Atractylodes Japonica Rhizome Extract Fermented with a Plant-Derived Lacticaseibacillus paracasei (Lactobacillus paracasei) IJH-SONE68 Improves the Wheat Gliadin-Induced Food Allergic Reaction in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of AJR Extract and LAB Culture Condition
2.2. Extraction and Preparation of Wheat Gliadins
2.3. Animal Treatment and Sensitization by Gliadin
2.4. Quantitative Evaluation of Symptoms in Gliadin-Challenged Mice
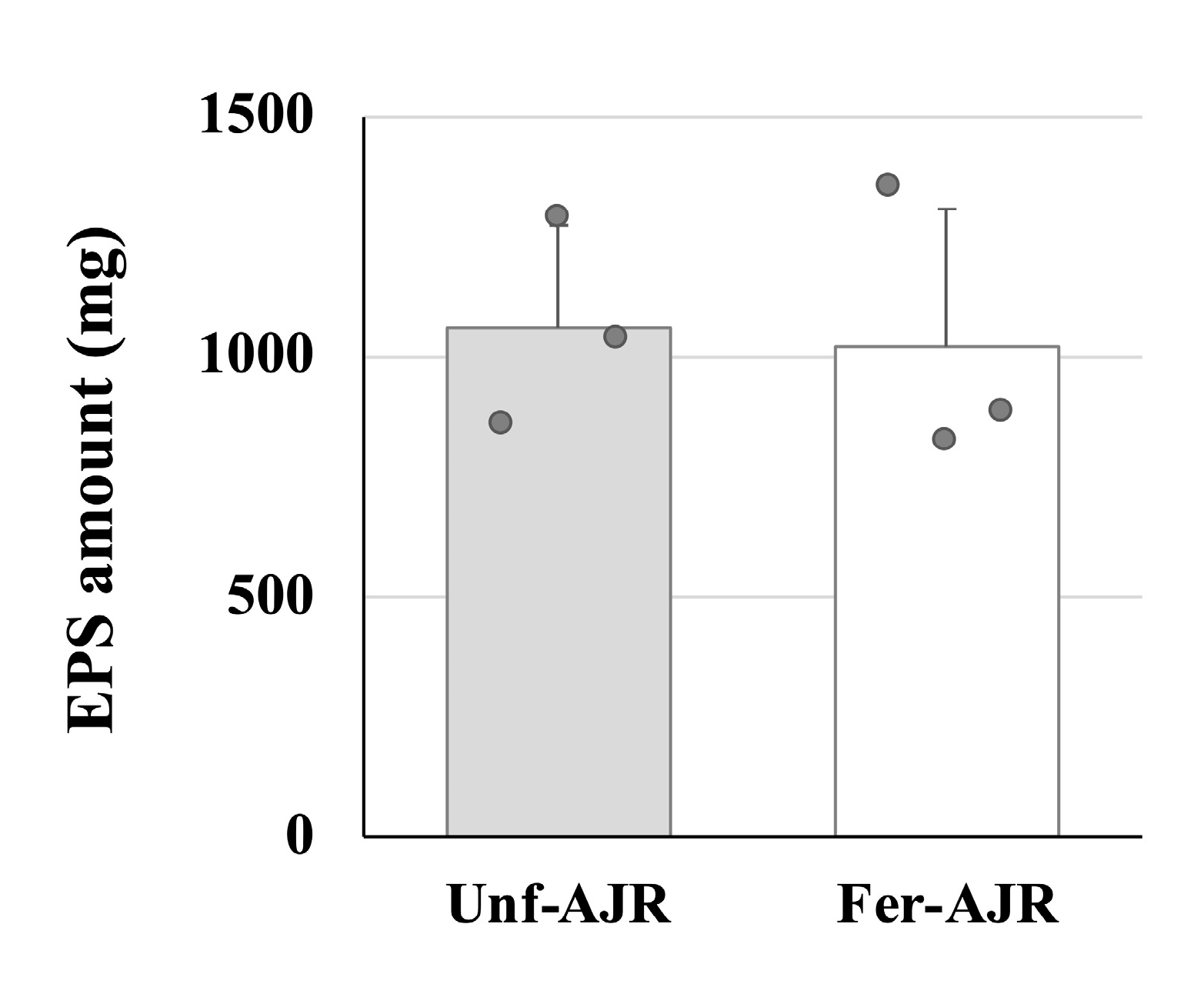
2.5. Purification of EPS from Culture Supernatant
2.6. Statistical Analyses
3. Results
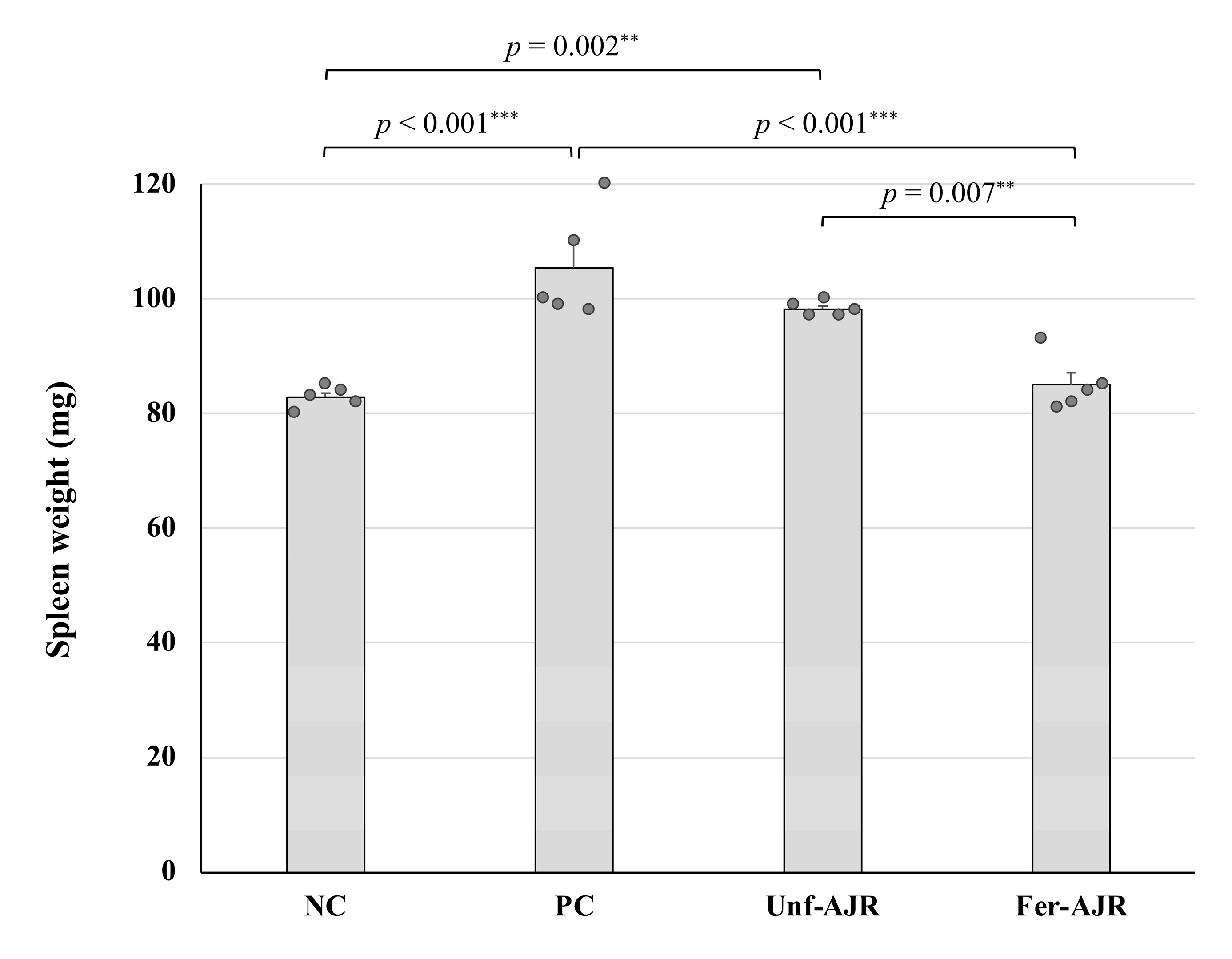
3.1. Ameliorative Effect on Gliadin-Induced Allergic Symptoms
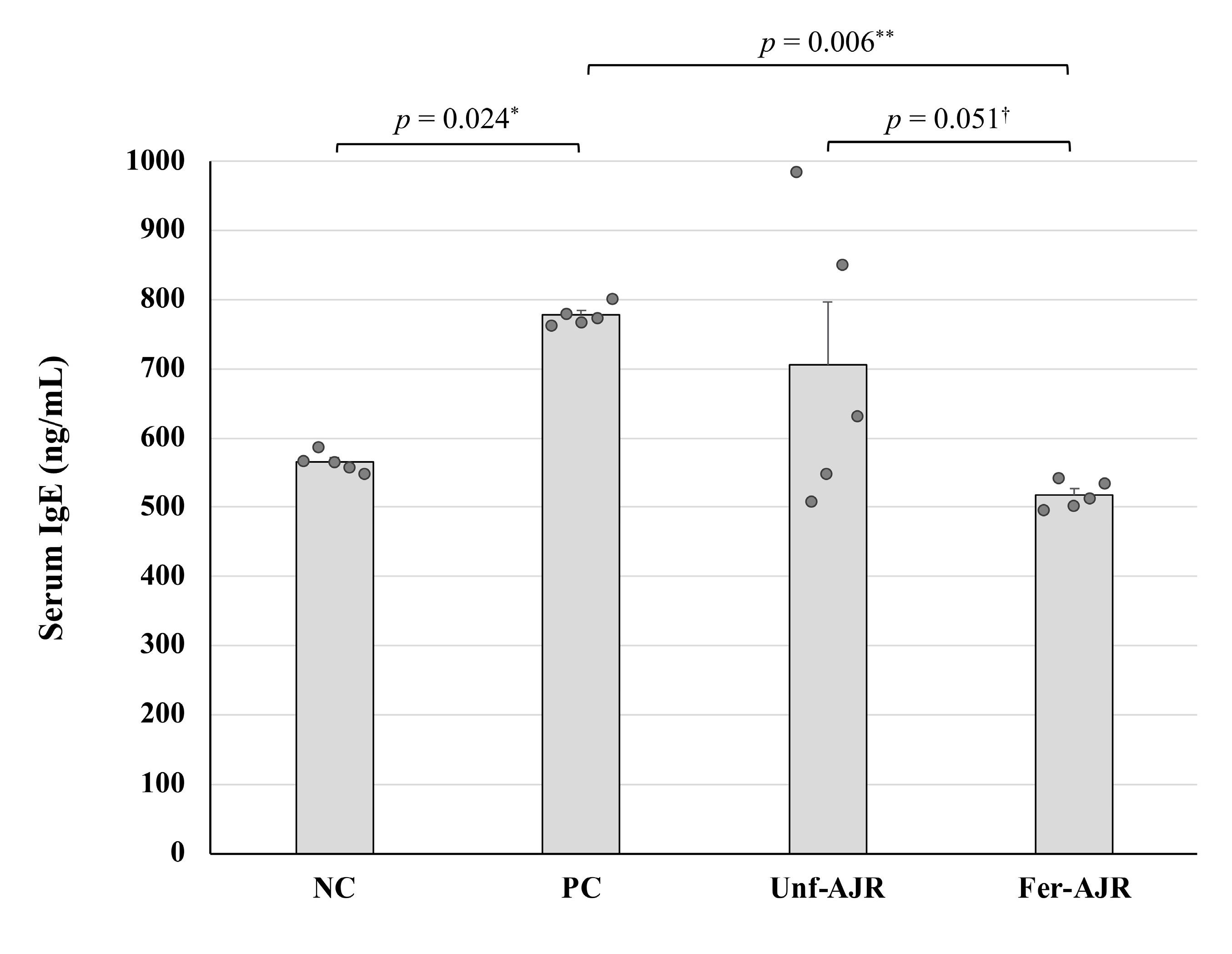
3.2. Repressive Effect on the Serum Total IgE Level
3.3. Fermented AJR Regulates Th1 and Th2 Cell Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEDS | atopic eczema/dermatitis syndrome |
| AJR | Atractylodes Japonica Rhizoma |
| DSS | dextran sulfate sodium |
| ELISA | enzyme-linked immunosorbent assay |
| EPS | exopolysaccharide |
| IFN | interferon |
| IgE | immunoglobulin E |
| IL | interleukin |
| LAB | lactic acid bacterium |
| MAPK | mitogen-activated protein kinase |
| MRS | de Man, Rogosa, and Sharpe |
| OVA | ovalbumin |
| SPF | specific-pathogen-free |
| TCA | trichloroacetic acid |
| WDEIA | wheat-dependent exertion-induced anaphylaxis |
References
- Pasha, I.; Saeed, F.; Sultan, M.T.; Batool, R.; Aziz, M.; Ahmed, W. Wheat allergy and intolerence; recent updates and perspectives. Crit. Rev. Food Sci. Nutr. 2016, 56, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Inomata, N. Wheat allergy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 238–243. [Google Scholar]
- Samasca, G.; Sur, G.; Iancu, M.; Lupan, I.; Deleanu, D. Current trends and investigative developments in wheat allergy. Int. Rev. Immunol. 2015, 34, 538–541. [Google Scholar] [PubMed]
- Scibilia, J.; Rossi Carlo, M.; Losappio Laura, M.; Mirone, C.; Farioli, L.; Pravettoni, V.; Pastorello, E.A. Favorable prognosis of wheat allergy in adults. J. Investig. Allergol. Clin. Immunol. 2019, 29, 118–123. [Google Scholar] [CrossRef]
- Palosuo, K.; Varjonen, E.; Kekki, O.M.; Klemola, T.; Kalkkinen, N.; Alenius, H.; Reunala, T. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J. Allergy Clin. Immunol. 2001, 108, 634–638. [Google Scholar] [CrossRef]
- Morita, E.; Kameyoshi, Y.; Mihara, S.; Hiragun, T.; Yamamoto, S. Gamma-Gliadin: A presumptive allergen causing wheat-dependent exercise-induced anaphylaxis. Br. J. Dermatol. 2001, 145, 182–184. [Google Scholar] [PubMed]
- Morita, E.; Yamamura, Y.; Mihara, S.; Kameyoshi, Y.; Yamamoto, S. Food-dependent exercise-induced anaphylaxis: A report of two cases and determination of wheat-gamma-gliadin as the presumptive allergen. Br. J. Dermatol. 2000, 143, 1059–1063. [Google Scholar] [CrossRef]
- Palosuo, K.; Alenius, H.; Varjonen, E.; Koivuluhta, M.; Mikkola, J.; Keskinen, H.; Kalkkinen, N.; Reunala, T. A novel wheat gliadin as a cause of exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 1999, 103, 912–917. [Google Scholar]
- Varjonen, E.; Vainio, E.; Kalimo, K. Life-threatening, recurrent anaphylaxis caused by allergy to gliadin and exercise. Clin. Exp. Allergy 1997, 27, 162–166. [Google Scholar] [CrossRef]
- Morita, E.; Matsuo, H.; Chinuki, Y.; Takahashi, H.; Dahlström, J.; Tanaka, A. Food-dependent exercise-induced anaphylaxis—Importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergol. Int. 2009, 58, 493–498. [Google Scholar]
- Shimato, Y.; Ota, M.; Asai, K.; Atsumi, T.; Tabuchi, Y.; Makino, T. Comparison of byakujutsu (Atractylodes rhizome) and sojutsu (Atractylodes lancea rhizome) on anti-inflammatory and immunostimulative effects in vitro. J. Nat. Med. 2018, 72, 192–201. [Google Scholar] [PubMed]
- Zhang, W.J.; Zhao, Z.Y.; Chang, L.K.; Cao, Y.; Wang, S.; Kang, C.Z.; Wang, H.Y.; Zhou, L.; Huang, L.Q.; Guo, L.P. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021, 266, 113415. [Google Scholar] [PubMed]
- Han, N.R.; Moon, P.D.; Nam, S.Y.; Ryu, K.J.; Yoou, M.S.; Choi, J.H.; Hwang, S.Y.; Kim, H.M.; Jeong, H.J. Inhibitory effects of atractylone on mast cell-mediated allergic reactions. Chem. Biol. Interact. 2016, 258, 59–68. [Google Scholar]
- Zhang, N.; Park, D.K.; Park, H.J. The inhibitory activity of atractylenolide Ш, a sesquiterpenoid, on IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA). J. Ethnopharmacol. 2013, 145, 278–285. [Google Scholar]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and antinociceptive constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [PubMed]
- di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; Vincentini, O.; Silano, M.; Giuliani, G.; de Angelis, M.; Gobbetti, M. Synthesis of isoflavone aglycones and equol in soy milks fermented by food-related lactic acid bacteria and their effect on human intestinal Caco-2 cells. J. Agric. Food Chem. 2010, 58, 10338–10346. [Google Scholar]
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar]
- Shakya, S.; Danshiitsoodol, N.; Noda, M.; Sugiyama, M. Role of phenolic acid metabolism in enhancing bioactivity of mentha extract fermented with plant-derived Lactobacillus plantarum SN13T. Probiotics Antimicrob. Proteins 2024, 16, 1052–1064. [Google Scholar]
- Shakya, S.; Danshiitsoodol, N.; Noda, M.; Inoue, Y.; Sugiyama, M. 3-Phenyllactic acid generated in medicinal plant extracts fermented with plant-derived lactic acid bacteria inhibits the biofilm synthesis of Aggregatibacter actinomycetemcomitans. Front. Microbiol. 2022, 13, 991144. [Google Scholar]
- Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-oxidant and anti-inflammatory substance generated newly in Paeoniae Radix Alba extract fermented with plant-derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. [Google Scholar] [CrossRef]
- Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 release inhibitors generated by fermentation of Artemisia princeps Pampanini herb extract with Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. [Google Scholar]
- Noda, M.; Sugimoto, S.; Hayashi, I.; Danshiitsoodol, N.; Fukamachi, M.; Sugiyama, M. A novel structure of exopolysaccharide produced by a plant-derived lactic acid bacterium Lactobacillus paracasei IJH-SONE68. J. Biochem. 2018, 164, 87–92. [Google Scholar]
- Tanaka, M.; Nagano, T.; Yano, H.; Matsuda, T.; Ikeda, T.M.; Haruma, K.; Kato, Y. Impact of ω-5 gliadin on wheat-dependent exercise-induced anaphylaxis in mice. Biosci. Biotechnol. Biochem. 2011, 75, 313–317. [Google Scholar] [PubMed]
- Noda, M.; Sultana, N.; Hayashi, I.; Fukamachi, M.; Sugiyama, M. Exopolysaccharide produced by Lactobacillus paracasei IJH-SONE68 prevents and improves the picryl chloride-induced contact dermatitis. Molecules 2019, 24, 2970. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Danshiitsoodol, N.; Kanno, K.; Uchida, T.; Sugiyama, M. The exopolysaccharide produced by Lactobacillus paracasei IJH-SONE68 prevents and ameliorates inflammatory responses in DSS–induced ulcerative colitis. Microorganisms 2021, 9, 2243. [Google Scholar] [CrossRef]
- Shindo, T.; Kanazawa, Y.; Saito, Y.; Kojima, K.; Ohsawa, M.; Teshima, R. Effective induction of oral anaphylaxis to ovalbumin in mice sensitized by feeding of the antigen with aid of oil emulsion and salicylate. J. Toxicol. Sci. 2012, 37, 307–315. [Google Scholar]
- Kameda, K.; Takahashi, E.; Kimoto, T.; Morita, R.; Sakai, S.; Nagao, M.; Fujisawa, T.; Kido, H. A murine model of food allergy by epicutaneous adjuvant-free allergen sensitization followed by oral allergen challenge combined with aspirin for enhanced detection of hypersensitivity manifestations and immunotherapy monitoring. Nutrients 2023, 15, 757. [Google Scholar] [CrossRef]
- Panthavee, W.; Noda, M.; Danshiitsoodol, N.; Kumagai, T.; Sugiyama, M. Characterization of exopolysaccharides produced by thermophilic lactic acid bacteria isolated from tropical fruits of Thailand. Biol. Pharm. Bull. 2017, 40, 621–629. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [PubMed]
- Steel, R.G.D. A rank sum test for comparing all treatments. Technometrics 1960, 2, 197–207. [Google Scholar]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar]
- Wang, C.C.; Lin, Y.R.; Liao, M.H.; Jan, T.R. Oral supplementation with areca-derived polyphenols attenuates food allergic responses in ovalbumin-sensitized mice. BMC Complement. Altern. Med. 2013, 13, 154. [Google Scholar]
- Chen, B.; Wu, Y.; Wu, H.; Meng, X.; Chen, H. Establishment of food allergy model in dextran sulfate sodium induced colitis mice. Foods 2023, 12, 1007. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Denery-Papini, S.; Larré, C.; Gaudin, J.C.; Brossard, C.; Bodinier, M. Wheat gliadins modified by deamidation are more efficient than native gliadins in inducing a Th2 response in Balb/c mice experimentally sensitized to wheat allergens. Mol. Nutr. Food Res. 2012, 56, 336–344. [Google Scholar]
- Zhang, T.; Hu, Z.; Cheng, Y.; Xu, H.; Velickovic, T.C.; He, K.; Sun, F.; He, Z.; Liu, Z.; Wu, X. Changes in allergenicity of ovalbumin in vitro and in vivo on conjugation with quercetin. J. Agric. Food Chem. 2020, 68, 4027–4035. [Google Scholar]
- Lee, D.; Kim, H.S.; Shin, E.; Do, S.G.; Lee, S.K.; Kim, Y.M.; Lee, M.B.; Min, K.Y.; Koo, J.; Kim, S.J.; et al. Polysaccharide isolated from Aloe vera gel suppresses ovalbumin-induced food allergy through inhibition of Th2 immunity in mice. Biomed. Pharmacother. 2018, 101, 201–210. [Google Scholar]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial signature in IgE-mediated food allergies. Genome Med. 2020, 12, 92. [Google Scholar]
- Joseph, C.L.; Sitarik, A.R.; Kim, H.; Huffnagle, G.; Fujimura, K.; Yong, G.J.M.; Levin, A.M.; Zoratti, E.; Lynch, S.; Ownby, D.R.; et al. Infant gut bacterial community composition and food-related manifestation of atopy in early childhood. Pediatr. Allergy Immunol. 2022, 33, e13704. [Google Scholar] [CrossRef] [PubMed]
- Nermes, M.; Salminen, S.; Isolauri, E. Is there a role for probiotics in the prevention or treatment of food allergy? Curr. Allergy Asthma Rep. 2013, 13, 622–630. [Google Scholar] [CrossRef]
- Rao, R.K.; Samak, G. Protection and restitution of gut barrier by probiotics: Nutritional and clinical implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Fu, S.; Wang, C.; Xie, M.; Wang, Y. Yogurt-sourced probiotic bacteria alleviate shrimp tropomyosin-induced allergic mucosal disorders, potentially through microbiota and metabolism modifications. Allergol. Int. 2019, 68, 506–514. [Google Scholar] [CrossRef]
- Fu, W.; Chen, C.; Xie, Q.; Gu, S.; Tao, S.; Xue, W. Pediococcus acidilactici strain alleviates gluten-induced food allergy and regulates gut microbiota in mice. Front. Cell Infect. Microbiol. 2022, 12, 845142. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Y.; Jian, D.I.; Olson, E.; Ng, P.K.W.; Gangur, V. Development and validation of a mouse-based primary screening method for testing relative allergenicity of proteins from different wheat genotypes. J. Immunol. Methods 2019, 464, 95–104. [Google Scholar] [CrossRef]
- Castan, L.; Villemin, C.; Claude, M.; Aubert, P.; Durand, T.; Neunlist, M.; Brossard, C.; Magnan, A.; Bodinier, M.; Bouchaud, G. Acid-hydrolyzed gliadins worsen food allergies through early sensitization. Mol. Nutr. Food Res. 2018, 62, 1800159. [Google Scholar] [CrossRef]
- Zhou, C.; Ludmila, T.; Sun, N.; Wang, C.; Pu, Q.; Huang, K.; Che, H. BALB/c mice can be used to evaluate allergenicity of different food protein extracts. Food Agric. Immunol. 2016, 27, 589–603. [Google Scholar] [CrossRef]
- Joo, Y.H.; Lee, D.H.; Lee, N.T.; Chung, N. Model development for prediction of the allergic response to the wheat proteins ω-5 gliadin and HMW-glutenin. Appl. Biol. Chem. 2016, 59, 827–831. [Google Scholar] [CrossRef]
- Jin, Y.; Ebaugh, S.; Martens, A.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A mouse model of anaphylaxis and atopic dermatitis to salt-soluble wheat protein extract. Int. Arch. Allergy Immunol. 2017, 174, 7–16. [Google Scholar] [CrossRef]
- Castan, L.; Cheminant, M.A.; Colas, L.; Brouard, S.; Magnan, A.; Bouchaud, G. Food allergen-sensitized CCR9+ lymphocytes enhance airways allergic inflammation in mice. Allergy 2018, 73, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, F. Lymphocytes and Lymphatic Organs. In Wintrobe’s Clinical Hematology, 13th ed.; Greer, J.P., Arber, D.A., Glader, B., List, A.F., Means, R.T., Jr., Rodgers, G.M., Foerster, J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 227–410. [Google Scholar]
- Haley, P.J. The lymphoid system: A review of species differences. Toxicol. Pathol. 2017, 30, 111–123. [Google Scholar]
- Dale, D.C. The mysteries of the spleen. J. Leukoc. Biol. 2016, 100, 249–251. [Google Scholar] [PubMed]
- Uy, P.P.D.; Francisco, D.M.; Trivedi, A.; O'Loughlin, M.; Wu, G.Y. Vascular diseases of the spleen: A review. J. Clin. Transl. Hepatol. 2017, 5, 152–164. [Google Scholar] [PubMed]
- Pivkin, I.V.; Peng, Z.; Karniadakis, G.E.; Buffet, P.A.; Dao, M.; Suresh, S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 7804–7809. [Google Scholar]
- Li, H.; Lu, L.; Li, X.; Buffet, P.A.; Dao, M.; Karniadakis, G.E.; Suresh, S. Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc. Natl. Acad. Sci. USA 2018, 115, 9574–9579. [Google Scholar] [CrossRef]
- Behshad, R.; Cooper, K.D.; Korman, N.J. A retrospective case series review of the peroxisome proliferator-activated receptor ligand rosiglitazone in the treatment of atopic dermatitis. Arch. Dermatol. 2008, 144, 84–88. [Google Scholar]
- Kang, Y.M.; Lee, K.Y.; An, H.J. Inhibitory effects of Helianthus tuberosus ethanol extract on Dermatophagoides farinae body-induced atopic dermatitis mouse model and human keratinocytes. Nutrients 2018, 10, 1657. [Google Scholar] [CrossRef]
- Jin, B.R.; Chung, K.S.; Cheon, S.Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.J.; An, H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, H.S.; Jeon, Y.J.; Madusanka, D.M.D.; Dias, M.K.H.; Jee, Y.; Ahn, G. Oral administration of Sargassum horneri improves the HDM/DNCB-induced atopic dermatitis in NC/Nga mice. Nutrients 2020, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, S.; Wakamatsu, E.; Ishida, Y.; Obata, Y.; Kurashima, Y.; Kiyono, H.; Abe, R. The spleen is the site where mast cells are induced in the development of food allergy. Int. Immunol. 2017, 29, 31–45. [Google Scholar] [PubMed]
- Zhen, B.X.; Cai, Q.; Li, F. Chemical components and protective effects of Atractylodes japonica Koidz. ex Kitam against acetic acid-induced gastric ulcer in rats. World J. Gastroenterol. 2023, 29, 5848–5864. [Google Scholar]
- Tang, F.; Fan, K.; Wang, K.; Bian, D. Atractylodin attenuates lipopolysaccharide-induced acute lung injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J. Pharmacol. Sci. 2018, 136, 203–211. [Google Scholar] [PubMed]
- Chae, H.S.; Kim, Y.M.; Chin, Y.W. Atractylodin inhibits interleukin-6 by blocking NPM-ALK activation and MAPKs in HMC-1. Molecules 2016, 21, 1169. [Google Scholar]
- Yu, C.; Xiong, Y.; Chen, D.; Li, Y.; Xu, B.; Lin, Y.; Tang, Z.; Jiang, C.; Wang, L. Ameliorative effects of atractylodin on intestinal inflammation and co-occurring dysmotility in both constipation and diarrhea prominent rats. Korean J. Physiol. Pharmacol. 2017, 21, 1–9. [Google Scholar]
- Jun, X.; Fu, P.; Lei, Y.; Cheng, P. Pharmacological effects of medicinal components of Atractylodes lancea (Thunb.) DC. Chin. Med. 2018, 13, 59. [Google Scholar]
- Chuang, C.H.; Cheng, Y.C.; Lin, S.C.; Lehman, C.W.; Wang, S.P.; Chen, D.Y.; Tsai, S.W.; Lin, C.C. Atractylodin suppresses dendritic cell maturation and ameliorates collagen-induced arthritis in a mouse model. J. Agric. Food Chem. 2019, 67, 6773–6784. [Google Scholar]
- Lin, Y.; Yang, C.; Lin, C.; Hsia, T.; Chao, W.; Lin, C. Atractylodin ameliorates ovalbumin induced asthma in a mouse model and exerts immunomodulatory effects on Th2 immunity and dendritic cell function. Mol. Med. Rep. 2020, 22, 4909–4918. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Noda, M.; Danshiitsoodol, N.; Sugiyama, M. Atractylodes Japonica Rhizome Extract Fermented with a Plant-Derived Lacticaseibacillus paracasei (Lactobacillus paracasei) IJH-SONE68 Improves the Wheat Gliadin-Induced Food Allergic Reaction in Mice. Nutrients 2025, 17, 1151. https://doi.org/10.3390/nu17071151
Ma Q, Noda M, Danshiitsoodol N, Sugiyama M. Atractylodes Japonica Rhizome Extract Fermented with a Plant-Derived Lacticaseibacillus paracasei (Lactobacillus paracasei) IJH-SONE68 Improves the Wheat Gliadin-Induced Food Allergic Reaction in Mice. Nutrients. 2025; 17(7):1151. https://doi.org/10.3390/nu17071151
Chicago/Turabian StyleMa, Qingmiao, Masafumi Noda, Narandalai Danshiitsoodol, and Masanori Sugiyama. 2025. "Atractylodes Japonica Rhizome Extract Fermented with a Plant-Derived Lacticaseibacillus paracasei (Lactobacillus paracasei) IJH-SONE68 Improves the Wheat Gliadin-Induced Food Allergic Reaction in Mice" Nutrients 17, no. 7: 1151. https://doi.org/10.3390/nu17071151
APA StyleMa, Q., Noda, M., Danshiitsoodol, N., & Sugiyama, M. (2025). Atractylodes Japonica Rhizome Extract Fermented with a Plant-Derived Lacticaseibacillus paracasei (Lactobacillus paracasei) IJH-SONE68 Improves the Wheat Gliadin-Induced Food Allergic Reaction in Mice. Nutrients, 17(7), 1151. https://doi.org/10.3390/nu17071151






