Association Between Dietary Soy Isoflavones Intake and the Risk of Hyperemesis Gravidarum: A Cross-Sectional Study in Chinese Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Dietary Assessment
2.3. Estimation of Dietary Soy Isoflavones
2.4. Pregnancy-Unique Quantification of Emesis and Nausea (PUQE Questionnaire)
2.5. Diagnostic Criteria for HG
- Before 16 weeks of gestation, severe nausea and frequent vomiting impair normal eating and significantly limit daily activities [22].
- Received medical attention and therapeutic measures for NVP [23].
- A PUQE score of ≥13 points [23].
- A weight loss of >5% of pre-pregnancy body weight due to nausea or vomiting [24].
- Exclude other potential causes of vomiting, such as gastrointestinal or urinary tract infections, viral hepatitis, or pre-existing medical conditions [24].
2.6. Assessment of Other Variables
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, L.A.W.; Shaw, V.; Grooten, I.J.; Koot, M.H.; Dean, C.R.; Painter, R.C. Diagnosis and treatment of hyperemesis gravidarum. CMAJ 2024, 196, E477–E485. [Google Scholar] [CrossRef] [PubMed]
- Fejzo, M.S.; Trovik, J.; Grooten, I.J.; Sridharan, K.; Roseboom, T.J.; Vikanes, A.; Painter, R.C.; Mullin, P.M. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat. Rev. Dis. Primers 2019, 5, 62. [Google Scholar] [CrossRef]
- Ali, A.I.; Nori, W.; Abdulrahman, H.B. Hyperemesis gravidarum and risks of placental dysfunction disorders. J. Pak. Med. Assoc. 2021, 71 (Suppl. S9), S24–S28. [Google Scholar]
- Fiaschi, L.; Nelson-Piercy, C.; Gibson, J.; Szatkowski, L.; Tata, L.J. Adverse Maternal and Birth Outcomes in Women Admitted to Hospital for Hyperemesis Gravidarum: A Population-Based Cohort Study. Paediatr. Perinat. Epidemiol. 2018, 32, 40–51. [Google Scholar] [CrossRef]
- Nijsten, K.; Jansen, L.; Limpens, J.; Finken, M.; Koot, M.H.; Grooten, I.J.; Roseboom, T.J.; Painter, R.C. Long-term health outcomes of children born to mothers with hyperemesis gravidarum: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 227, 414–429. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.; van Dam, M.; Painter, R.C.; Postma, A. Wernicke’s encephalopathy in hyperemesis gravidarum: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 84–93. [Google Scholar]
- Austin, K.; Wilson, K.; Saha, S. Hyperemesis Gravidarum. Nutr. Clin. Pract. 2019, 34, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.W.; Hasler, W.L.; Nugent, C.E.; Owyang, C. Progesterone and estrogen are potential mediators of gastric slow-wave dysrhythmias in nausea of pregnancy. Am. J. Physiol. 1996, 270, G506–G514. [Google Scholar] [CrossRef]
- Jarnfelt-Samsioe, A.; Eriksson, B.; Waldenstrom, J.; Samsioe, G. Some new aspects on emesis gravidarum. Relations to clinical data, serum electrolytes, total protein and creatinine. Gynecol. Obstet. Investig. 1985, 19, 174. [Google Scholar]
- Flaxman, S.M.; Sherman, P.W. Morning sickness: A mechanism for protecting mother and embryo. Q. Rev. Biol. 2000, 75, 113. [Google Scholar] [CrossRef]
- Rosenberg, M.J.; Meyers, A.; Roy, V. Efficacy, cycle control, and side effects of low- and lower-dose oral contraceptives: A randomized trial of 20 μg and 35 μg estrogen preparations. Contraception 1999, 60, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, Q.; Wang, S.; Huang, X.; Jin, S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. Can. Med. Assoc. J. 2010, 182, 1857–1862. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef]
- Qin, H.; Lin, Z.; Vasquez, E.; Luan, X.; Guo, F.; Xu, L. High soy isoflavone or soy-based food intake during infancy and in adulthood is associated with an increased risk of uterine fibroids in premenopausal women: A meta-analysis. Nutr. Res. 2019, 71, 30–42. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, E.; Liu, R.; Zhang, Y.; Wang, C.; Gao, S.; Su, S.; Gao, X.; Wu, Q.; Yang, X.; et al. The China birth cohort study (CBCS). Eur. J. Epidemiol. 2022, 37, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, Z.; Shi, J.; Liu, S.; Li, L.; Ding, L.; Zhao, J.; Pan, Y.; Lei, H.; He, T.; et al. The association between prepregnancy dietary fatty acids and risk of gestational diabetes mellitus: A prospective cohort study. Clin. Nutr. 2024, 43, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.L.; Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2019; p. 695. [Google Scholar]
- Cheng, Y.; Yan, H.; Dibley, M.J.; Shen, Y.; Li, Q.; Zeng, L. Validity and reproducibility of a semi-quantitative food frequency questionnaire for use among pregnant women in rural China. Asia Pac. J. Clin. Nutr. 2008, 17, 166–177. [Google Scholar]
- Cheng, Y.; Dibley, M.J.; Zhang, X.; Zeng, L.; Yan, H. Assessment of dietary intake among pregnant women in a rural area of western China. BMC Public Health 2009, 9, 222. [Google Scholar] [CrossRef]
- Birkeland, E.; Stokke, G.; Tangvik, R.J.; Torkildsen, E.A.; Boateng, J.; Wollen, A.L.; Albrechtsen, S.; Flaatten, H.; Trovik, J. Norwegian PUQE (Pregnancy-Unique Quantification of Emesis and nausea) identifies patients with hyperemesis gravidarum and poor nutritional intake: A prospective cohort validation study. PLoS ONE 2015, 10, e119962. [Google Scholar] [CrossRef]
- Koren, G.; Boskovic, R.; Hard, M.; Maltepe, C.; Navioz, Y.; Einarson, A. Motherisk-PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am. J. Obstet. Gynecol. 2002, 186, S228–S231. [Google Scholar] [CrossRef]
- Elkins, J.R.; Oxentenko, A.S.; Nguyen, L.A.B. Hyperemesis Gravidarum and Nutritional Support. Am. J. Gastroenterol. 2022, 117, 2–9. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists (ACOG). Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy. Obstet. Gynecol. 2018, 131, e15–e30. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Piercy, C.; Dean, C.; Shehmar, M.; Gadsby, R.; O’Hara, M.; Hodson, K.; Nana, M. The Management of Nausea and Vomiting in Pregnancy and Hyperemesis Gravidarum (Green-top Guideline No. 69). BJOG Int. J. Obstet. Gynaecol. 2024, 131, e1–e30. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.F.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Park, K. Association between Soy Food and Dietary Soy Isoflavone Intake and the Risk of Cardiovascular Disease in Women: A Prospective Cohort Study in Korea. Nutrients 2021, 13, 1407. [Google Scholar] [CrossRef]
- Woo, H.W.; Kim, M.K.; Lee, Y.H.; Shin, D.H.; Shin, M.H.; Choi, B.Y. Sex-specific associations of habitual intake of soy protein and isoflavones with risk of type 2 diabetes. Clin. Nutr. 2021, 40, 127–136. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Soy product intake and premenopausal hysterectomy in a follow-up study of Japanese women. Eur. J. Clin. Nutr. 2001, 55, 773–777. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.C.; Lin, F.; Cheng, S.; Fu, J.; Chen, Y. Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci. 2010, 101, 501–507. [Google Scholar] [CrossRef]
- Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur. J. Epidemiol. 2020, 35, 567–578. [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Tokinobu, A.; Arakawa, M. Maternal consumption of soy and isoflavones during pregnancy and risk of childhood behavioural problems: The Kyushu Okinawa Maternal and Child Health Study. Int. J. Food Sci. Nutr. 2021, 72, 1118–1127. [Google Scholar] [CrossRef]
- Chan, S.G.; Ho, S.C.; Kreiger, N.; Darlington, G.; So, K.F.; Chong, P.Y. Dietary sources and determinants of soy isoflavone intake among midlife Chinese Women in Hong Kong. J. Nutr. 2007, 137, 2451–2455. [Google Scholar]
- Carson, D.A.; Bhat, S.; Hayes, T.; Gharibans, A.A.; Andrews, C.N.; O’Grady, G.; Varghese, C. Abnormalities on Electrogastrography in Nausea and Vomiting Syndromes: A Systematic Review, Meta-Analysis, and Comparison to Other Gastric Disorders. Dig. Dis. Sci. 2022, 67, 773–785. [Google Scholar]
- Munawwarah, S.; Nanda, K.R.; Hasriantirisna, H.; Yahya, F.D. Supplementary Feeding (Soy Biscuits) on the Frequency of Nausea and Vomiting of Pregnant Women Experiencing Hyperemesis Gravidarum in Health Crisis Situations. Adv. Healthc. Res. 2025, 3, 45–59. [Google Scholar]
- Pepper, G.V.; Craig, R.S. Rates of nausea and vomiting in pregnancy and dietary characteristics across populations. Proc. R. Soc. B-Biol. Sci. 2006, 273, 2675–2679. [Google Scholar]
- Crozier, S.R.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Nausea and vomiting in early pregnancy: Effects on food intake and diet quality. Matern. Child Nutr. 2017, 13, e12389. [Google Scholar] [PubMed]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun, A.M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar]
- Verberg, M.F.; Gillott, D.J.; Al-Fardan, N.; Grudzinskas, J.G. Hyperemesis gravidarum, a literature review. Hum. Reprod. Update 2005, 11, 527–539. [Google Scholar]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar]
- Beyazit, F.; Turkon, H.; Pek, E.; Ozturk, F.H.; Unsal, M. Elevated circulating nitric oxide levels correlates with enhanced oxidative stress in patients with hyperemesis gravidarum. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2018, 38, 668–673. [Google Scholar] [CrossRef]
- Simsek, Y.; Simsek, G.; Bayar, M.N.; Arikan, O.K. Olfactory dysfunction and oxidative stress in pregnant women with hyperemesis gravidarum. Arch. Gynecol. Obstet. 2021, 304, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wei, H. Effect of dietary genistein on antioxidant enzyme activities in SENCAR mice. Nutr. Cancer 1996, 25, 1–7. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Zhao, J.; Chen, Y. Genistein improves inflammatory response and colonic function through NF-kappaB signal in DSS-induced colonic injury. Oncotarget 2017, 8, 61385–61392. [Google Scholar] [CrossRef]
- Metzger, C.E.; Narayanan, S.A.; Zawieja, D.C.; Bloomfield, S.A. A moderately elevated soy protein diet mitigates inflammatory changes in gut and in bone turnover during chronic TNBS-induced inflammatory bowel disease. Appl. Physiol. Nutr. Metab. 2019, 44, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Juritsch, A.F.; Moreau, R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr. Rev. 2018, 76, 618–638. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Wopperer, A.L.; Chrisfield, B.J.; Tao, L.; Cooper, T.K.; Vanamala, J.; Elias, R.J.; Hayes, J.E.; Lambert, J.D. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo. J. Nutr. Biochem. 2017, 40, 201–208. [Google Scholar] [CrossRef]
- Grooten, I.J.; Den Hollander, W.J.; Roseboom, T.J.; Kuipers, E.J.; Jaddoe, V.W.; Gaillard, R.; Painter, R.C. Helicobacter pylori infection: A predictor of vomiting severity in pregnancy and adverse birth outcome. Am. J. Obstet. Gynecol. 2017, 216, 511–512. [Google Scholar]
- Shaban, M.M.; Kandil, H.O.; Elshafei, A.H. Helicobacter pylori seropositivity in patients with hyperemesis gravidarum. Am. J. Med. Sci. 2014, 347, 101–105. [Google Scholar] [CrossRef]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen genistein as an anti-staphylococcal agent. Microbes Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Kim, D.H. In vitro anti-Helicobacter pylori activity of irisolidone isolated from the flowers and rhizomes of Pueraria thunbergiana. Planta Med. 2001, 67, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 1244S–1249S. [Google Scholar] [CrossRef]
- Agyei, D. Bioactive Proteins and Peptides from Soybeans. Recent Pat. Food Nutr. Agric. 2015, 7, 100–107. [Google Scholar] [CrossRef]
- Montgomery, K.S. Soy protein. J. Perinat. Educ. 2003, 12, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Jednak, M.A.; Shadigian, E.M.; Kim, M.S.; Woods, M.L.; Hooper, F.G.; Owyang, C.; Hasler, W.L. Protein meals reduce nausea and gastric slow wave dysrhythmic activity in first trimester pregnancy. Am. J. Physiol. 1999, 277, G855–G861. [Google Scholar] [CrossRef]
- Waliat, S.; Arshad, M.S.; Hanif, H.; Ejaz, A.; Khalid, W.; Kauser, S.; Al-Farga, A. A review on bioactive compounds in sprouts: Extraction techniques, food application and health functionality. Int. J. Food Prop. 2023, 26, 647–665. [Google Scholar] [CrossRef]
- Chon, S.U. Total polyphenols and bioactivity of seeds and sprouts in several legumes. Curr. Pharm. Des. 2013, 19, 6112–6124. [Google Scholar] [CrossRef]
- Hu, F. Vitamin D and hyperemesis gravidarum: A mendelian randomization study. J. Gynecol. Obstet. Hum. Reprod. 2023, 52, 102678. [Google Scholar] [CrossRef]
- Hemati, N.; Asis, M.; Moradi, S.; Mollica, A.; Stefanucci, A.; Nikfar, S.; Mohammadi, E.; Farzaei, M.H.; Abdollahi, M. Effects of genistein on blood pressure: A systematic review and meta-analysis. Food Res. Int. 2020, 128, 108764. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wang, T.; Wang, Y.; Zhao, L.; Fang, Y. The effect of genistein on glucose control and insulin sensitivity in postmenopausal women: A meta-analysis. Maturitas 2017, 97, 44–52. [Google Scholar] [PubMed]
- Murphy, P.A.; Barua, K.; Hauck, C.C. Solvent extraction selection in the determination of isoflavones in soy foods. J. Chromatogr. B 2002, 777, 129–138. [Google Scholar]
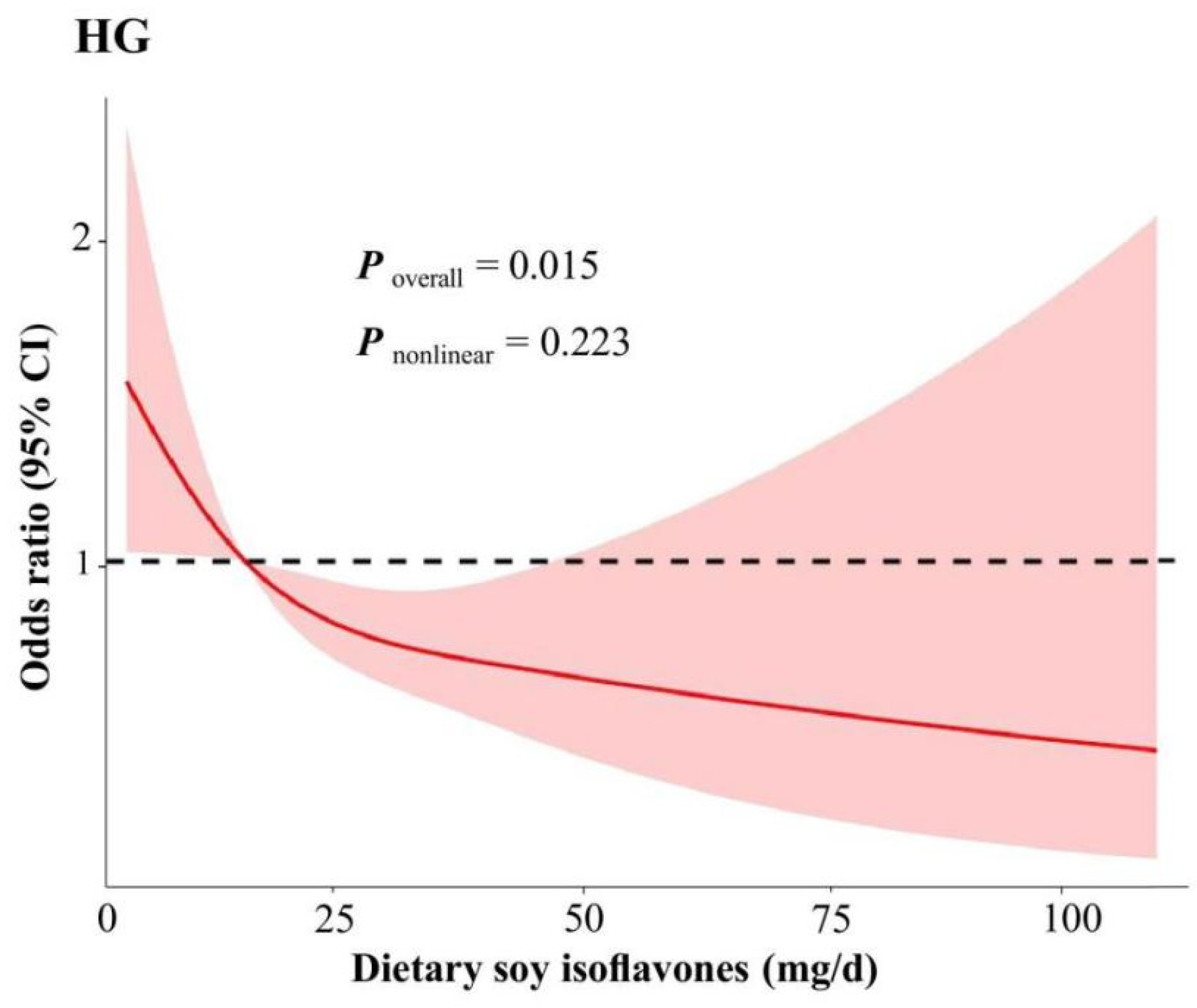
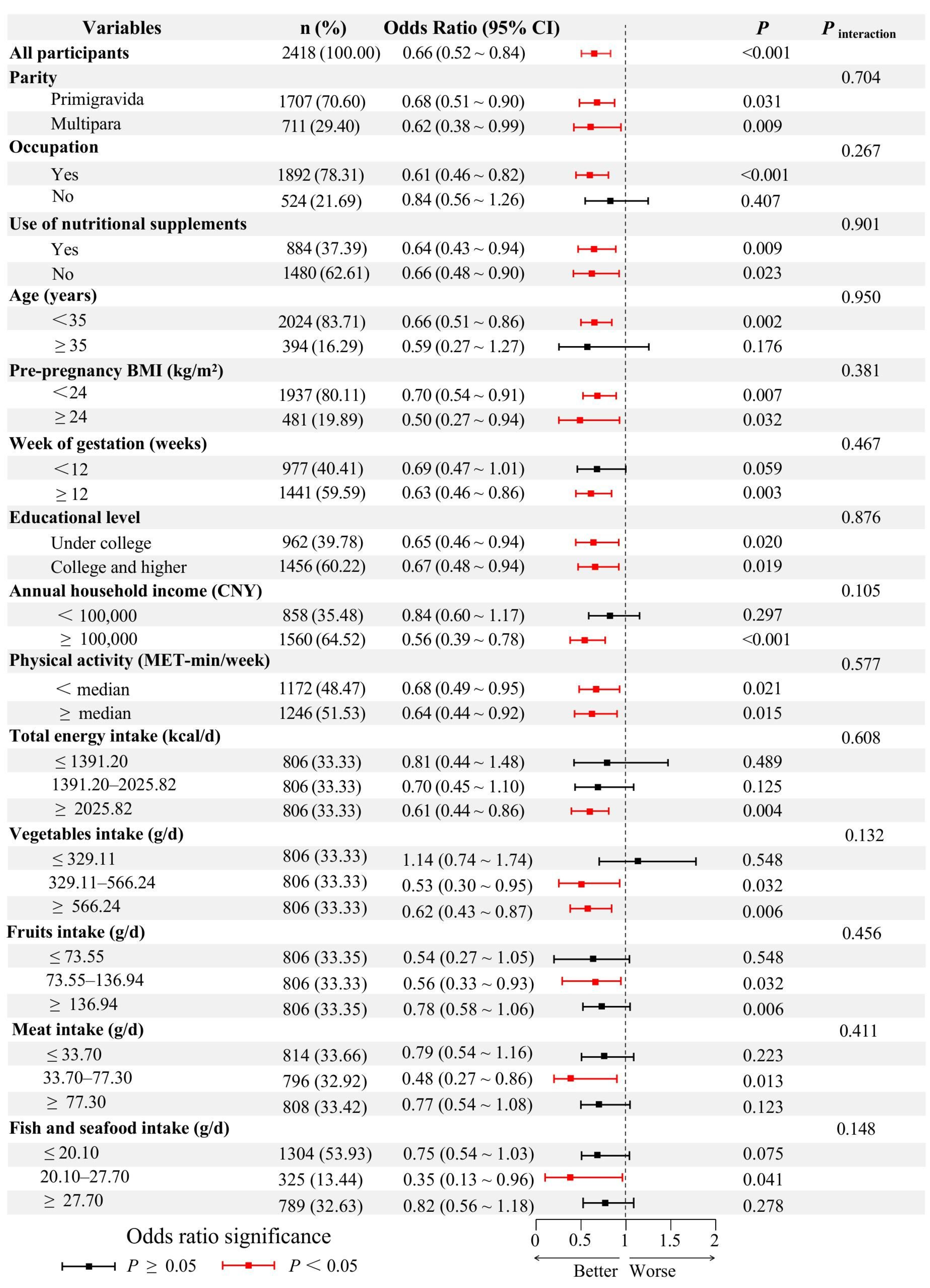
| Characteristic | Quartiles of Dietary Soy Isoflavones Intake | p | |||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | ||
| No. of participants (n) | 605 | 604 | 604 | 605 | |
| Age (years), mean ± SD | 30.7 ± 3.4 | 31.4 ± 3.7 | 31.4 ± 3.4 | 31.2 ± 3.3 | 0.001 |
| Pre-pregnancy BMI (kg/m2), mean ± SD | 21.8 ± 3.3 | 21.8 ± 3.2 | 21.9 ± 3.4 | 21.9 ± 3.9 | 0.932 |
| Week of gestation (weeks), median (IQR) | 12.0 (8.3, 12.5) | 12.2 (10.2, 12.7) | 12.2 (10.2, 12.7) | 12.0 (9.3, 12.7) | <0.001 |
| Physical activity (MET-min/week), median (IQR) | 17.26 (11.31, 19.64) | 17.27 (11.10, 19.33) | 17.27 (11.43, 19.60) | 17.27 (11.31, 19.72) | 0.879 |
| Total energy intake (kcal/d), median (IQR) | 1863.13 (1428.04, 2313.11) | 1318.75 (1015.86, 1871.18) | 1560.38 (1234.65, 2044.45) | 1915.35 (1501.38, 2557.71) | <0.001 |
| Vegetables intake (g/day), median (IQR) | 325.85 (222.92, 478.39) | 342.32 (225.98, 510.91) | 470.08 (329.48, 652.87) | 629.29 (456.79, 914.70) | <0.001 |
| Fruits intake (g/day), median (IQR) | 80.68 (48.43, 131.72) | 80.89 (49.66, 132.75) | 106.97 (70.08, 171.11) | 142.45 (92.63, 220.95) | <0.001 |
| Meat intake (g/day), median (IQR) | 54.70 (28.50, 90.90) | 43.70 (26.10, 77.30) | 59.70 (31.10, 95.90) | 77.30 (38.70, 130.50) | <0.001 |
| Fish and seafood intake (g/day), median (IQR) | 20.10 (15.10, 30.30) | 20.10 (15.10, 27.70) | 20.10 (15.10, 41.00) | 27.70 (20.10, 42.90) | <0.001 |
| Occupation (yes), n (%) | 460 (76.16) | 476 (78.81) | 467 (77.45) | 489 (80.83) | 0.235 |
| Use of nutritional supplements (yes), n (%) | 210 (35.41) | 221 (37.39) | 215 (36.82) | 238 (39.93) | 0.437 |
| Smoking (yes), n (%) | 25 (4.13) | 20 (3.31) | 17 (2.81) | 16 (2.64) | 0.460 |
| Alcohol consumption (yes), n (%) | 29 (4.79) | 22 (3.64) | 16 (2.65) | 14 (2.31) | 0.073 |
| Educational level, n (%) | 0.164 | ||||
| Under college | 258 (42.64) | 227 (37.58) | 227 (37.58) | 250 (41.32) | |
| College and higher | 347 (57.36) | 377 (62.42) | 377 (62.42) | 355 (58.68) | |
| Annual household income, CNY, n (%) | 0.959 | ||||
| <100,000 | 217 (35.87) | 218 (36.09) | 213 (35.26) | 210 (34.71) | |
| ≥100,000 | 388 (64.13) | 386 (63.91) | 391 (64.74) | 395 (65.29) | |
| Parity, n (%) | 0.045 | ||||
| Primigravida | 449 (74.21) | 405 (67.05) | 420 (69.54) | 433 (71.57) | |
| Multipara | 156 (25.79) | 199 (32.95) | 184 (30.46) | 172 (28.43) | |
| Variables | Participants (%) | OR (95% CI) | p |
|---|---|---|---|
| Age (continuous) | 2418 (100.00) | 0.98 (0.93, 1.02) | 0.297 |
| Week of gestation (continuous) | 2418 (100.00) | 1.15 (1.07, 1.24) | <0.001 |
| Physical activity (continuous) | 2418 (100.00) | 1.01 (0.98, 1.03) | 0.449 |
| Pre-pregnancy BMI a (<24 kg/m2/≥24 kg/m2) | 1937/481 (80.11/19.89) | 0.99 (0.70, 1.42) | 0.975 |
| Educational level b (Under college/College and higher) | 962/1456 (39.78/60.22) | 0.85 (0.64, 1.13) | 0.261 |
| Annual household income c (<100,000 CNY/≥100,000 CNY) | 858/1560 (35.48/64.52) | 0.84 (0.63, 1.12) | 0.243 |
| Parity d (Primigravida/Multipara) | 1707/711 (70.60/29.40) | 0.97 (0.71, 1.32) | 0.833 |
| Use of nutritional supplements e (no/yes) | 884/1480 (37.39/62.61) | 1.11 (0.83, 1.50) | 0.475 |
| Smoking e (no/yes) | 2340/78 (94.32/5.68) | 1.20 (0.57, 2.52) | 0.637 |
| Alcohol consumption e (no/yes) | 2337/81 (96.65/3.35) | 0.53 (0.19, 1.47) | 0.223 |
| Total energy intake | |||
| ≤1391.20 kcal/day | 806 (33.33) | 1.00 (Ref) | |
| 1391.20–2025.82 kcal/day | 806 (33.33) | 1.09 (0.76, 1.55) | 0.648 |
| ≥2025.82 kcal/day | 806 (33.33) | 1.20 (0.85, 1.69) | 0.296 |
| Vegetables intake | |||
| ≤329.11 g/day | 806 (33.33) | 1.00 (Ref) | |
| 329.11–566.24 g/day | 806 (33.33) | 0.81 (0.55, 1.09) | 0.140 |
| ≥566.24 g/day | 806 (33.33) | 0.81 (0.58, 1.14) | 0.228 |
| Fruits intake | |||
| ≤73.55 g/day | 806 (33.33) | 1.00 (Ref) | |
| 73.55–136.94 g/day | 806 (33.33) | 0.79 (0.56, 1.12) | 0.189 |
| ≥136.94 g/day | 806 (33.33) | 0.90 (0.64, 1.26) | 0.547 |
| Meat intake | |||
| ≤33.70 g/day | 814 (33.66) | 1.00 (Ref) | |
| 33.70–77.30 g/day | 796 (32.92) | 0.61 (0.44, 0.86) | 0.004 |
| ≥77.30 g/day | 808 (33.42) | 0.56 (0.39, 0.79) | 0.001 |
| Fish and seafood intake | |||
| ≤20.10 g/day | 1304 (53.93) | 1.00 (Ref) | |
| 20.10–27.70 g/day | 728 (13.44) | 0.71 (0.46, 1.11) | 0.140 |
| ≥27.70 g/day | 189 (32.63) | 0.50, (0.35, 0.71) | <0.001 |
| Quartiles of Dietary Soy Isoflavones Intake (OR, 95% CI) | Ptrend a | ||||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | ||
| Median (IQR), mg/d | 7.60 (5.89, 8.77) | 11.93 (10.87, 12.99) | 18.18 (16.19, 21.65) | 36.34 (30.15, 46.69) | |
| Case/total | 69/605 | 53/604 | 52/604 | 38/605 | |
| Unadjusted Model | 1.00 (Ref) | 0.74 (0.51, 1.09) | 0.73 (0.50, 1.07) | 0.52 (0.34, 0.79) | <0.001 |
| Partially Adjusted Model | 1.00 (Ref) | 0.68 (0.46, 1.01) | 0.69 (0.47, 1.01) | 0.51 (0.33, 0.77) | <0.001 |
| Fully Adjusted Model | 1.00 (Ref) | 0.76 (0.47, 1.15) | 0.77 (0.52, 1.16) | 0.56 (0.36, 0.88) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, X.; Zhang, L.; Cheng, W.; Jin, Y.; Ma, Q.; Ma, L.; Zhang, S.; Lin, J. Association Between Dietary Soy Isoflavones Intake and the Risk of Hyperemesis Gravidarum: A Cross-Sectional Study in Chinese Pregnant Women. Nutrients 2025, 17, 1282. https://doi.org/10.3390/nu17071282
Chen S, Zhang X, Zhang L, Cheng W, Jin Y, Ma Q, Ma L, Zhang S, Lin J. Association Between Dietary Soy Isoflavones Intake and the Risk of Hyperemesis Gravidarum: A Cross-Sectional Study in Chinese Pregnant Women. Nutrients. 2025; 17(7):1282. https://doi.org/10.3390/nu17071282
Chicago/Turabian StyleChen, Siyang, Xinyu Zhang, Lan Zhang, Wenjie Cheng, Yuan Jin, Qian Ma, Le Ma, Shunming Zhang, and Jing Lin. 2025. "Association Between Dietary Soy Isoflavones Intake and the Risk of Hyperemesis Gravidarum: A Cross-Sectional Study in Chinese Pregnant Women" Nutrients 17, no. 7: 1282. https://doi.org/10.3390/nu17071282
APA StyleChen, S., Zhang, X., Zhang, L., Cheng, W., Jin, Y., Ma, Q., Ma, L., Zhang, S., & Lin, J. (2025). Association Between Dietary Soy Isoflavones Intake and the Risk of Hyperemesis Gravidarum: A Cross-Sectional Study in Chinese Pregnant Women. Nutrients, 17(7), 1282. https://doi.org/10.3390/nu17071282








