The Prevalence, Risk Factors, and Clinical Outcomes of Vitamin C Deficiency in Adult Hospitalised Patients: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
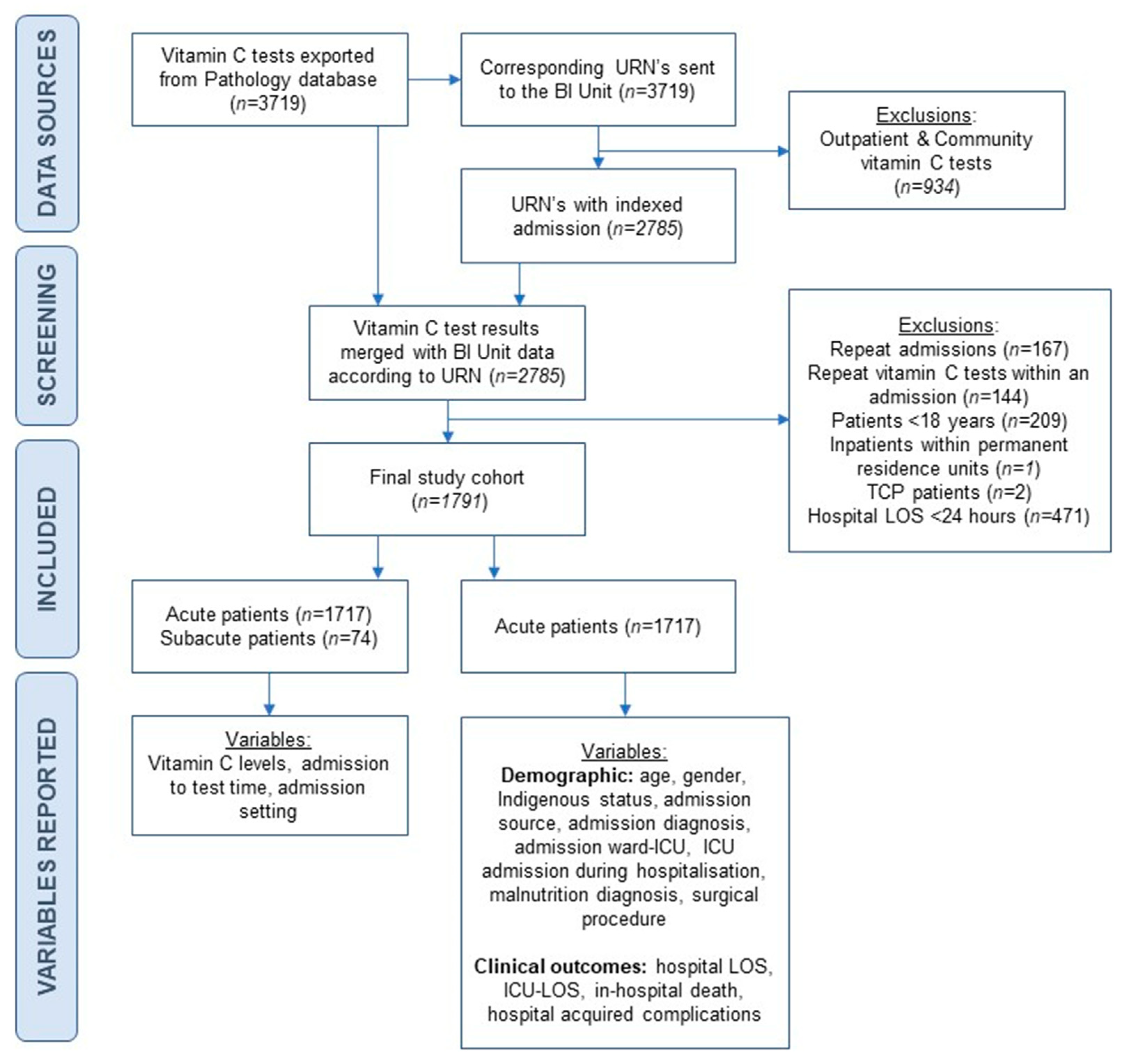
2.1. Study Design and Patients
2.2. Biochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Prevalence of VCD
3.3. Characteristics of Patients with VCD Within Acute Care Wards
3.4. Risk Factors Associated with VCD Status
3.5. VCD Status Associated with Hospital Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300–310. [Google Scholar] [CrossRef]
- Travica, N.; Ried, K.; Sali, A.; Huson, I.; Scholey, A.; Pipingas, A. Plasma vitamin C concentrations and cognitive function: A cross-sectional study. Front. Aging Neurosci. 2019, 11, 72–92. [Google Scholar]
- Sharma, Y.; Miller, M.; Shahi, R.; Doyle, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Vitamin C deficiency in Australian hospitalised patients: An observational study. Intern. Med. J. 2019, 49, 189–196. [Google Scholar] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar]
- Ravindran, P.; Wiltshire, S.; Das, K.; Wilson, R.B. Vitamin C deficiency in an Australian cohort of metropolitan surgical patients. Pathology 2018, 50, 654–658. [Google Scholar]
- Oudemans-van Straaten, H.; Man, A.; de Waard, M. Vitamin C revisited. Crit. Care 2014, 18, 460–472. [Google Scholar] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Powers, C.D.; Sternberg, M.R.; Patel, S.B.; Pfeiffer, C.M.; Storandt, R.J.; Schleicher, R.L. Vitamin C Status of US Adults Assessed as Part of the National Health and Nutrition Examination Survey Remained Unchanged between 2003–2006 and 2017–2018. J. Appl. Lab. Med. 2023, 8, 272–284. [Google Scholar] [CrossRef]
- World Health Organization. Scurvy and Its Prevention and Control in Major Emergencies. Available online: https://apps.who.int/iris/handle/10665/66962 (accessed on 10 April 2023).
- Anwar, A.; Hyder, S.; Mohamed Nor, N.; Younis, M. Government health expenditures and health outcome nexus: A study on OECD countries. Front. Public Health 2023, 11, 1123759. [Google Scholar]
- Golder, J.E.; Bauer, J.D.; Barker, L.A.; Lemoh, C.N.; Gibson, S.J.; Davidson, Z.E. Prevalence, risk factors, and clinical outcomes of vitamin C deficiency in adult hospitalized patients in high-income countries: A scoping review. Nutr. Rev. 2024, 82, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Ryoo, S.M.; Park, J.E.; Jo, Y.H.; Jang, D.H.; Suh, G.J.; Kim, T.; Kim, Y.J.; Kim, S.; Cho, H.; et al. Combination therapy of vitamin C and thiamine for septic shock: A multi-centre double-blinded randomized, controlled study. Intensive Care Med. 2020, 46, 2015–2025. [Google Scholar] [PubMed]
- Pena, G.; Kuang, B.; Cowled, P.; Howell, S.; Dawson, J.; Philpot, R.; Fitridge, R. Micronutrient Status in Diabetic Patients with Foot Ulcers. Adv. Wound Care 2020, 9, 9–15. [Google Scholar] [CrossRef]
- Nixon, D.W.; Heymsfield, S.B.; Cohen, A.E.; Kutner, M.H.; Ansley, J.; Lawson, D.H.; Rudman, D. Protein-calorie undernutrition in hospitalized cancer patients. Am. J. Med. 1980, 68, 683–690. [Google Scholar] [CrossRef]
- Gariballa, S. Poor vitamin C status is associated with increased depression symptoms following acute illness in older people. Int. J. Vitam. Nutr. Res. 2014, 84, 12–17. [Google Scholar] [CrossRef]
- Gan, R.; Hoffer, L.J.; Eintracht, S. Vitamin C Deficiency in a University Teaching Hospital. J. Am. Coll. Nutr. 2008, 27, 428–433. [Google Scholar] [CrossRef]
- Fain, O.; Pariés, J.; Jacquart, B.; Le Moël, G.; Kettaneh, A.; Stirnemann, J.; Héron, C.; Sitbon, M.; Taleb, C.; Letellier, E.; et al. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003, 14, 419–425. [Google Scholar] [CrossRef]
- Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Relationship between vitamin C deficiency and cognitive impairment in older hospitalised patients: A cross-sectional study. Antioxidants 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Golder, J.E.; Bauer, J.D.; Barker, L.A.; Lemoh, C.N.; Gibson, S.J.; Davidson, Z.E. Exploring the relationship between vitamin C deficiency and protein-energy malnutrition in adult hospitalised patients: A cross-sectional study. Nutr. Diet. 2024. online ahead of print. [Google Scholar]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar]
- Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study. Nutrients 2021, 13, 2117. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. AR-DRG Version 11.0. Available online: https://www.ihacpa.gov.au/resources/ar-drg-version-110 (accessed on 1 November 2023).
- World Health Organisation. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2019/en (accessed on 14 August 2023).
- Independent Health and Aged Care Pricing Authority. Australian Coding Standards for the International Statistical Classification of Disease and Related Health Problems, Tenth Revision (ICD-10-AM). Available online: https://www.ihacpa.gov.au/resources/icd-10-amachiacs-twelfth-edition (accessed on 10 November 2023).
- Australian Government. Hospital-Acquired Complications (HACs). Available online: https://www.safetyandquality.gov.au/our-work/indicators/hospital-acquired-complications (accessed on 8 June 2023).
- Bujang, M.A.; Sa’at, N.; Sidik, T.; Joo, L.C. Sample Size Guidelines for Logistic Regression from Observational Studies with Large Population: Emphasis on the Accuracy Between Statistics and Parameters Based on Real Life Clinical Data. Malays. J. Med. Sci. 2018, 25, 122–130. [Google Scholar]
- Fernandez, G.A.; Vatcheva, K.P. A comparison of statistical methods for modeling count data with an application to hospital length of stay. BMC Med. Res. Methodol. 2022, 22, 211. [Google Scholar]
- Cass, A.R.; Charlton, K. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. J. Hum. Nutr. Diet. 2022, 35, 1043–1058. [Google Scholar]
- Armstrong, E.; Jamieson, R.; Porter, J. Food cooking methods contribute to the reduced vitamin C content of foods prepared in hospitals and care facilities: A systematic review. Int. J. Food Sci. Technol. 2019, 54, 291–299. [Google Scholar]
- Paillaud, E.; Merlier, I.; Dupeyron, C.; Scherman, E.; Poupon, J.; Bories, P.N. Oral candidiasis and nutritional deficiencies in elderly hospitalised patients. Br. J. Nutr. 2004, 92, 861–867. [Google Scholar] [PubMed]
- Khalife, R.; Grieco, A.; Khamisa, K.; Tinmouh, A.; McCudden, C.; Saidenberg, E. Scurvy, an old story in a new time: The hematologist’s experience. Blood Cells Mol. Dis. 2019, 76, 40–44. [Google Scholar] [CrossRef]
- Gordon, B.L.; Galati, J.S.; Yang, S.; Longman, R.S.; Lukin, D.; Scherl, E.J.; Battat, R. Prevalence and factors associated with vitamin C deficiency in inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 4834–4845. [Google Scholar]
- Fennessy, G.J.; Warrillow, S.J. Gastrointestinal problems in intensive care. Anaesth. Intensive Care Med. 2015, 16, 165–170. [Google Scholar]
- Llibre-Nieto, G.; Lira, A.; Vergara, M.; Solé, C.; Casas, M.; Puig-Diví, V.; Solé, G.; Humanes, A.; Grau, L.; Barradas, J.M.; et al. Micronutrient deficiencies in patients with decompensated liver cirrhosis. Nutrients 2021, 13, 1249. [Google Scholar] [CrossRef]
- Story, D.A.; Ronco, C.; Bellomo, R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit. Care Med. 1999, 27, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with community acquired pneumonia exhibit depleted vitamin c status and elevated oxidative stress. Nutrients 2020, 12, 1318. [Google Scholar] [CrossRef]
- Laven, G.T.; Huang, C.; DeVivo, M.J.; Stover, S.L.; Kuhlemeier, K.V.; Fine, P.R. Nutritional status during the acute stage of spinal cord injury. Arch. Phys. Med. Rehabil. 1989, 70, 277–282. [Google Scholar] [PubMed]
- Bollet, A.J.; Owens, S. Evaluation of nutritional status of selected hospitalized patients. Am. J. Clin. Nutr. 1973, 26, 931–938. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Evans-Olders, R.; Eintracht, S.; Hoffer, L.J. Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 2010, 26, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Chakravorty, N.; Annan, G.; Habibzadeh, N.; Schorah, C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam. Nutr. Res. 1994, 64, 212–219. [Google Scholar]
- Wang, Y.; Liu, X.J.; Robitaille, L.; Eintracht, S.; MacNamara, E.; Hoffer, L.J. Effects of vitamin C and vitamin D administration on mood and distress in acutely hospitalized patients. Am. J. Clin. Nutr. 2013, 98, 705–711. [Google Scholar] [CrossRef]
- Schmuck, A.; Ravel, A.; Coudray, C.; Alary, J.; Franco, A.; Roussel, A.-M. Antioxidant vitamins in hospitalized elderly patients: Analysed dietary intakes and biochemical status. Eur. J. Clin. Nutr. 1996, 50, 473–478. [Google Scholar]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Marik, P.E.; Liggett, A. Adding an orange to the banana bag: Vitamin C deficiency is common in alcohol use disorders. Crit. Care 2019, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Mwala, N.N.; Borkent, J.W.; van der Meij, B.S.; de van der Schueren, M.A.E. Challenges in Identifying Malnutrition in Obesity; An Overview of the State of the Art and Directions for Future Research. Nutr. Res. Rev. 2024, 1–27. [Google Scholar]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar]
- Watterson, C.; Fraser, A.; Banks, M.; Isenring, E.; Miller, M.; Silvester, C.; Hoevenaars, R.; Bauer, J.; Vivanti, A.; Ferguson, M. Evidence based practice guidelines for the nutritional management of malnutrition in adult patients across the continuum of care. Nutr. Diet. 2009, 66, S1–S34. [Google Scholar]
- Bailey, R.L. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar]
- Emanuel, A.S.; McCully, S.N.; Gallagher, K.M.; Updegraff, J.A. Theory of Planned Behavior explains gender difference in fruit and vegetable consumption. Appetite 2012, 59, 693–697. [Google Scholar]
- Australian Bureau of Statistics. Insights into Australian Smokers, 2021–2022. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/smoking/2020-21 (accessed on 5 February 2025).
- Australian Bureau of Statistics. Alcohol Consumption. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/alcohol-consumption/latest-release (accessed on 5 February 2025).
- Carr, A.C.; Block, G.; Lykkesfeldt, J. Estimation of Vitamin C Intake Requirements Based on Body Weight: Implications for Obesity. Nutrients 2022, 14, 1460. [Google Scholar] [CrossRef]
- Australian Government. Australia’s Health 2018. Available online: https://www.aihw.gov.au/reports/australias-health/australias-health-2018/contents/indicators-of-australias-health/fruit-and-vegetable-intake (accessed on 5 February 2025).
- Hui, S.; Lim, A.; Koh, E.; Abasszade, J.; Morgan, A.; Tan, P.Y.; Lemoh, C.; Robertson, M. Prevalence and prognostic significance of vitamin C deficiency in patients with acute upper gastrointestinal bleeding: A prospective cohort study. Aliment. Pharmacol. Ther. 2023, 57, 313–322. [Google Scholar]
- Mayr, F.; Yende, S.; Angus, D. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar]
| Patient Demographics | Total Cohort (n = 1717) | VCD Cohort (n = 399) | Not VCD Cohort (n = 1318) |
|---|---|---|---|
| Age at admission in years (MD, IQR) | 65 (49, 78) | 63 (50, 75) * | 66 (48, 79) |
| Gender: | |||
| Male (n, %) | 860 (50.1) | 230 (57.6) †† | 630 (47.8) |
| Female (n, %) | 857 (49.9) | 169 (42.4) | 688 (52.2) |
| Indigenous status: | |||
| Not Aboriginal or Torres Strait Islander (n, %) | 1682 (98) | 386 (96.7) † | 1296 (98.3) |
| Aboriginal and/or Torres Strait Islander (n, %) | 22 (1.3) | 6 (1.5) | 16 (1.2) |
| Question not able to be asked (n, %) | 13 (0.7) | 7 (1.8) | 6 (0.5) |
| Admitted from: | |||
| Home (n, %) | 1333 (77.6) | 304 (76.2) | 1029 (78.1) |
| Residential aged care facility (n, %) | 58 (3.4) | 5 (1.25) † | 53 (4) |
| Hospital transfer (n, %) | 300 (17.5) | 85 (21.3) † | 215 (16.3) |
| Other (n, %) | 26 (1.5) | 5 (1.25) | 21 (1.6) |
| Admission ward-ICU (n, %) | 95 (5.5) | 30 (7.5) † | 65 (4.9) |
| ICU admission during hospitalisation (n, %) | 327 (19) | 122 (30.6) †† | 205 (15.6) |
| Major diagnostic category: | |||
| Respiratory (n, %) | 201 (11.7) | 28 (7) †† | 173 (13.1) |
| Circulatory (n, %) | 136 (7.9) | 25 (6.3) | 111 (8.4) |
| Digestive (n, %) | 360 (21) | 115 (28.8) †† | 245 (18.7) |
| Hepatobiliary and pancreas (n, %) | 84 (4.9) | 22 (5.5) | 62 (4.7) |
| Musculoskeletal and connective tissue (n, %) | 157 (9.1) | 34 (8.5) | 123 (9.3) |
| Skin, subcutaneous tissue and breast (n, %) | 132 (7.7) | 21(5.3) † | 111 (8.4) |
| Endocrine, nutrition and metabolic | 89 (5.2) | 24 (6) | 65 (4.9) |
| Infectious and parasitic diseases (n, %) | 104 (6.1) | 26 (6.5) | 78 (5.9) |
| Other (n, %) | 454 (26.4) | 104 (26.1) | 350 (26.6) |
| Malnutrition diagnosis (n, %) | 611 (35.6) | 183 (45.9) †† | 428 (32.5) |
| Surgical procedure (n, %) | 731 (42.6) | 209 (52.4) †† | 522 (39.6) |
| Clinical Outcomes | Total Cohort (n = 1717) | VCD Cohort (n = 399) | Not VCD Cohort (n = 1318) |
|---|---|---|---|
| LOS (hospital) in days (MD, IQR) | 12.8 (6.2, 27.1) | 14.4 (7.21, 31.2) * | 12.4 (5.9, 25.9) |
| Adjusted for in-hospital death (n = 1635) | 12.6 (6.1, 26.8) | 14.4 (7.2, 31.3) * | 12.0 (5.9, 25.0) |
| ICU-LOS (admission ward-ICU) in days, n = 95 (MD, IQR) | 4.1 (1.8, 8.1) | 4.7 (2.6, 7.5) | 3.8 (1.7, 8.7) |
| Adjusted for in-hospital death (n = 86) | 3.7 (1.8, 7.2) | 4.1 (2.5, 7.9) | 3.3 (1.6, 6.9) |
| ICU-LOS (ICU admission during hospitalisation) in days, n = 327 (MD, IQR) | 3.8 (1.7, 8) | 4.3 (1.9, 10.1) | 3.6 (1.6, 7) |
| Adjusted for in-hospital death (n = 282) | 3.4 (1.6, 6.8) | 4.0 (1.9, 8.9) * | 3.0 (1.6, 6.0) |
| In-hospital death (n, %) | 82 (4.8) | 24 (6) | 58 (4.4) |
| Hospital Acquired Complications: | |||
| Pressure Injury; all (n, %) | 16 (0.9) | 5 (1.3) | 11 (0.8) |
| Gastrointestinal bleeding; all (n, %) | 35 (2.0) | 17 (4.3) ††† | 18 (1.4) |
| Delirium; all (n, %) | 84 (4.9) | 31 (7.8) †† | 53 (4) |
| Surgical requiring unplanned return to theatre; all (n, %) | 74 (4.3) | 21 (5.3) | 53 (4) |
| Cardiac; all (n, %) | 64 (3.7) | 20 (5) | 44 (3.3) |
| Falls resulting in fracture or other intracranial injury; all (n, %) | 7 (0.4) | 2 (0.5) | 5 (0.4) |
| Healthcare associate infection | |||
| Urinary tract infection (n, %) | 55 (3.2) | 15 (3.8) | 40 (3) |
| Surgical site infection (n, %) | 2 (0.1) | 1 (0.3) | 1 (0.1) |
| Blood stream infection (n, %) | 19 (1.1) | 6 (1.5) | 13 (1) |
| High impact infections (n, %) | 69 (4.0) | 25 (6.3) †† | 44 (3.3) |
| Pneumonia infections (n, %) | 109 (6.3) | 35 (8.8) †† | 74 (5.6) |
| All other healthcare associated infections (n, %) | 40 (2.3) | 17 (4.3) †† | 23 (1.7) |
| Respiratory complications | |||
| Respiratory failure including ARDS (n, %) | 41 (2.4) | 16 (4) †† | 25 (1.9) |
| Aspiration pneumonia (n, %) | 34 (2.0) | 12 (3) † | 22 (1.7) |
| Pulmonary oedema (n, %) | 2 (0.1) | 1 (0.3) | 1 (0.1) |
| Demographic and Clinical Predictor Variables | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Admission age (years) | 1.00 (0.99, 1.01) | 0.670 | -- | -- |
| Male gender | 1.50 (1.20, 1.88) | <0.001 | 1.47 (1.17, 1.86) | 0.001 |
| Admitted from residential aged care facility | 0.32 (0.13, 0.80) | 0.016 | 0.43 (0.17, 1.09) | 0.076 |
| Admitted from hospital transfer | 1.37 (1.03, 1.81) | 0.029 | 1.17 (0.88, 1.57) | 0.278 |
| Respiratory diagnosis | 0.50 (0.33, 0.75) | 0.001 | 0.62 (0.40, 0.96) | 0.033 |
| Digestive diagnosis | 1.73 (1.34, 2.25) | <0.001 | 1.36 (1.02, 1.82) | 0.037 |
| Skin, subcutaneous tissue, and breast diagnosis | 0.62 (0.38, 1.00) | 0.050 | 0.75 (0.46, 1.24) | 0.263 |
| Protein–energy malnutrition diagnosis | 1.72 (1.37, 2.17) | <0.001 | 1.50 (1.19, 1.91) | <0.001 |
| Surgical procedure | 1.65 (1.31, 2.08) | <0.001 | 1.31 (1.02, 1.68) | 0.037 |
| Admission ward-ICU | 1.60 (1.02, 2.50) | 0.042 | 1.59 (1.00, 2.52) | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golder, J.; Bauer, J.; Barker, L.A.; Lemoh, C.; Gibson, S.; Davidson, Z.E. The Prevalence, Risk Factors, and Clinical Outcomes of Vitamin C Deficiency in Adult Hospitalised Patients: A Retrospective Observational Study. Nutrients 2025, 17, 1131. https://doi.org/10.3390/nu17071131
Golder J, Bauer J, Barker LA, Lemoh C, Gibson S, Davidson ZE. The Prevalence, Risk Factors, and Clinical Outcomes of Vitamin C Deficiency in Adult Hospitalised Patients: A Retrospective Observational Study. Nutrients. 2025; 17(7):1131. https://doi.org/10.3390/nu17071131
Chicago/Turabian StyleGolder, Janet, Judith Bauer, Lisa A. Barker, Christopher Lemoh, Simone Gibson, and Zoe E. Davidson. 2025. "The Prevalence, Risk Factors, and Clinical Outcomes of Vitamin C Deficiency in Adult Hospitalised Patients: A Retrospective Observational Study" Nutrients 17, no. 7: 1131. https://doi.org/10.3390/nu17071131
APA StyleGolder, J., Bauer, J., Barker, L. A., Lemoh, C., Gibson, S., & Davidson, Z. E. (2025). The Prevalence, Risk Factors, and Clinical Outcomes of Vitamin C Deficiency in Adult Hospitalised Patients: A Retrospective Observational Study. Nutrients, 17(7), 1131. https://doi.org/10.3390/nu17071131







