Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Food Intake, Body Weight, and Body Composition
2.3. Cytokines, Leptin, and Insulin Levels in Serum
2.4. Microbiome Analysis
2.5. Lipid Content in Feces
2.6. Statistical Analysis
3. Results
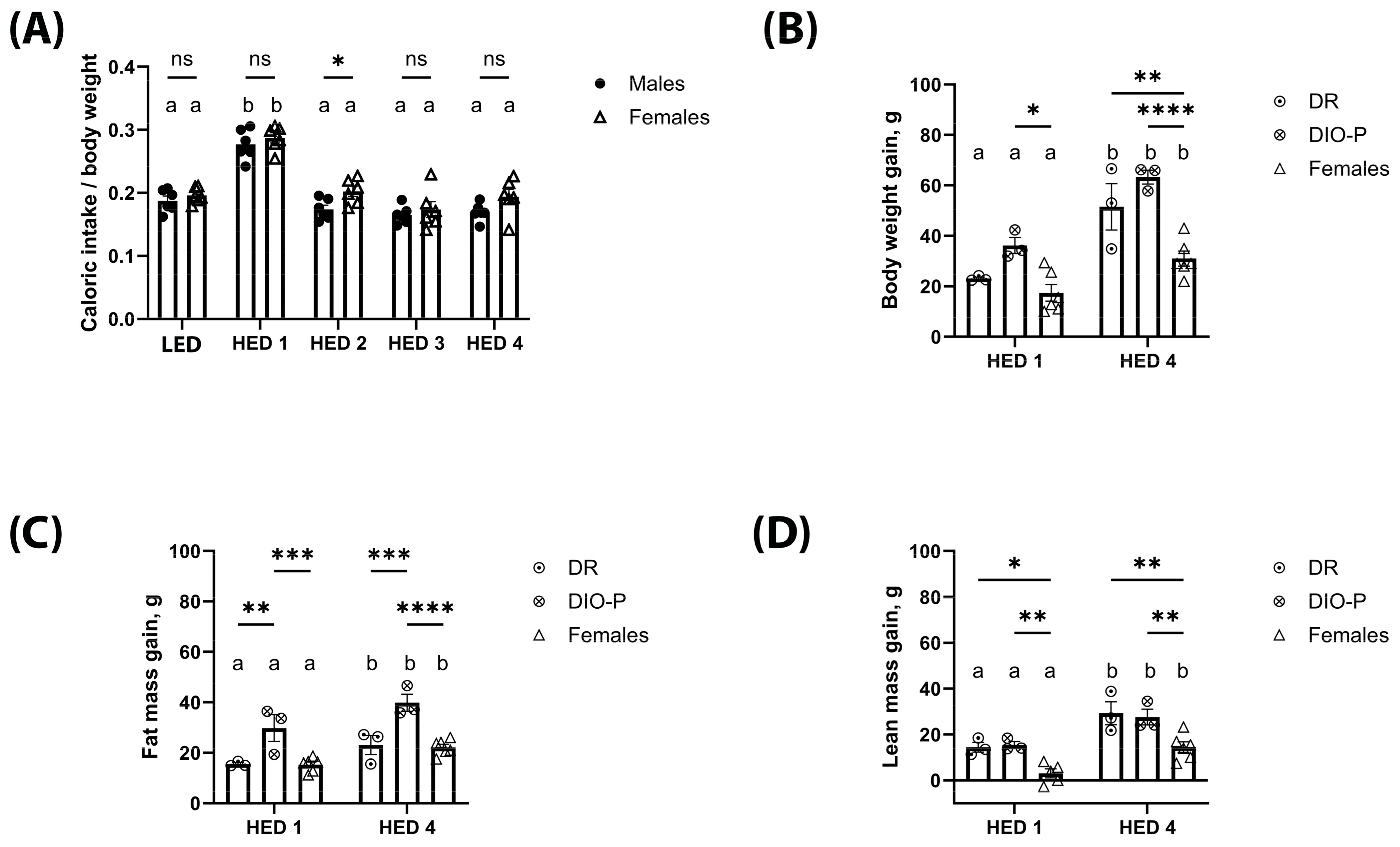
3.1. High-Energy-Density Diet Consumption Significantly Increased Body Weight and Fat Mass
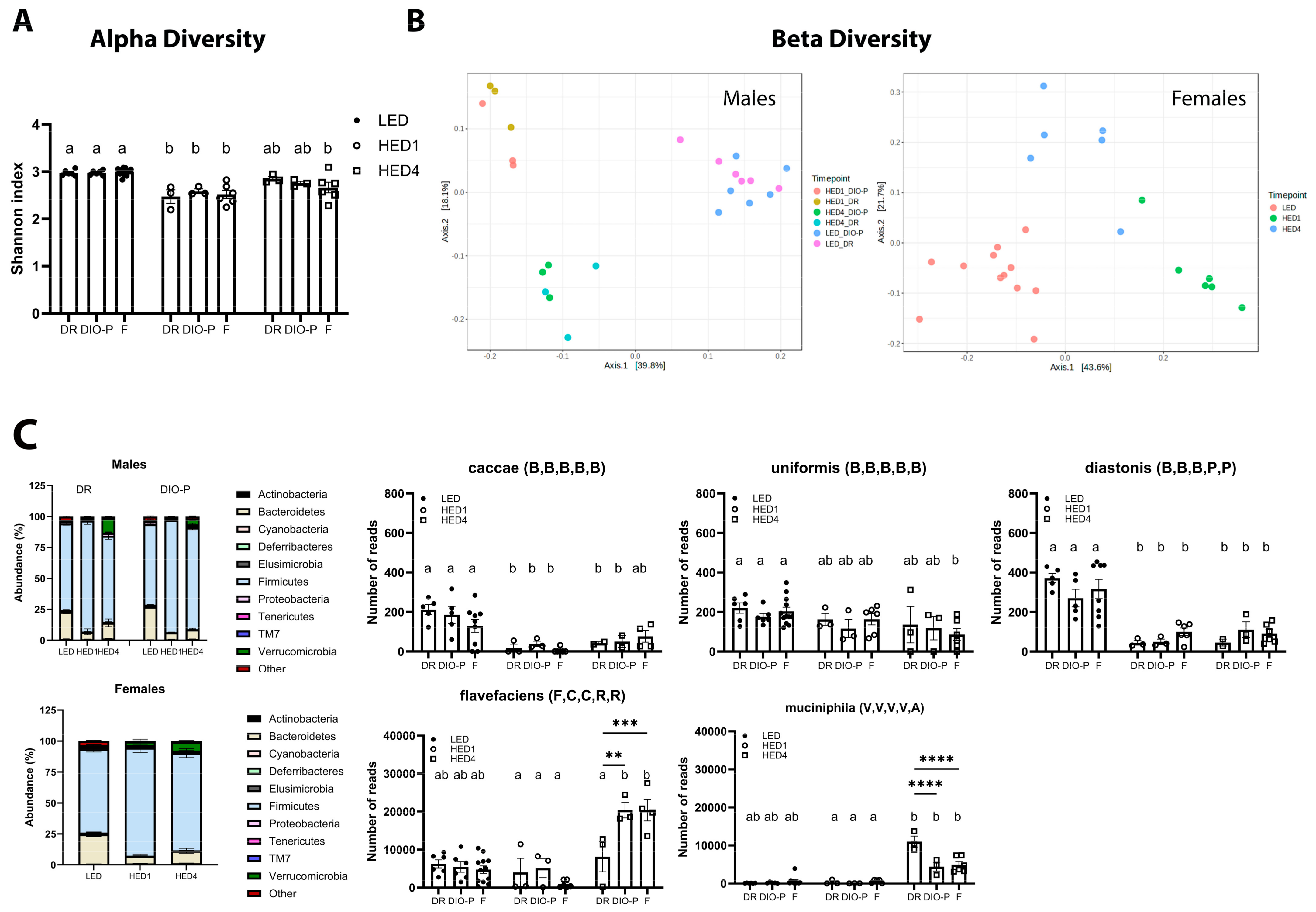
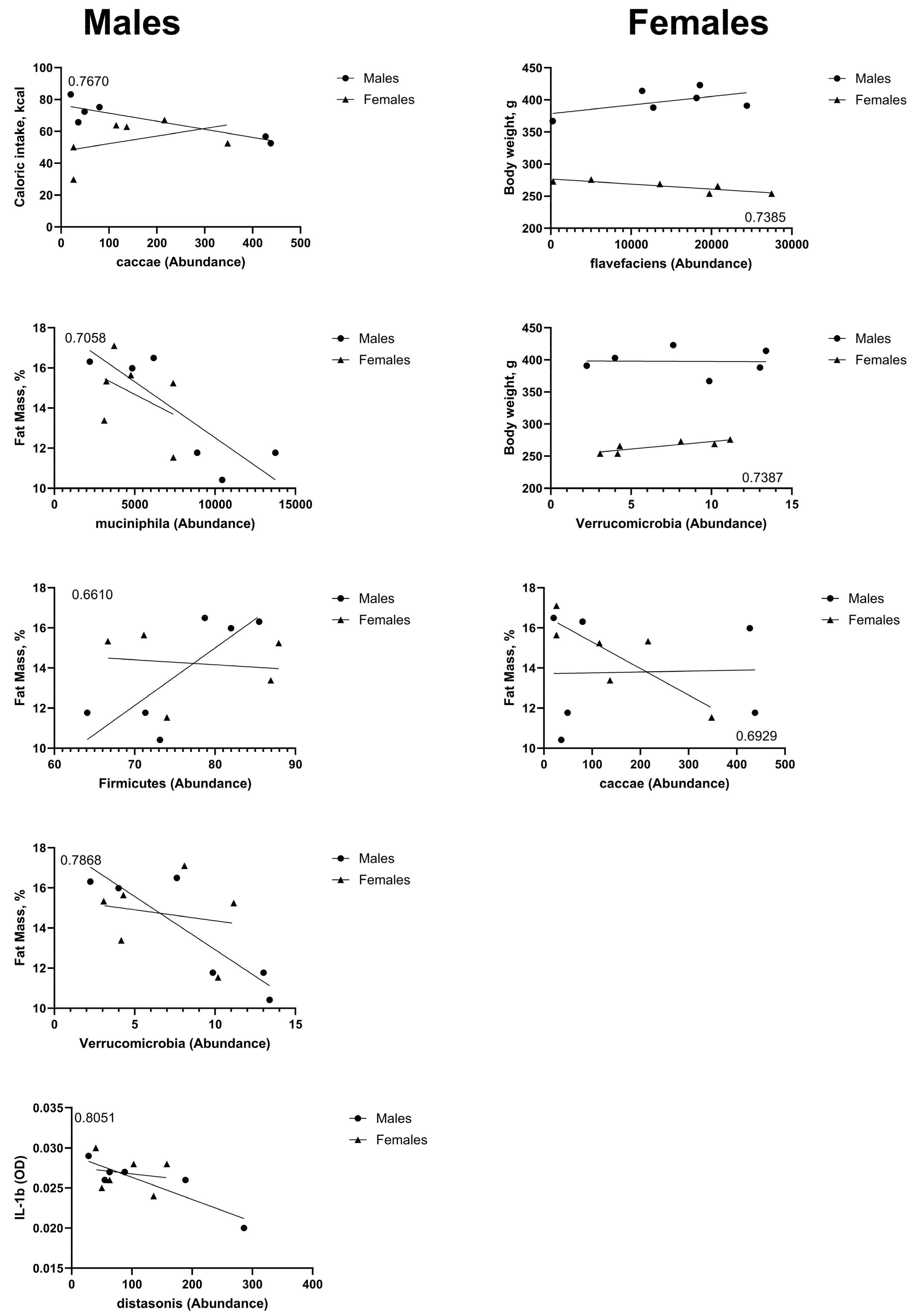
3.2. HED Diet Decreased Microbial Diversity Within a Week
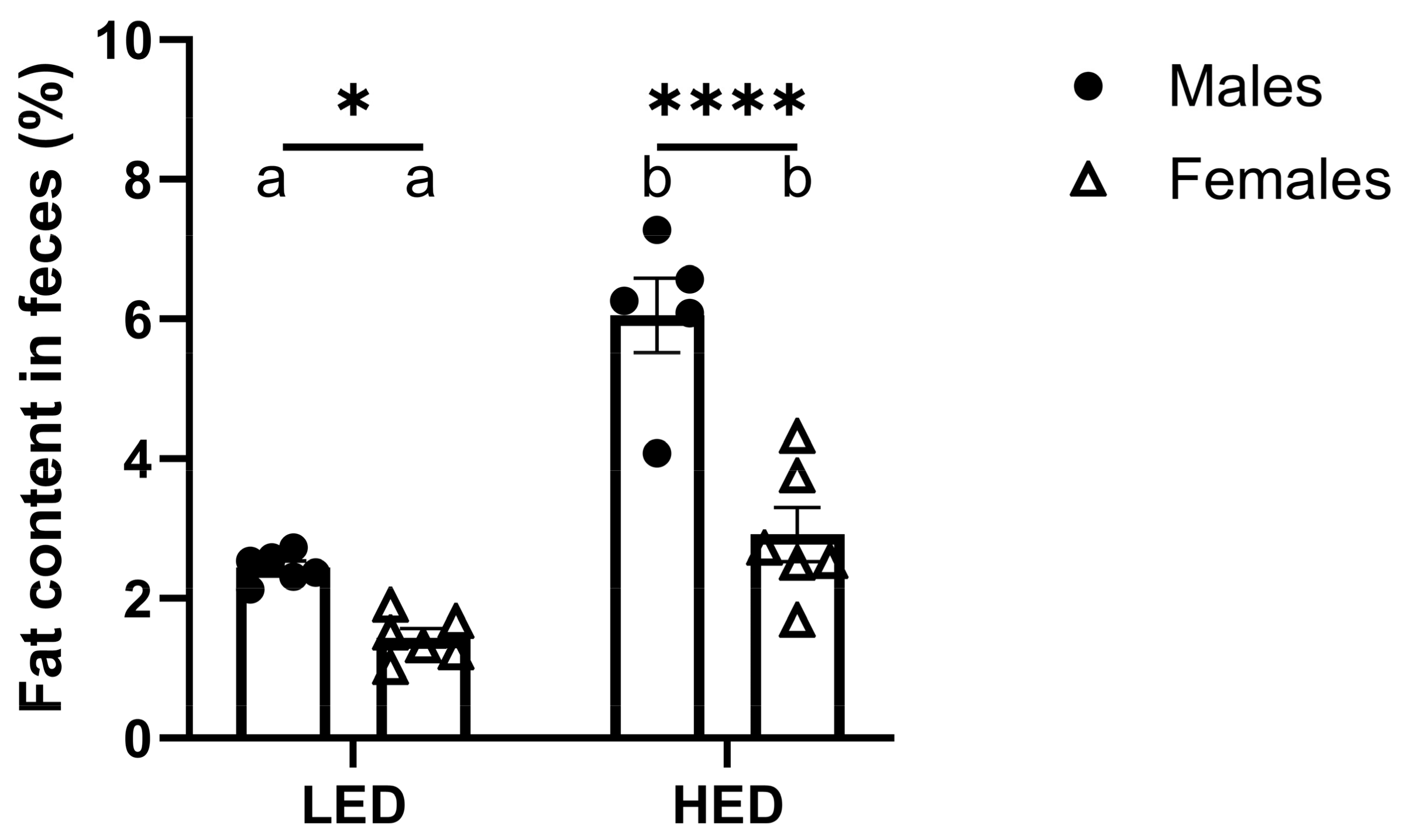
3.3. Male Rats Excrete More Fat in Their Feces than Female Rats
3.4. HED Diet Consumption Affects Cytokine and Chemokine Levels in Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HED | High energy density |
| LED | Low energy density |
| DIO-P | Diet-induced obesity prone |
| DR | Diet-induced obesity resistant |
| TGF-β | Transforming growth factor β |
| IP-10 | Interferon gamma-inducible protein 10 |
| RANTES | Regulated on activation, normal T cell expressed and secreted protein |
| FGFb | Basic fibroblast growth factor |
| GI track | Gastrointestinal track |
| IL-1β | Interleukin-1β |
| IL-1α | Interleukin-1α |
| IL-6 | Interleukin 6 |
| VEGF | Vascular endothelial growth factor |
| TNF-α | Tumor necrosis factor α |
| IFN-γ | Interferon γ |
| MCP-1 | Monocyte chemoattractant protein 1 |
| SCF | Stem cell factor |
| MIP-1α | Macrophage inflammatory protein 1α |
| IL-5 | Interleukin 5 |
| IL-15 | Interleukin 15 |
| IL-6 | Interleukin 6 |
References
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.M.O.; de Oliveira, D.G.; Rocha-Gomes, A.; Oliveira, G.A.; Bernardes, E.D.O.; Dias, P.L.; Reis, Í.G.; Severiano, C.M.; da Silva, A.A.; Lessa, M.R.; et al. Unripe banana flour (Musa cavendishii) promotes increased hypothalamic antioxidant activity, reduced caloric intake, and abdominal fat accumulation in rats on a high-fat diet. J. Food Biochem. 2022, 46, e14341. [Google Scholar] [CrossRef]
- Shen, C.-L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.-C.; Gao, W.; Wang, J.-S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet–induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Binayi, F.; Moslemi, M.; Khodagholi, F.; Hedayati, M.; Zardooz, H. Long-term high-fat diet disrupts lipid metabolism and causes inflammation in adult male rats: Possible intervention of endoplasmic reticulum stress. Arch. Physiol. Biochem. 2023, 129, 204–212. [Google Scholar] [CrossRef]
- Kim, J.S.; Kirkland, R.A.; Lee, S.H.; Cawthon, C.R.; Rzepka, K.W.; Minaya, D.M.; de Lartigue, G.; Czaja, K.; de La Serre, C.B. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiol. Behav. 2020, 225, 113082. [Google Scholar] [CrossRef]
- Kim, J.S.; Williams, K.C.; Kirkland, R.A.; Schade, R.; Freeman, K.G.; Cawthon, C.R.; Rautmann, A.W.; Smith, J.M.; Edwards, G.L.; Glenn, T.C.; et al. The gut-brain axis mediates bacterial driven modulation of reward signaling. Mol. Metab. 2023, 75, 101764. [Google Scholar] [CrossRef]
- Levin, B.E.; Triscari, J.; Sullivan, A.C. Relationship between sympathetic activity and diet-induced obesity in two rat strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1983, 245, R364–R371. [Google Scholar] [CrossRef]
- Nazari, S.; Moosavi, S.M.S. Temporal patterns of alterations in obesity index, lipid profile, renal function and blood pressure during the development of hypertension in male, but not female, rats fed a moderately high-fat diet. Arch. Physiol. Biochem. 2022, 128, 897–909. [Google Scholar] [CrossRef]
- Jang, I.; Hwang, D.; Lee, J.; Chae, K.; Kim, Y.; Kang, T.; Kim, C.; Shin, D.; Hwang, J.; Huh, Y.; et al. Physiological Difference between Dietary Obesity-Susceptible and Obesity-Resistant Sprague Dawley Rats in Response to Moderate High Fat Diet. Exp. Anim. 2003, 52, 99–107. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Davies, M.J.; Prewitt, R.L.; Lauterio, T.J. Development of Hypertension in a Rat Model of Diet-Induced Obesity. Hypertension 2000, 35, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, G.; Barbier de la Serre, C.; Espero, E.; Lee, J.; Raybould, H.E. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E187–E195. [Google Scholar] [CrossRef] [PubMed]
- Paulino, G.; Serre, C.B.d.l.; Knotts, T.A.; Oort, P.J.; Newman, J.W.; Adams, S.H.; Raybould, H.E. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E898–E903. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Sutoyo, D.A.; Atmaka, D.R.; Sidabutar, L. Dietary factors affecting firmicutes and bacteroidetes ratio in solving obesity problem: A literature review. Media Gizi Indones. 2020, 15, 94–109. [Google Scholar]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Zhou, Z.; Wu, S.; Zhao, J.; Jia, W.; Liu, M.; Shen, X.; He, F.; Cheng, R. Gut Microbiota Perturbation in Early Life Could Influence Pediatric Blood Pressure Regulation in a Sex-Dependent Manner in Juvenile Rats. Nutrients 2023, 15, 2661. [Google Scholar] [CrossRef]
- García-Cabrerizo, R.; Carbia, C.; O’Riordan, K.J.; Schellekens, H.; Cryan, J.F. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2021, 157, 1495–1524. [Google Scholar] [CrossRef]
- Bashaw, H.; Brownell, J.N.; Schall, J.I.; Stallings, V.A. Measures of Dietary Fat and Energy Absorption in Healthy Adults. Pancreas 2020, 49, 845–854. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.B.; Higuchi, S.; Banks, A.S.; Haeusler, R.A. Cyp2c-deficiency depletes muricholic acids and protects against high-fat diet-induced obesity in male mice but promotes liver damage. Mol. Metab. 2021, 53, 101326. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lopez-Pena, C.L.; McClements, D.J.; Decker, E.A.; Xiao, H. Safety evaluation and lipid-lowering effects of food-grade biopolymer complexes (ε-polylysine-pectin) in mice fed a high-fat diet. Food Funct. 2017, 8, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jadhav, V.; Odelade, A.; Chang, E.; Chang, A.; Harrison, S.H.; Maldonado-Devincci, A.M.; Graves, J.L.; Han, J. High fat diet reveals sex-specific fecal and liver metabolic alterations in C57BL/6J obese mice. Metabolomics 2023, 19, 97. [Google Scholar] [CrossRef]
- Priego, T.; Sanchez, J.; Pico, C.; Palou, A. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity 2008, 16, 819–826. [Google Scholar] [CrossRef]
- Eckel, L.A.; Moore, S.R. Diet-induced hyperphagia in the rat is influenced by sex and exercise. Am. J. Physiol. Reg. I 2004, 287, R1080–R1085. [Google Scholar] [CrossRef]
- Taraschenko, O.D.; Maisonneuve, I.M.; Glick, S.D. Resistance of male Sprague–Dawley rats to sucrose-induced obesity: Effects of 18-methoxycoronaridine. Physiol. Behav. 2011, 102, 126–131. [Google Scholar]
- Roca, P.; Rodriguez, A.M.; Oliver, P.; Bonet, M.L.; Quevedo, S.; Picó, C.; Palou, A. Brown adipose tissue response to cafeteria diet-feeding involves induction of the UCP2 gene and is impaired in female rats as compared to males. Pflügers Arch. 1999, 438, 628–634. [Google Scholar]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef]
- Salinero, A.E.; Anderson, B.M.; Zuloaga, K.L. Sex differences in the metabolic effects of diet-induced obesity vary by age of onset. Int. J. Obes. 2018, 42, 1088–1091. [Google Scholar]
- Yang, Y.; Smith, D.L., Jr.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [PubMed]
- Marco, A.; Schroeder, M.; Weller, A. Microstructural pattern of palatable food intake from weaning to adulthood in male and female OLETF rats. Behav. Neurosci. 2009, 123, 1251. [Google Scholar] [PubMed]
- Strohmayer, A.; Smith, G. The meal pattern of genetically obese (ob/ob) mice. Appetite 1987, 8, 111–123. [Google Scholar]
- Maric, I.; Krieger, J.P.; van der Velden, P.; Borchers, S.; Asker, M.; Vujicic, M.; Asterholm, I.W.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 828522. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.Y.; Ewald, J.; Pang, Z.Q.; Shiri, T.; Xia, J.G. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Tran, M.; Forget, P.; Van den Neucker, A.; Van Kreel, B. Improved Steatocrit Results Obtained by Acidification of Fecal Homogenates Are Due to Improved Fat Extraction. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 157–160. [Google Scholar] [CrossRef]
- Tschöp, M.; Heiman, M.L. Rodent obesity models: An overview. Exp. Clin. Endocrinol. Diabetes 2001, 109, 307–319. [Google Scholar] [CrossRef]
- Levin, B.E.; Triscari, J.; Hogan, S.; Sullivan, A.C. Resistance to Diet-Induced Obesity—Food-Intake, Pancreatic Sympathetic Tone, and Insulin. Am. J. Physiol. 1987, 252, R471–R478. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Graham, B.; Yakubu, F.; Lin, D.; Peters, J.C.; Hill, J.O. Metabolic Differences between Obesity-Prone and Obesity-Resistant Rats. Am. J. Physiol. 1990, 259, R1103–R1110. [Google Scholar] [CrossRef] [PubMed]
- Minaya, D.M.; Turlej, A.; Joshi, A.; Nagy, T.; Weinstein, N.; DiLorenzo, P.; Hajnal, A.; Czaja, K. Consumption of a high energy density diet triggers microbiota dysbiosis, hepatic lipidosis, and microglia activation in the nucleus of the solitary tract in rats. Nutr. Diabetes 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Minaya, D.M.; Di Lorenzo, P.M.; Hajnal, A.; Czaja, K. Roux-en-Y gastric bypass surgery triggers rapid DNA fragmentation in vagal afferent neurons in rats. Acta Neurobiol. Exp. 2019, 79, 432–444. [Google Scholar] [CrossRef]
- Braga Tibaes, J.R.; Azarcoya-Barrera, J.; Wollin, B.; Veida-Silva, H.; Makarowski, A.; Vine, D.; Tsai, S.; Jacobs, R.; Richard, C. Sex Differences Distinctly Impact High-Fat Diet-Induced Immune Dysfunction in Wistar Rats. J. Nutr. 2022, 152, 1347–1357. [Google Scholar] [CrossRef]
- Minaya, D.M.; Robertson, K.L.; Rowland, N.E. Circadian and economic factors affect food acquisition in rats restricted to discrete feeding opportunities. Physiol. Behav. 2017, 181, 10–15. [Google Scholar] [CrossRef]
- Taraschenko, O.D.; Maisonneuve, I.M.; Glick, S.D. Sex differences in high fat-induced obesity in rats: Effects of 18-methoxycoronaridine. Physiol. Behav. 2011, 103, 308–314. [Google Scholar] [CrossRef]
- Huang, K.P.; Ronveaux, C.C.; Knotts, T.A.; Rutkowsky, J.R.; Ramsey, J.J.; Raybould, H.E. Sex differences in response to short-term high fat diet in mice. Physiol. Behav. 2020, 221, 112894. [Google Scholar] [CrossRef]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex Differences in High-fat Diet-induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Choi, D.K.; Oh, T.S.; Choi, J.W.; Mukherjee, R.; Wang, X.; Liu, H.; Yun, J.W. Gender difference in proteome of brown adipose tissues between male and female rats exposed to a high fat diet. Cell. Physiol. Biochem. 2011, 28, 933–948. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R46–R54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yeh, C.-Y.; Bello, N.T. High-fat diet attenuates morphine withdrawal effects on sensory-evoked locus coeruleus norepinephrine neural activity in male obese rats. Nutr. Neurosci. 2022, 25, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Alternating Diet as a Preventive and Therapeutic Intervention for High Fat Diet-induced Metabolic Disorder. Sci. Rep. 2016, 6, 26325. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.W.; Russell, L.; Helfer, G.; Thomson, L.M.; Dalby, M.J.; Morgan, P.J. Photoperiod regulates lean mass accretion, but not adiposity, in growing F344 rats fed a high fat diet. PLoS ONE 2015, 10, e0119763. [Google Scholar] [CrossRef]
- Rojas, J.M.; Printz, R.L.; Niswender, K.D. Insulin detemir attenuates food intake, body weight gain and fat mass gain in diet-induced obese Sprague–Dawley rats. Nutr. Diabetes 2011, 1, e10. [Google Scholar] [CrossRef]
- Estrany, M.E.; Proenza, A.M.; Gianotti, M.; Lladó, I. High-fat diet feeding induces sex-dependent changes in inflammatory and insulin sensitivity profiles of rat adipose tissue. Cell Biochem. Funct. 2013, 31, 504–510. [Google Scholar] [CrossRef]
- Fourny, N.; Lan, C.; Bernard, M.; Desrois, M. Male and Female Rats Have Different Physiological Response to High-Fat High-Sucrose Diet but Similar Myocardial Sensitivity to Ischemia-Reperfusion Injury. Nutrients 2021, 13, 2914. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Tan, H.Z.; Zhai, Q.X.; Chen, W. Investigations of spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.F.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.Y.; Wang, Y.J.; Liu, C.; Wang, W.Z.; Wang, J.; et al. Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, P.P.; Wang, J. High-Fat Diet Alters the Intestinal Microbiota in Streptozotocin-Induced Type 2 Diabetic Mice. Microorganisms 2019, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Iwao, M.; Gotoh, K.; Arakawa, M.; Endo, M.; Honda, K.; Seike, M.; Murakami, K.; Shibata, H. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci. Rep. 2020, 10, 18768. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Jackson, C.D.; Weis, C.; Poirier, L.A.; Bechtel, D.H. Interactions of varying levels of dietary fat, carbohydrate, and fiber on food consumption and utilization, weight gain and fecal fat contents in female sprague-dawley rats. Nutr. Res. 1996, 16, 1735–1747. [Google Scholar] [CrossRef]
- Shang, W.; Si, X.; Zhou, Z.; Li, Y.; Strappe, P.; Blanchard, C. Characterization of fecal fat composition and gut derived fecal microbiota in high-fat diet fed rats following intervention with chito-oligosaccharide and resistant starch complexes. Food Funct. 2017, 8, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Jackman, M.R.; Kramer, R.E.; MacLean, P.S.; Bessesen, D.H. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1083–E1091. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.D.; Kramer, M.L.; Bishop, P. Estrogen and Substrate Metabolism. Sports Med. 2000, 29, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Escrich, R.; Costa, I.; Moreno, M.; Cubedo, M.; Vela, E.; Escrich, E.; Moral, R. A high-corn-oil diet strongly stimulates mammary carcinogenesis, while a high-extra-virgin-olive-oil diet has a weak effect, through changes in metabolism, immune system function and proliferation/apoptosis pathways. J. Nutr. Biochem. 2019, 64, 218–227. [Google Scholar] [CrossRef]
- Almog, T.; Kandel Kfir, M.; Levkovich, H.; Shlomai, G.; Barshack, I.; Stienstra, R.; Lustig, Y.; Leikin Frenkel, A.; Harari, A.; Bujanover, Y.; et al. Interleukin-1α deficiency reduces adiposity, glucose intolerance and hepatic de-novo lipogenesis in diet-induced obese mice. BMJ Open Diabetes Res. Care 2019, 7, e000650. [Google Scholar] [CrossRef]
- Kundu, A.; Richa, S.; Dey, P.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Lee, S.-Y.; Lee, B.-H.; Lee, K.Y.; Kacew, S.; et al. Protective effect of EX-527 against high-fat diet-induced diabetic nephropathy in Zucker rats. Toxicol. Appl. Pharmacol. 2020, 390, 114899. [Google Scholar] [CrossRef]
- Pinhal, C.S.; Lopes, A.; Torres, D.B.; Felisbino, S.L.; Rocha Gontijo, J.A.; Boer, P.A. Time-course morphological and functional disorders of the kidney induced by long-term high-fat diet intake in female rats. Nephrol. Dial. Transplant. 2013, 28, 2464–2476. [Google Scholar] [CrossRef]
- Yadav, H.; Quijano, C.; Kamaraju, A.K.; Gavrilova, O.; Malek, R.; Chen, W.; Zerfas, P.; Zhigang, D.; Wright, E.C.; Stuelten, C.; et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011, 14, 67–79. [Google Scholar] [CrossRef]
- Tsurutani, Y.; Fujimoto, M.; Takemoto, M.; Irisuna, H.; Koshizaka, M.; Onishi, S.; Ishikawa, T.; Mezawa, M.; He, P.; Honjo, S.; et al. The roles of transforming growth factor-β and Smad3 signaling in adipocyte differentiation and obesity. Biochem. Biophys. Res. Commun. 2011, 407, 68–73. [Google Scholar] [CrossRef]
- Mendes, L.O.; Scarano, W.R.; Rochel-Maia, S.S.; Fioruci-Fontaneli, B.A.; Chuffa, L.G.; Martinez, F.E. Testosterone therapy differently regulates the anti- and pro-inflammatory cytokines in the plasma and prostate of rats submitted to chronic ethanol consumption (UChB). Am. J. Reprod. Immunol. 2014, 72, 317–325. [Google Scholar] [CrossRef]
- De Paula, G.C.; Simões, R.F.; Garcia-Serrano, A.M.; Duarte, J.M. High-fat and high-sucrose diet-induced hypothalamic inflammation shows sex specific features in mice. Neurochem. Res. 2024, 49, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Liva, S.M.; Voskuhl, R.R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.D.; Almer, G.; Kaiser, M.; Zelzer, S.; Meinitzer, A.; Scharnagl, H.; Sedej, S.; Gruber, H.-J.; Herrmann, M. The effects of long-term moderate exercise and Western-type diet on oxidative/nitrosative stress, serum lipids and cytokines in female Sprague Dawley rats. Eur. J. Nutr. 2022, 61, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Aronica, S.M.; Fanti, P.; Kaminskaya, K.; Gibbs, K.; Raiber, L.; Nazareth, M.; Bucelli, R.; Mineo, M.; Grzybek, K.; Kumin, M.; et al. Estrogen disrupts chemokine-mediated chemokine release from mammary cells: Implications for the interplay between estrogen and IP-10 in the regulation of mammary tumor formation. Breast Cancer Res. Treat. 2004, 84, 235–245. [Google Scholar] [CrossRef]
- Aloui, F.; Charradi, K.; Hichami, A.; Subramaniam, S.; Khan, N.A.; Limam, F.; Aouani, E. Grape seed and skin extract reduces pancreas lipotoxicity, oxidative stress and inflammation in high fat diet fed rats. Biomed. Pharmacother. 2016, 84, 2020–2028. [Google Scholar] [CrossRef]
- Laurentius, T.; Kob, R.; Fellner, C.; Nourbakhsh, M.; Bertsch, T.; Sieber, C.C.; Bollheimer, L.C. Long-Chain Fatty Acids and Inflammatory Markers Coaccumulate in the Skeletal Muscle of Sarcopenic Old Rats. Dis. Markers 2019, 2019, 9140789. [Google Scholar] [CrossRef]
- Lewis, D.K.; Bake, S.; Thomas, K.; Jezierski, M.K.; Sohrabji, F. A high cholesterol diet elevates hippocampal cytokine expression in an age and estrogen-dependent manner in female rats. J. Neuroimmunol. 2010, 223, 31–38. [Google Scholar] [CrossRef]
- Tabata, Y.; Miyao, M.; Inamoto, T.; Ishii, T.; Hirano, Y.; Yamaoki, Y.; Ikada, Y. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng. 2000, 6, 279–289. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, Y.; Wang, Y.; Wang, L.; Zhang, L.; Zhao, Z.; Li, S. Lactobacillus plantarum S9 alleviates lipid profile, insulin resistance, and inflammation in high-fat diet-induced metabolic syndrome rats. Sci. Rep. 2022, 12, 15490. [Google Scholar] [CrossRef]
- Dharavath, R.N.; Arora, S.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. High fat-low protein diet induces metabolic alterations and cognitive dysfunction in female rats. Metab. Brain Dis. 2019, 34, 1531–1546. [Google Scholar] [CrossRef]
- Amengual-Cladera, E.; Lladó, I.; Gianotti, M.; Proenza, A.M. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism 2012, 61, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Alsiö, J.; Västermark, A.; Risérus, U.; Schiöth, H.B. Adipose tissue stearoyl-CoA desaturase 1 index is increased and linoleic acid is decreased in obesity-prone rats fed a high-fat diet. Lipids Health Dis. 2013, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Dunn-Meynell, A.A. Differential effects of exercise on body weight gain and adiposity in obesity-prone and -resistant rats. Int. J. Obes. 2006, 30, 722–727. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359. [Google Scholar] [CrossRef]
- Gómez-Crisóstomo, N.P.; De la Cruz-Hernández, E.N.; Méndez Méndez, E.R.; Hernández-Landero, M.F.; Camacho Liévano, J.U.; Martínez-Abundis, E. Differential effect of high-fat, high-sucrose and combined high-fat/high-sucrose diets consumption on fat accumulation, serum leptin and cardiac hypertrophy in rats. Arch. Physiol. Biochem. 2020, 126, 258–263. [Google Scholar] [CrossRef]
- Zengin, A.; Kropp, B.; Chevalier, Y.; Junnila, R.; Sustarsic, E.; Herbach, N.; Fanelli, F.; Mezzullo, M.; Milz, S.; Bidlingmaier, M.; et al. Low-carbohydrate, high-fat diets have sex-specific effects on bone health in rats. Eur. J. Nutr. 2016, 55, 2307–2320. [Google Scholar] [CrossRef]
- El-Haschimi, K.; Pierroz, D.D.; Hileman, S.M.; Bjørbaek, C.; Flier, J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Investig. 2000, 105, 1827–1832. [Google Scholar] [CrossRef]
- Buettner, C.; Pocai, A.; Muse, E.D.; Etgen, A.M.; Myers, M.G., Jr.; Rossetti, L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006, 4, 49–60. [Google Scholar] [CrossRef]
- Zhu, Q.; Qi, N.T.; Shen, L.; Lo, C.C.; Xu, M.F.; Duan, Q.; Ollberding, N.J.; Wu, Z.; Hui, D.Y.; Tso, P.; et al. Sexual Dimorphism in Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 2175. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Torres, C.; Crespo-Castrillo, A.; Diaz-Pacheco, S.; Healy, K.; Stanton, C.; Chowen, J.A.; Garcia-Segura, L.M.; Arevalo, M.A.; Cryan, J.F.; et al. High-fat diet alters stress behavior, inflammatory parameters and gut microbiota in Tg APP mice in a sex-specific manner. Neurobiol. Dis. 2021, 159, 105495. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.B.; Li, Y.S.; Li, X.Y.; Yang, X.Y.; Chen, H.Y.; Zhu, Y.; Lu, N.H.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef]

| Males | Females | ||||
|---|---|---|---|---|---|
| Cytokines | Category | LED | HED | LED | HED |
| IL-6 | Pro- and anti-inflammatory | 0.021 ± 0.001 | 0.027 ± 0.003 | 0.018 ± 0.001 | 0.020 ± 0.002 |
| IL-5 | Proinflammatory | 0.059 ± 0.007 | 0.047 ± 0.003 | 0.053 ± 0.003 | 0.058 ± 0.005 |
| IL-15 | Proinflammatory | 0.051 ± 0.006 | 0.045 ± 0.004 | 0.047 ± 0.003 | 0.046 ± 0.002 |
| IL-1β | Proinflammatory | 0.070 ± 0.007 | 0.056 ± 0.002 | 0.065 ± 0.003 | 0.062 ± 0.004 |
| Leptin | Proinflammatory | 0.050 ± 0.006 | 0.041 ± 0.002 | 0.046 ± 0.003 | 0.043 ± 0.003 |
| VEGF | Proinflammatory | 0.029 ± 0.003 | 0.027 ± 0.001 | 0.028 ± 0.002 | 0.028 ± 0.003 |
| TNF-α | Proinflammatory | 0.057 ± 0.007 | 0.044 ± 0.004 | 0.053 ± 0.003 | 0.050 ± 0.005 |
| MCP-1 | Proinflammatory | 0.027 ± 0.003 | 0.022 ± 0.001 | 0.028 ± 0.003 | 0.023 ± 0.001 |
| IFN-γ | Proinflammatory | 0.056 ± 0.008 | 0.045 ± 0.004 | 0.052 ± 0.003 | 0.047 ± 0.003 |
| MIP-1α | Proinflammatory | 0.019 ± 0.001 | 0.017 ± 0.002 | 0.017 ± 0.001 | 0.020 ± 0.001 |
| SCF | Stem cell factor | 0.074 ± 0.010 | 0.076 ± 0.016 | 0.067 ± 0.004 | 0.058 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minaya, D.M.; Hoss, A.; Bhagat, A.; Guo, T.L.; Czaja, K. Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats. Nutrients 2025, 17, 1147. https://doi.org/10.3390/nu17071147
Minaya DM, Hoss A, Bhagat A, Guo TL, Czaja K. Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats. Nutrients. 2025; 17(7):1147. https://doi.org/10.3390/nu17071147
Chicago/Turabian StyleMinaya, Dulce M., Adam Hoss, Ayushi Bhagat, Tai L. Guo, and Krzysztof Czaja. 2025. "Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats" Nutrients 17, no. 7: 1147. https://doi.org/10.3390/nu17071147
APA StyleMinaya, D. M., Hoss, A., Bhagat, A., Guo, T. L., & Czaja, K. (2025). Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats. Nutrients, 17(7), 1147. https://doi.org/10.3390/nu17071147







