Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges
Abstract
1. Introduction
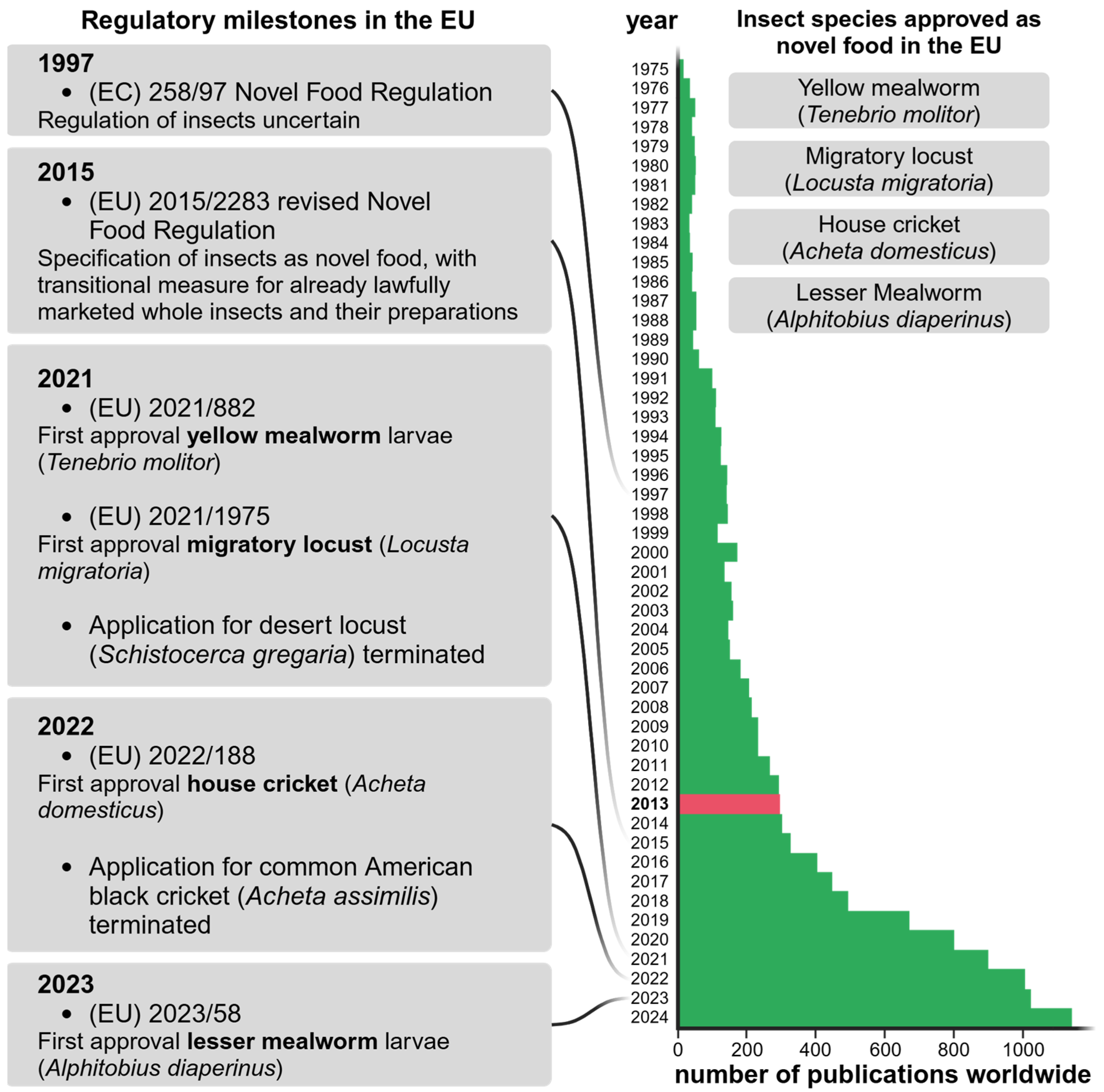
2. Regulations in the European Union
3. About the Success of Insects as Novel Foods
3.1. Challenges of Insect Foods in Western Countries
3.2. Edible Insects Around the World
3.3. Benefits of Eating Insects
4. Nutritional Profile of Edible Insects
| Food | Energy (kcal/100 g) | Protein (g/100 g) | Fat (g/100 g) | Carbohydrates (g/100 g) | Fibre (g/100 g) | Reference |
|---|---|---|---|---|---|---|
| Coleoptera (beetles) 1) | 490.30 | 40.69 | 33.40 | 13.20 2) | 10.74 | [15] |
| Tenebrio molitor larvae 1) | 557.12 | 48.35 | 38.51 | 4.82 2) | 8.48 | [15] |
| Alphitobius diaperinus larvae 1) | n.d. | 48.60 | 29.60 | n.d. | 4.60 | [70,71] |
| Orthoptera (crickets, locusts) 1) | 426.25 | 61.32 | 13.41 | 12.98 2) | 9.55 | [15] |
| Acheta domesticus 1) | 455.19 | 67.22 | 21.14 | 4.57 2) | 19.18 | [15] |
| Locusta migratoria 1) | 512.34 | 65.87 | 23.81 | 1.50 2) | 12.78 | [72,73] |
| Chicken, fresh meat, raw | 165.87 | 19.90 | 9.60 | 0 | 0 | [66] |
| Pork, fresh meat, raw | 231.36 | 17.11 | 18.31 | 0 | 0 | [66] |
| Beef, fresh meat, raw | 155.59 | 19.60 | 8.58 | 0 | 0 | [66] |
| Fish, fresh, raw | 99.67 | 19.32 | 2.39 | 0 | 0 | [66] |
| Soybean, dried | 386.47 | 38.20 | 18.27 | 6.29 | 21.96 | [66] |
| Wheat flour | 348.47 | 10.04 | 0.98 | 72.34 | 2.75 | [66] |
| Fungi, dried | 291.11 | 48.24 | 2.92 | 6.58 | 22.32 | [66] |
4.1. Proteins and Amino Acid Profile
4.2. Lipids
4.3. Micronutrients and Bioactive Compounds
5. Bioavailability
6. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Year of Application | Status of Application | Product and Species | EU Application Number | EFSA Question Number and Output |
|---|---|---|---|---|
| 2018 | Approval as a novel food in 2023 (EU) 2023/58 | Frozen and freeze-dried forms of lesser mealworm larvae (Alphitobius diaperinus) | NF 2018/0125 [21] | EFSA-Q-2018-00282 [22,106] |
| 2018 | Ongoing/positive EFSA safety assessment available | Frozen, dried, and powder forms of house crickets (Acheta domesticus) | NF 2018/0128 [107] | EFSA-Q-2018-00543 [108,109] |
| 2018 | Approval as a novel food in 2021 (EU) 2021/882 | Dried yellow mealworms (Tenebrio molitor) | NF 2018/0241 [110] | EFSA-Q-2018-00262 [111,112] |
| 2018 | Application withdrawn in 2022 | Dried tropical house crickets (Gryllodes sigillatus) | NF 2018/0260 [17] | EFSA-Q-2018-00263 [18] |
| 2018 | Ongoing EFSA risk assessment | Heat-treated locust nymphs or adults of migratory locusts (Locusta migratoria) | NF 2018/0395 [113] | EFSA-Q-2018-00513 [114] |
| 2018 | Ongoing/positive EFSA safety assessment available | Frozen and dried forms of whole yellow mealworm larvae (Tenebrio molitor) | NF 2018/0396 [115] | EFSA-Q-2018-00746 [116,117] |
| 2018 | Ongoing EFSA risk assessment | Honeybee drone brood (Apis mellifera) | NF 2018/0754 [19] | EFSA-Q-2019-0020 [20] |
| 2018 | Application withdrawn in 2024 | Black soldier fly (Hermetia illucens) meal | NF 2018/0765 [118] | EFSA-Q-2019-00046 [119] |
| 2018 | Approval as a novel food in 2022 (EU) 2022/169 | Frozen, dried, and powder forms of yellow mealworm larvae (Tenebrio molitor) | NF 2018/0802 [120] | EFSA-Q-2019-00101 [121,122] |
| 2018 | Approval as a novel food in 2021 (EU) 2021/1975 | Whole and ground migratory locusts (Locusta migratoria) | NF 2018/0803 [123] | EFSA-Q-2019-00115 [124,125] |
| 2018 | Approval as a novel food in 2022 (EU) 2022/188 | Whole and ground house crickets (Acheta domesticus) | NF 2018/0804 [126] | EFSA-Q-2019-00121 [127,128] |
| 2019 | Approval as a novel food in 2023 (EU) 2023/5 | Defatted whole house cricket powder (Acheta domesticus) | NF 2019/1227 [129] | EFSA-Q-2019-00589 [130,131] |
| 2019 | Approval as a novel food in 2025 (EU) 2025/89 | UV-treated powder of whole yellow mealworm larvae (Tenebrio molitor) | NF 2019/1142 [132] | EFSA-Q-2019-00748 [82,133] |
| 2020 | Ongoing/positive EFSA safety assessment available | House cricket powder (Acheta domesticus) | NF 2020/1860 [134] | EFSA-Q-2021-00262 [135,136] |
| 2020 | Ongoing EFSA risk assessment | Protein-rich flour from yellow mealworm larvae (Tenebrio molitor) | NF 2020/1959 [137] | EFSA-Q-2021-00105 [138] |
| 2021 | Ongoing EFSA risk assessment | Vitamin D3-containing UV-treated mealworm oil | NF 2021/0039 [139] | EFSA-Q-2022-00534 [140] |
| 2023 | Ongoing EFSA risk assessment | Dried defatted powder of Hermetia Illucens larvae | NF 2023/15216 [141] | EFSA-Q-2023-00703 [26] |
References
- van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO, Ed.; FAO Forestry Paper; FAO: Rome, Italy, 2013; ISBN 978-92-5-107595-1.
- Evans, J.; Alemu, M.H.; Flore, R.; Frøst, M.B.; Halloran, A.; Jensen, A.B.; Maciel-Vergara, G.; Meyer-Rochow, V.B.; Münke-Svendsen, C.; Olsen, S.B.; et al. ‘Entomophagy’: An Evolving Terminology in Need of Review. J. Insects Food Feed 2015, 1, 293–305. [Google Scholar] [CrossRef]
- Su, Z.-H.; Sasaki, A.; Minami, H.; Ozaki, K. Arthropod Phylotranscriptomics With a Special Focus on the Basal Phylogeny of the Myriapoda. Genome Biol. Evol. 2024, 16, evae189. [Google Scholar] [CrossRef]
- Giribet, G.; Edgecombe, G.D. The Phylogeny and Evolutionary History of Arthropods. Curr. Biol. 2019, 29, R592–R602. [Google Scholar] [CrossRef] [PubMed]
- Sadava, D.E.; Hillis, D.M.; Heller, H.C.; Hacker, S.D. Purves Die Arthropoden sind die artenreichste aller Tiergruppen. In Purves Biologie; Markl, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 963–979. ISBN 978-3-662-58171-1. [Google Scholar]
- Celniker, S.E.; Rubin, G.M. The Drosophila Melanogaster Genome. Annu. Rev. Genomics Hum. Genet. 2003, 4, 89–117. [Google Scholar] [CrossRef]
- Van Itterbeeck, J.; Pelozuelo, L. How Many Edible Insect Species Are There? A Not So Simple Question. Diversity 2022, 14, 143. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World. Wagening. Univ. Neth. 2017. Available online: https://www.wur.nl/en/research-results/chair-groups/plant-sciences/laboratory-of-entomology/edible-insects/worldwide-species-list.htm (accessed on 3 December 2024).
- Liceaga, A.M.; Aguilar-Toalá, J.E.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Insects as an Alternative Protein Source. Annu. Rev. Food Sci. Technol. 2022, 13, 19–34. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on Insects as Food and Feed: A Global Comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Van Huis, A.; Halloran, A.; Van Itterbeeck, J.; Klunder, H.; Vantomme, P. How Many People on Our Planet Eat Insects: 2 Billion? J. Insects Food Feed 2022, 8, 1–4. [Google Scholar] [CrossRef]
- Van Huis, A.; Rumpold, B. Strategies to Convince Consumers to Eat Insects? A Review. Food Qual. Prefer. 2023, 110, 104927. [Google Scholar] [CrossRef]
- Meyer-Rochow, V. Can Insects Help to Ease the Problem of World Food Shortage? Search 1975, 6, 261–262. [Google Scholar]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- EFSA Scientific Committee Risk Profile Related to Production and Consumption of Insects as Food and Feed. EFSA J. 2015, 13, 4257. [CrossRef]
- SAS EAP Group—MICRONUTRIS Summary of the Dossier: Dried Crickets (Gryllodes sigillatus) 2018-0260. Available online: https://food.ec.europa.eu/document/download/aab48a45-43ca-46af-a5c6-66316e27e9e7_en?filename=novel-food_sum_ongoing-app_2018-0260.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Dried Crickets (Gryllodes sigillatus) as a Novel Food (NF 2018/0260). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2018-00263 (accessed on 22 January 2025).
- Finnish Beekeepers’ Association Summary of the Dossier: Honye Bee Drone Brood (Apis mellifera Male Pupae) 2018-0754. Available online: https://food.ec.europa.eu/document/download/2fcd9057-70f8-4704-8885-9f3c20fb47c4_en?filename=novel-food_sum_ongoing-app_2018-0754.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Apis mellifera Male Pupae as a Novel Food (NF 2018/0754). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00201 (accessed on 22 January 2025).
- Ynsect NL, B.V. (Proti-Farm Holding NV) Summary of the Dossier: Whole and Grinded Alphitobius diaperinus Larvae Products 2018-0125. Available online: https://food.ec.europa.eu/document/download/6037c899-1013-4f58-955b-b05dba6b188f_en?filename=novel-food_sum_ongoing-app_2018-0125.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Frozen and Freeze-Dried Formulations of the Lesser Mealworm (Alphitobius diaperinus Larva) as a Novel Food (NF 2018/0125). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2018-00282 (accessed on 23 January 2025).
- European Union Summary of Applications and Notifications—European Commission. Available online: https://food.ec.europa.eu/food-safety/novel-food/authorisations/summary-applications-and-notifications_en (accessed on 17 December 2024).
- International Platform of Insects for Food & Feed (IPIFF) Information Note on the Establishment of EU Import Conditions for Insects Intended for Human Consumption. Available online: https://ipiff.org/wp-content/uploads/2020/09/Information-note-Imports-insects-intended-for-human-consumption-and-authorised-list-of-countries-28-09-2020.pdf (accessed on 22 January 2025).
- European Union Union List of Novel Foods—European Commission. Available online: https://food.ec.europa.eu/food-safety/novel-food/authorisations/union-list-novel-foods_en (accessed on 31 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Application for Authorisation of Dried Defatted Powder of Hermetia Illucens Larvae as a Novel Food (NF 2023/15216). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2023-00703 (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Application for Authorisation of Hermetia Illucens Larvae Refined Fat (Refined Black Soldier Fly Larvae Fat) as a Novel Food. Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2023-00847 (accessed on 22 January 2025).
- European Union Decisions Terminating the Procedure—European Commission. Available online: https://food.ec.europa.eu/food-safety/novel-food/decisions-terminating-procedure_en (accessed on 7 January 2025).
- Melgar-Lalanne, G.; Hernández-Álvarez, A.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef]
- Engström, A. The Eating Insects Startups: Here Is the List of Entopreneurs Around the World! Available online: https://www.bugburger.se/foretag/the-eating-insects-startups-here-is-the-list-of-entopreneurs-around-the-world/ (accessed on 4 February 2025).
- House, J. Insects Are Not ‘the New Sushi’: Theories of Practice and the Acceptance of Novel Foods. Soc. Cult. Geogr. 2019, 20, 1285–1306. [Google Scholar] [CrossRef]
- Mancini, S.; Sogari, G.; Espinosa Diaz, S.; Menozzi, D.; Paci, G.; Moruzzo, R. Exploring the Future of Edible Insects in Europe. Foods 2022, 11, 455. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Osei-Owusu, J.; Yunusa, B.M.; Rahayu, T.; Fernando, I.; Shah, M.A.; Centoducati, G. Prospects of Edible Insects as Sustainable Protein for Food and Feed—A Review. J. Insects Food Feed 2023, 10, 191–217. [Google Scholar] [CrossRef]
- Maciejewska, M.; Dąbrowska, A.; Cano-Lamadrid, M. Sustainable Protein Sources: Functional Analysis of Tenebrio molitor Hydrolysates and Attitudes of Consumers in Poland and Spain Toward Insect-Based Foods. Foods 2025, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Lammers, P.; Ullmann, L.M.; Fiebelkorn, F. Acceptance of Insects as Food in Germany: Is It about Sensation Seeking, Sustainability Consciousness, or Food Disgust? Food Qual. Prefer. 2019, 77, 78–88. [Google Scholar] [CrossRef]
- Detilleux, L.; Bayendi Loudit, S.; Le Gall, P.; Francis, F.; Caparros Megido, R.; Dogot, T. Consumers of Insect-Based Foods: A Cross-Cultural Study between Belgium and Gabon. J. Insect Sci. 2024, 24, 2. [Google Scholar] [CrossRef]
- Russell, P.S.; Knott, G. Encouraging Sustainable Insect-Based Diets: The Role of Disgust, Social Influence, and Moral Concern in Insect Consumption. Food Qual. Prefer. 2021, 92, 104187. [Google Scholar] [CrossRef]
- Ruby, M.B.; Rozin, P. Disgust, Sushi Consumption, and Other Predictors of Acceptance of Insects as Food by Americans and Indians. Food Qual. Prefer. 2019, 74, 155–162. [Google Scholar] [CrossRef]
- Florença, S.G.; Guiné, R.P.F.; Gonçalves, F.J.A.; Barroca, M.J.; Ferreira, M.; Costa, C.A.; Correia, P.M.R.; Cardoso, A.P.; Campos, S.; Anjos, O.; et al. The Motivations for Consumption of Edible Insects: A Systematic Review. Foods 2022, 11, 3643. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Kejonen, A. Could Western Attitudes towards Edible Insects Possibly Be Influenced by Idioms Containing Unfavourable References to Insects, Spiders and Other Invertebrates? Foods 2020, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Psarianos, M.; Schlüter, O.K.; Ojha, S. Protein from Insects—A New Biosphere of Opportunity. In Future Proteins; Elsevier: Amsterdam, The Netherlands, 2023; pp. 173–194. ISBN 978-0-323-91739-1. [Google Scholar]
- Van Peer, M.; Frooninckx, L.; Coudron, C.; Berrens, S.; Álvarez, C.; Deruytter, D.; Verheyen, G.; Van Miert, S. Valorisation Potential of Using Organic Side Streams as Feed for Tenebrio molitor, Acheta domesticus and Locusta migratoria. Insects 2021, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food Waste Valorisation and Circular Economy Concepts in Insect Production and Processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Schlüter, O.; Rumpold, B.; Holzhauser, T.; Roth, A.; Vogel, R.F.; Quasigroch, W.; Vogel, S.; Heinz, V.; Jäger, H.; Bandick, N.; et al. Safety Aspects of the Production of Foods and Food Ingredients from Insects. Mol. Nutr. Food Res. 2017, 61, 1600520. [Google Scholar] [CrossRef]
- Van Huis, A. Welfare of Farmed Insects. J. Insects Food Feed 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Jankowski, W.M.; Przychodniak, D.; Gromek, W.; Majsiak, E.; Kurowski, M. Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review. Foods 2025, 14, 270. [Google Scholar] [CrossRef]
- Ojha, S.; Bekhit, A.E.-D.; Grune, T.; Schlüter, O.K. Bioavailability of Nutrients from Edible Insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; ISBN 978-92-5-109551-5.
- United Nations Department for Economic and Social Affairs. World Population Prospects 2024: Summary of Results; United Nations: New York, NY, USA, 2024; ISBN 978-92-1-003169-1.
- Searchinger, T.D. Creating a Sustainable Food Future: A Menu of Solutions to Sustainably Feed More than 9 Billion People by 2050; World Resources Institute: Washington, DC, USA, 2019; ISBN 978-1-56973-963-1. [Google Scholar]
- Van Huis, A. Edible Insects: Challenges and Prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Future Proteins: Sources, Processing, Applications and the Bioeconomy; Tiwari, B.K., Healy, L.E., EBSCOhost, Eds.; Academic Press: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-91739-1. [Google Scholar]
- Weindl, I.; Ost, M.; Wiedmer, P.; Schreiner, M.; Neugart, S.; Klopsch, R.; Kühnhold, H.; Kloas, W.; Henkel, I.M.; Schlüter, O.; et al. Sustainable Food Protein Supply Reconciling Human and Ecosystem Health: A Leibniz Position. Glob. Food Secur. 2020, 25, 100367. [Google Scholar] [CrossRef]
- Yield and Nutritional Value or the Commercially More Important Fish Species; Torry Research Station, Ed.; FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1989; ISBN 978-92-5-102870-4. [Google Scholar]
- Van Raamsdonk, L.W.D.; Van Der Fels-Klerx, H.J.; De Jong, J. New Feed Ingredients: The Insect Opportunity. Food Addit. Contam. Part A 2017, 34, 1384–1397. [Google Scholar] [CrossRef]
- Glencross, B.; Fracalossi, D.M.; Hua, K.; Izquierdo, M.; Mai, K.; Øverland, M.; Robb, D.; Roubach, R.; Schrama, J.; Small, B.; et al. Harvesting the Benefits of Nutritional Research to Address Global Challenges in the 21st Century. J. World Aquac. Soc. 2023, 54, 343–363. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; De Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- De Vries, M.; De Boer, I.J.M. Comparing Environmental Impacts for Livestock Products: A Review of Life Cycle Assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for Food: A Water Footprint Perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. A Global Assessment of the Water Footprint of Farm Animal Products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3.
- Smil, V. Worldwide Transformation of Diets, Burdens of Meat Production and Opportunities for Novel Food Proteins. Enzyme Microb. Technol. 2002, 30, 305–311. [Google Scholar] [CrossRef]
- Vinci, G.; Prencipe, S.A.; Masiello, L.; Zaki, M.G. The Application of Life Cycle Assessment to Evaluate the Environmental Impacts of Edible Insects as a Protein Source. Earth 2022, 3, 925–938. [Google Scholar] [CrossRef]
- Plant Protein Foods; Manickavasagan, A., Lim, L.-T., Ali, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-91205-5. [Google Scholar]
- Max Rubner-Institut (MRI) Der Bundeslebensmittelschlüssel (BLS) 2020. Available online: https://www.blsdb.de/ (accessed on 22 February 2025).
- D-A-C-H-Referenzwerte Für Die Nährstoffzufuhr; Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Eds.; 2. Auflage, 7. aktualisierte Ausgabe 2021; Deutsche Gesellschaft für Ernährung: Bonn, Germany, 2021; ISBN 978-3-88749-261-8. [Google Scholar]
- Stull, V.J. Impacts of Insect Consumption on Human Health. J. Insects Food Feed 2021, 7, 695–713. [Google Scholar] [CrossRef]
- Van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional Qualities and Enhancement of Edible Insects. Annu. Rev. Nutr. 2021, 41, 551–576. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Leni, G.; Soetemans, L.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Bastiaens, L.; Caligiani, A.; Sforza, S. Protein Hydrolysates from Alphitobius Diaperinus and Hermetia Illucens Larvae Treated with Commercial Proteases. J. Insects Food Feed 2020, 6, 393–404. [Google Scholar] [CrossRef]
- Fombong, F.T.; Kinyuru, J.; Ng’ang’a, J.; Ayieko, M.; Tanga, C.M.; Vanden Broeck, J.; Van Der Borght, M. Affordable Processing of Edible Orthopterans Provides a Highly Nutritive Source of Food Ingredients. Foods 2021, 10, 144. [Google Scholar] [CrossRef]
- Purschke, B.; Tanzmeister, H.; Meinlschmidt, P.; Baumgartner, S.; Lauter, K.; Jäger, H. Recovery of Soluble Proteins from Migratory Locust (Locusta migratoria) and Characterisation of Their Compositional and Techno-Functional Properties. Food Res. Int. 2018, 106, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-Protein Conversion Factors for Edible Insects on the Swiss Market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- Jonas-Levi, A.; Martinez, J.-J.I. The High Level of Protein Content Reported in Insects for Food and Feed Is Overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- WHO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; World Health Organization: Albany, NY, USA, 2007; ISBN 978-92-4-120935-9. [Google Scholar]
- Elmadfa, I.; Leitzmann, C. Ernährung des Menschen; utb Ernährungswissenschaften, Ökotrophologie, Diätetik/Diätologie; 6., überarbeitete und aktualisierte Auflage; Verlag Eugen Ulmer: Stuttgart, Germany, 2019; ISBN 978-3-8252-8748-1. [Google Scholar]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible Insects as a Food Source: A Review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- DeFoliart, G. Insect Fatty Acids: Similar to Those of Poultry and Fish in Their Degree of Unsaturation, but Higher in the Polyunsaturates. Food Insects Newsl. 1991, 4, 1–4. [Google Scholar]
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on UV-Treated Powder of Whole Yellow Mealworm (Tenebrio molitor) Larvae (NF 2019/1142). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00748 (accessed on 23 January 2025).
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. A Systematic Review of Nutrient Composition Data Available for Twelve Commercially Available Edible Insects, and Comparison with Reference Values. Trends Food Sci. Technol. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Van Loon, J.J.A.; Van Loon, L.J.C. Consideration of Insects as a Source of Dietary Protein for Human Consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Ros-Baró, M.; Casas-Agustench, P.; Díaz-Rizzolo, D.A.; Batlle-Bayer, L.; Adrià-Acosta, F.; Aguilar-Martínez, A.; Medina, F.-X.; Pujolà, M.; Bach-Faig, A. Edible Insect Consumption for Human and Planetary Health: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 11653. [Google Scholar] [CrossRef]
- Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; Food and Agriculture Organization of the United Nations, Ed.; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6.
- Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, Bethesda, Md., USA; 4–8 December 1989; FAO, Weltgesundheitsorganisation, Eds.; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991; ISBN 978-92-5-103097-4.
- Malla, N.; Nørgaard, J.V.; Roos, N. Protein Quality of Edible Insects in the View of Current Assessment Methods. Anim. Front. 2023, 13, 50–63. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Sousa, R.; Recio, I.; Heimo, D.; Dubois, S.; Moughan, P.J.; Hodgkinson, S.M.; Portmann, R.; Egger, L. In Vitro Digestibility of Dietary Proteins and in Vitro DIAAS Analytical Workflow Based on the INFOGEST Static Protocol and Its Validation with in Vivo Data. Food Chem. 2023, 404, 134720. [Google Scholar] [CrossRef]
- Poelaert, C.; Francis, F.; Alabi, T.; Megido, R.C.; Crahay, B.; Bindelle, J.; Beckers, Y. Protein Value of Two Insects, Subjected to Various Heat Treatments, Using Growing Rats and the Protein Digestibility-Corrected Amino Acid Score. J. Insects Food Feed 2018, 4, 77–87. [Google Scholar] [CrossRef]
- Lampová, B.; Kopecká, A.; Šmíd, P.; Kulma, M.; Kurečka, M.; Ogrinc, N.; Heath, D.; Kouřimská, L.; Doskočil, I. Evaluating Protein Quality in Edible Insects: A Comparative Analysis of House Cricket, Yellow Mealworm, and Migratory Locust Using DIAAS Methodologies. LWT 2024, 213, 117062. [Google Scholar] [CrossRef]
- Hammer, L.; Moretti, D.; Abbühl-Eng, L.; Kandiah, P.; Hilaj, N.; Portmann, R.; Egger, L. Mealworm Larvae (Tenebrio molitor) and Crickets (Acheta domesticus) Show High Total Protein in Vitro Digestibility and Can Provide Good-to-Excellent Protein Quality as Determined by in Vitro DIAAS. Front. Nutr. 2023, 10, 1150581. [Google Scholar] [CrossRef]
- Jensen, L.D.; Miklos, R.; Dalsgaard, T.K.; Heckmann, L.H.; Nørgaard, J.V. Nutritional Evaluation of Common (Tenebrio molitor) and Lesser (Alphitobius diaperinus) Mealworms in Rats and Processing Effect on the Lesser Mealworm. J. Insects Food Feed 2019, 5, 257–266. [Google Scholar] [CrossRef]
- Ochiai, M.; Suzuki, Y.; Suzuki, R.; Iwata, K.; Murayama, M. Low Protein Digestibility-Corrected Amino Acid Score and Net Nitrogen-to-Protein Conversion Factor Value of Edible Insects. Food Chem. 2024, 454, 139781. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; House, J.D. Potential Impact of the Digestible Indispensable Amino Acid Score as a Measure of Protein Quality on Dietary Regulations and Health. Nutr. Rev. 2017, 75, 658–667. [Google Scholar] [CrossRef]
- Fanelli, N.S.; Bailey, H.M.; Thompson, T.W.; Delmore, R.; Nair, M.N.; Stein, H.H. Digestible Indispensable Amino Acid Score (DIAAS) Is Greater in Animal-Based Burgers than in Plant-Based Burgers If Determined in Pigs. Eur. J. Nutr. 2022, 61, 461–475. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for Digestible Indispensable Amino Acid Scores (DIAAS) for Some Dairy and Plant Proteins May Better Describe Protein Quality than Values Calculated Using the Concept for Protein Digestibility-Corrected Amino Acid Scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Bøgh, K.L.; Madsen, C.B. Food Allergens: Is There a Correlation between Stability to Digestion and Allergenicity? Crit. Rev. Food Sci. Nutr. 2016, 56, 1545–1567. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.; Barroso, F.G.; Fabrikov, D.; Sánchez-Muros, M.J. In Vitro Crude Protein Digestibility of Insects: A Review. Insects 2022, 13, 682. [Google Scholar] [CrossRef]
- Vangsoe, M.T.; Thogersen, R.; Bertram, H.C.; Heckmann, L.-H.L.; Hansen, M. Ingestion of Insect Protein Isolate Enhances Blood Amino Acid Concentrations Similar to Soy Protein in A Human Trial. Nutrients 2018, 10, 1357. [Google Scholar] [CrossRef]
- Hermans, W.J.; Senden, J.M.; Churchward-Venne, T.A.; Paulussen, K.J.; Fuchs, C.J.; Smeets, J.S.; Van Loon, J.J.; Verdijk, L.B.; Van Loon, L.J. Insects Are a Viable Protein Source for Human Consumption: From Insect Protein Digestion to Postprandial Muscle Protein Synthesis in Vivo in Humans: A Double-Blind Randomized Trial. Am. J. Clin. Nutr. 2021, 114, 934–944. [Google Scholar] [CrossRef]
- Dai, J.; Lov, J.; Martin-Arrowsmith, P.W.; Gritsas, A.; Churchward-Venne, T.A. The Acute Effects of Insect vs. Beef-Derived Protein on Postprandial Plasma Aminoacidemia, Appetite Hormones, Appetite Sensations, and Energy Intake in Healthy Young Men. Eur. J. Clin. Nutr. 2022, 76, 1548–1556. [Google Scholar] [CrossRef]
- Lanng, S.K.; Oxfeldt, M.; Pedersen, S.S.; Johansen, F.T.; Risikesan, J.; Lejel, T.; Bertram, H.C.; Hansen, M. Influence of Protein Source (Cricket, Pea, Whey) on Amino Acid Bioavailability and Activation of the mTORC1 Signaling Pathway after Resistance Exercise in Healthy Young Males. Eur. J. Nutr. 2023, 62, 1295–1308. [Google Scholar] [CrossRef]
- The State of Food Security and Nutrition in the World 2024; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: New York, NY, USA; WFP: Rome, Italy; WHO: Geneva, Switzerland, 2024; ISBN 978-92-5-138882-2.
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Frozen and Freeze-dried Formulations of the Lesser Mealworm (Alphitobius diaperinus Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07325. [Google Scholar] [CrossRef]
- Belgian Insect Industry Federation (BiiF) Summary of the Dossier: Frozen, Dried and Powder Forms of House Crickets (Acheta domesticus) 2018-0128. Available online: https://food.ec.europa.eu/document/download/fc1d368f-ce33-4e81-931e-e9e858f3fe75_en?filename=novel-food_sum_ongoing-app_2018-0128.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Acheta domesticus as a Novel Food (NF 2018/0128). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2018-00543 (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Cámara, M.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Jos, Á.; Maciuk, A.; Mangelsdorf, I.; et al. Safety of Frozen, Dried and Powder Forms of House Crickets (Acheta domesticus) as a Novel Food Pursuant. EFSA J. 2024, 22, e9101. [Google Scholar] [CrossRef]
- SAS EAP Group—MICRONUTRIS Summary of the Dossier: Dried Mealworms (Tenebrio molitor) 2018-0241. Available online: https://food.ec.europa.eu/document/download/f24ab911-2170-43a5-b058-17881db9ce44_en?filename=novel-food_sum_ongoing-app_2018-0241.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Dried Mealworms (Tenebrio molitor) as a Novel Food (NF 2018/0241). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2018-00262 (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Dried Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- Belgian Insect Industry Federation (BiiF) Summary of the Dossier: Migratory Locust (Locusta migratoria) 2018-0395. Available online: https://food.ec.europa.eu/document/download/c9581ad6-4456-494c-991e-a16d22a53d98_en?filename=novel-food_sum_ongoing-app_2018-0395.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Locusta migratoria as a Novel Food (NF 2018/0395). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2018-00513 (accessed on 23 January 2025).
- Belgian Insect Industry Federation (BiiF) Summary of the Dossier: Frozen and Dried Forms of Whole Yellow Mealworm (Tenebrio molitor Larva) 2018-0396. Available online: https://food.ec.europa.eu/document/download/23e24fd5-6890-4107-b84d-2ec7ce719605_en?filename=novel-food_sum_ongoing-app_2019-0396.pdf (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Cámara, M.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Jos, Á.; Maciuk, A.; Mangelsdorf, I.; et al. Safety of Frozen and Dried Forms of Whole Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2025, 23, e9155. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Mealworm (Tenebrio molitor) as a Novel Food (NF 2018/0396). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2018-00746 (accessed on 23 January 2025).
- Enorm Biofactory A/S Summary of the Dossier: Hermetia Meal 2018-0765. Available online: https://food.ec.europa.eu/document/download/e5edf5ac-5155-4072-a70c-54e8365fced6_en?filename=novel-food_sum_ongoing-app_2018-0765.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Hermetia illucens Meal as a Novel Food (NF 2018/0765). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00046 (accessed on 23 January 2025).
- Fair Insects BV (A Protix Company) Summary of the Dossier: Whole and Ground Mealworm (Tenebrio molitor) Larvae 2018-0802. Available online: https://food.ec.europa.eu/document/download/c293ee6b-b952-46dd-a1c0-28aab7174951_en?filename=novel-food_sum_ongoing-app_2018-0802.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Whole and Ground Mealworms (Tenebrio molitor) Larvae as a Novel Food (NF 2018/0802). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00101 (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Frozen and Dried Formulations from Whole Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06778. [Google Scholar] [CrossRef]
- Fair Insects BV (A Protix Company) Summary of the Dossier: Whole and Ground Grasshopper (Locusta migratoria) 2018-0803. Available online: https://food.ec.europa.eu/document/download/03af8cdd-56fc-468a-960b-c7fe358fb5c9_en?filename=novel-food_sum_ongoing-app_2018-0803.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Whole and Ground Grasshoppers (Locusta migratoria) as a Novel Food (NF 2018/0803). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00115 (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Frozen and Dried Formulations from Migratory Locust (Locusta migratoria) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06667. [Google Scholar] [CrossRef]
- Fair Insects BV (A Protix Company) Summary of the Dossier: Whole and Ground Cricket (Acheta domesticus) 2018-0804. Available online: https://food.ec.europa.eu/document/download/e696b4cd-6659-4d7c-a216-7e8a0e75bf58_en?filename=novel-food_sum_ongoing-app_2018-0804.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Whole and Ground Crickets (Acheta domesticus) as a Novel Food (NF 2018/0804). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00121 (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Frozen and Dried Formulations from Whole House Crickets (Acheta domesticus) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06779. [Google Scholar] [CrossRef]
- Cricket One No., Ltd. Summary of the Dossier: Defatted Whole Cricket (Acheta domesticus) Powder 2019-1227. Available online: https://food.ec.europa.eu/document/download/163beed7-d900-434a-beaa-0f9e208bea3a_en?filename=novel-food_sum_ongoing-app_2019-1227.pdf (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Partially Defatted House Cricket (Acheta domesticus) Powder as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07258. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Defatted Whole Cricket (Acheta domesticus) Powder as a Novel Food (NF 2019/1227). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2019-00589 (accessed on 23 January 2025).
- Nutri’Earth Summary of the Dossier: Mealworm (Tenebrio molitor) Flour 2019-1142. Available online: https://food.ec.europa.eu/document/download/531f0e98-9c78-41c0-a6e3-ba2a00ef9e5a_en?filename=novel-food_sum_ongoing-app_2019-1142.pdf (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA Panel); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of UV-treated Powder of Whole Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2023, 21, e08009. [Google Scholar] [CrossRef]
- Italian Cricket Farm S.r.l Summary of the Dossier: Cricket Flour Acheta domesticus 2020-1860. Available online: https://food.ec.europa.eu/document/download/1c0a3fa1-5284-419a-8a59-fbc889dec385_en?filename=novel-food_sum_ongoing-app_2020-1860.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Acheta domesticus Powder as a Novel Food (NF 2020/1860). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2021-00262 (accessed on 23 January 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Acheta Domesticus Powder as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8919. [Google Scholar] [CrossRef]
- Ynsect Summary of Application: Tenebrio molitor Protein Concentrate 2020-1959. Available online: https://food.ec.europa.eu/document/download/299034a8-ad5d-437e-be5e-e69ba334af85_en?filename=novel-food_sum_ongoing-app_2020-1959.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Request for a Scientific Opinion on Protein-Rich Flour from Fresh Larvae of Mealworm (Tenebrio molitor) as a Novel Food (NF 2020/1959). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2021-00105 (accessed on 23 January 2025).
- Nutri’Earth Summary of the Application: Vitamin D3 Containing UV-Treated Mealworm Oil 2021-0039. Available online: https://food.ec.europa.eu/document/download/3c1d5748-17e8-44ce-ab3d-a9d391db163e_en?filename=novel-food_sum_ongoing-app_2021-0039.pdf (accessed on 23 January 2025).
- European Food Safety Authority (EFSA) Open EFSA—Application for Authorisation of Vitamin D3 Containing UV-Treated Mealworm Oil as a Novel Food. Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2022-00534 (accessed on 23 January 2025).
- InnovaFeed SAS Summary of Dossier: Dried Defatted Powder of Hermetia illucens Larvae 2023-15216. Available online: https://food.ec.europa.eu/document/download/b904232d-a776-4a6a-93ed-449c6ede6774_en?filename=novel-food_sum_ongoing-app_2023-15216.pdf (accessed on 23 January 2025).

| Benefits | Challenges |
|---|---|
| Worldwide traditions of eating wild edible insects | (International) legislation |
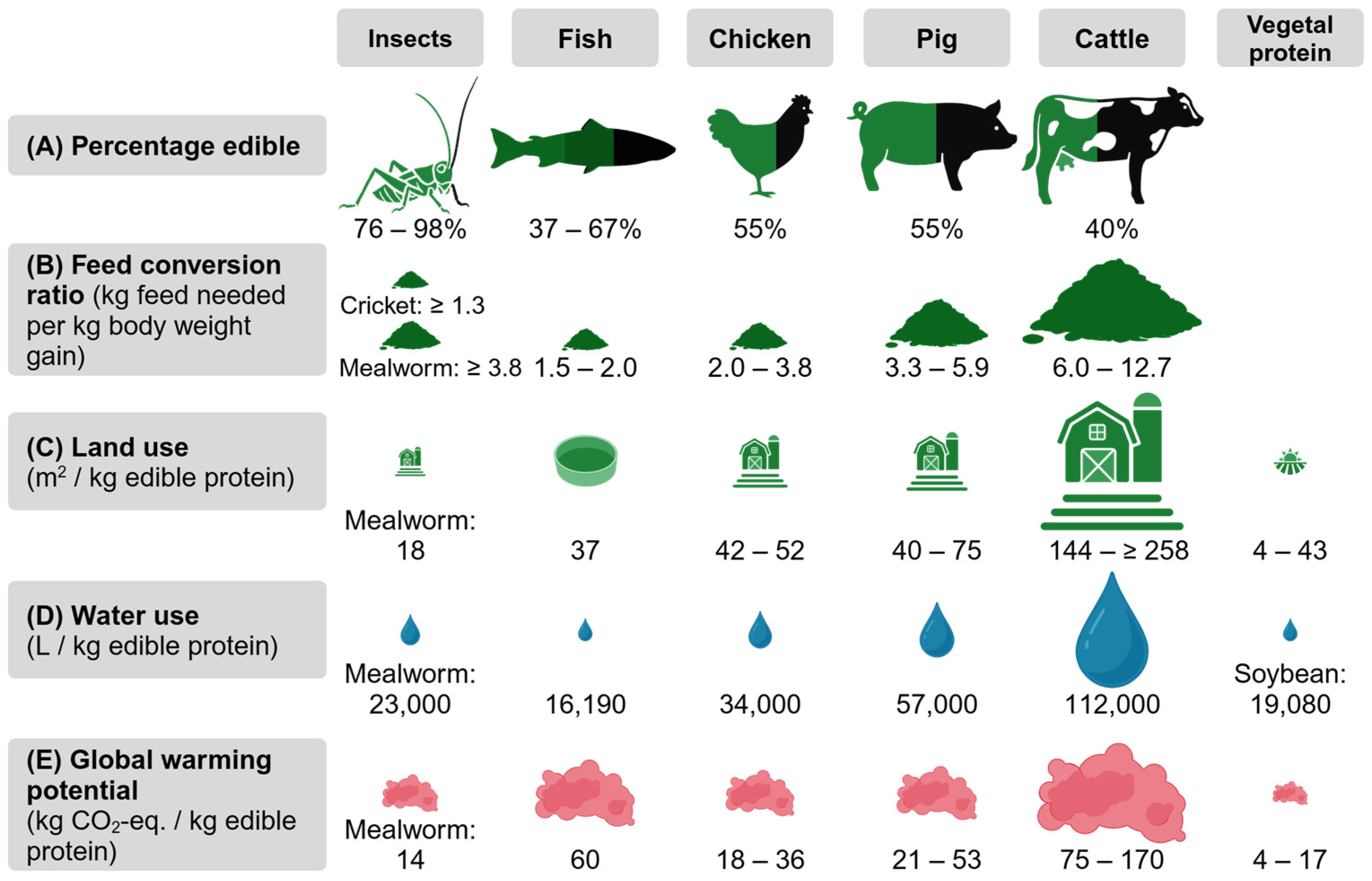
| Less land use (with some species requiring 14 times (or more) less land than livestock [33,57,58]) | Edible insect-based novel foods have no clear place in Western cuisine |
| Lower water use (five times (or more) less than livestock depending on insect species [33,60,61]) | Consumer acceptance (disgust, food neophobia, curiosity, tradition) |
| Lower feed conversion ratios (up to twelve times less feed is needed to gain 1 kg of edible insects compared with livestock [1,55]) | Food safety (standardized rearing conditions, contamination, insect welfare) |
| Bioconversion of organic side streams to high-value food (circular economy) | Allergenicity |
| Lower global warming potential (ten times lower than livestock depending on the insect species [33,57,58]) | |
| High nutritional value for human nutrition |
| Food | Indispensable Amino Acids (mg/g Protein) | Reference | ||||||||
| His | Ile | Leu | Lys | Met | Phe | Thr | Trp | Val | ||
| Coleoptera (beetles) 1) | 26.3 | 45.6 | 74.2 | 50.6 | 16.2 | 47.1 | 35.2 | 10.1 | 51.9 | [15] |
| Tenebrio molitor larvae 1) | 34.9 | 48.8 | 88.8 | 60.1 | 15.5 | 39.9 | 39.1 | 9.2 | 64.7 | [15] |
| Alphitobius diaperinus larvae 1) | 39.7 | 46.1 | 73.2 | 70.5 | 15.9 | 51.7 | 43.1 | 14.7 | 57.6 | [70] |
| Orthoptera (crickets, locusts) 1) | 21.2 | 39.6 | 74.8 | 53.9 | 19.3 | 46.6 | 35.8 | 8.1 | 50.3 | [15] |
| Acheta domesticus 1) | 23.1 | 41.2 | 83.4 | 52.4 | 17.3 | 31.0 | 35.6 | 7.0 | 50.3 | [15] |
| Locusta migratoria 1) | 14.0 | 25.0 | 46.0 | 29.0 | 16.0 | 37.0 | 19.0 | 4.0 | 41.0 | [73,74] |
| Chicken, fresh meat, raw | 26.6 | 56.2 | 77.5 | 88.8 | 27.9 | 39.6 | 44.0 | 12.2 | 51.4 | [66] |
| Pork, fresh meat, raw | 38.7 | 49.5 | 75.5 | 86.3 | 27.9 | 38.2 | 49.0 | 11.7 | 55.9 | [66] |
| Beef, fresh meat, raw | 33.0 | 49.6 | 80.8 | 83.8 | 24.8 | 41.0 | 44.9 | 11.1 | 54.3 | [66] |
| Fish, raw | 22.9 | 49.5 | 84.6 | 99.5 | 34.6 | 42.0 | 46.3 | 11.7 | 53.2 | [66] |
| Soybean, dried | 21.7 | 46.6 | 74.3 | 49.7 | 15.2 | 51.6 | 39.0 | 11.8 | 46.1 | [66] |
| Wheat flour | 19.2 | 40.2 | 71.8 | 21.0 | 14.8 | 48.2 | 28.0 | 10.6 | 42.9 | [66] |
| Fungi, dried | 20.4 | 39.4 | 43.1 | 60.8 | 8.3 | 26.5 | 31.2 | 8.5 | 32.1 | [66] |
| Daily amino acid requirement in human nutrition | 15 | 30 | 59 | 45 | 16 | 38 2) | 23 | 6 | 39 | [76] |
| Dispensable Amino Acids (mg/g Protein) | ||||||||||
| Ala | Arg | Asx | Cys | Glx | Gly | Pro | Ser | Tyr | ||
| Coleoptera (beetles) 1) | 69.5 | 53.9 | n.d. | 14.6 | 123.7 | 55.2 | 64.1 | 42.6 | 55.7 | [15] |
| Tenebrio molitor larvae 1) | 79.1 | 56.1 | 81.0 | 9.2 | 123.2 | 56.4 | 69.8 | 51.8 | 77.4 | [15,74] |
| Alphitobius diaperinus larvae 1) | 65.8 | 53.5 | 93.8 | 9.6 | 130.1 | 42.0 | 63.6 | 44.1 | 84.9 | [70] |
| Orthoptera (crickets, locusts) 1) | 77.4 | 53.6 | n.d. | 12.8 | 64.5 | 54.0 | 53.9 | 41.9 | 61.5 | [15] |
| Acheta domesticus 1) | 82.4 | 59.2 | 93.0 | 9.1 | 104.7 | 48.0 | 55.2 | 50.9 | 46.4 | [15,74] |
| Locusta migratoria 1) | 75.0 | 37.0 | 43.0 | 8.0 | 68.0 | 40.0 | 76.0 | 23.0 | 58.0 | [73,74] |
| Chicken, fresh meat, raw | 62.7 | 60.6 | 98.9 | 13.1 | 160.7 | 61.0 | 45.7 | 40.1 | 33.1 | [66] |
| Pork, fresh meat, raw | 60.3 | 59.8 | 95.1 | 11.8 | 153.0 | 55.9 | 47.0 | 43.7 | 40.6 | [66] |
| Beef, fresh meat, raw | 66.2 | 59.9 | 93.3 | 11.5 | 162.8 | 60.4 | 47.8 | 41.8 | 33.0 | [66] |
| Fish, raw | 60.1 | 62.8 | 106.4 | 12.8 | 157.6 | 44.7 | 33.0 | 43.1 | 35.1 | [66] |
| Soybean, dried | 40.1 | 61.8 | 104.5 | 15.4 | 169.9 | 37.2 | 47.6 | 44.2 | 32.7 | [66] |
| Wheat flour | 32.4 | 37.6 | 42.0 | 21.0 | 320.5 | 36.8 | 127.0 | 57.8 | 28.0 | [66] |
| Fungi, dried | 112.9 | 71.6 | 104.1 | 5.1 | 158.4 | 61.3 | 124.8 | 67.7 | 23.6 | [66] |
| Food | PDCAAS | DIAAS | First Limiting Amino Acid | Reference |
|---|---|---|---|---|
| Tenebrio molitor larvae | 0.86 1) c) | Met + Cys | [91] | |
| 64.0 2) | Met + Cys | [88] | ||
| 94.4 3) | Lys | [92] | ||
| 106.4 3) | Met + Cys | [93] | ||
| Alphitobius diaperinus larvae | 0.76 1) | n.d. | [94] | |
| 83.0 2) | Met + Cys | [88] | ||
| Acheta domesticus | 0.84 1) c) | Leu | [91] | |
| 89.0 2) | Met + Cys | [88] | ||
| 88.5 3) | Met + Cys | [92] | ||
| 119.3 3) | Leu | [93] | ||
| Locusta migratoria | 0.44 1) ps) | Trp | [95] | |
| 86.2 3) | Met + Cys | [92] | ||
| Chicken breast | 1.01 2) | 108.0 4) | n.d. | [96] |
| 136.5 3) | Leu | [93] | ||
| Pork | 119.0 2) | none | [97] | |
| Beef | 0.92 4) | n.d. | [87] | |
| 119.0 2) | none | [97] | ||
| Soy protein isolate | 1.02 2) | 98.0 2) | Met + Cys | [98] |
| Tofu | 0.56 2) | 52.0 3) | Met + Cys | [53] |
| Wheat | 0.51 2) | 54.0 2) | Lys | [98] |
| Whey protein concentrate | 1.34 2) | 133.0 2) | His | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachtigall, L.; Grune, T.; Weber, D. Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges. Nutrients 2025, 17, 1245. https://doi.org/10.3390/nu17071245
Nachtigall L, Grune T, Weber D. Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges. Nutrients. 2025; 17(7):1245. https://doi.org/10.3390/nu17071245
Chicago/Turabian StyleNachtigall, Lukas, Tilman Grune, and Daniela Weber. 2025. "Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges" Nutrients 17, no. 7: 1245. https://doi.org/10.3390/nu17071245
APA StyleNachtigall, L., Grune, T., & Weber, D. (2025). Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges. Nutrients, 17(7), 1245. https://doi.org/10.3390/nu17071245







