-Creatinine Ratio—A Retrospective Single-Center Study" Nutrients 17, no. 8: 1293. https://doi.org/10.3390/nu17081293
APA StyleHillinger, P., Markl-Le Levé, A., Woyke, S., Ronzani, M., Kreutziger, J., Schmid, S., & Rugg, C. (2025). The Impact of Protein Feed on the Urea-to-Creatinine Ratio—A Retrospective Single-Center Study. Nutrients, 17(8), 1293. https://doi.org/10.3390/nu17081293






