Bicarbonate-Rich Mineral Water Mitigates Hypoxia-Induced Osteoporosis in Mice via Gut Microbiota and Metabolic Pathway Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Drinking Water for Experiment
2.2. Animals and Experimental Groups
2.3. Hypobaric and Hypoxic Exposure
2.4. Mouse Bone Densitometry
2.5. Micro-CT Scanning and 3D Reconstruction
2.6. Three-Point Bending Test
- (1)
- Cross-sectional moment of inertia
- (2)
- Maximum bending stress:
- (3)
- Maximum strain:
2.7. Preparation and Staining of Mouse Femur Sections
2.8. Routine Blood Tests and Analysis of Serum Indicators
2.9. 16S rRNA Sequencing
2.10. Untargeted Metabolomics
2.11. Statistical Analysis
3. Results
3.1. Minerals in Water
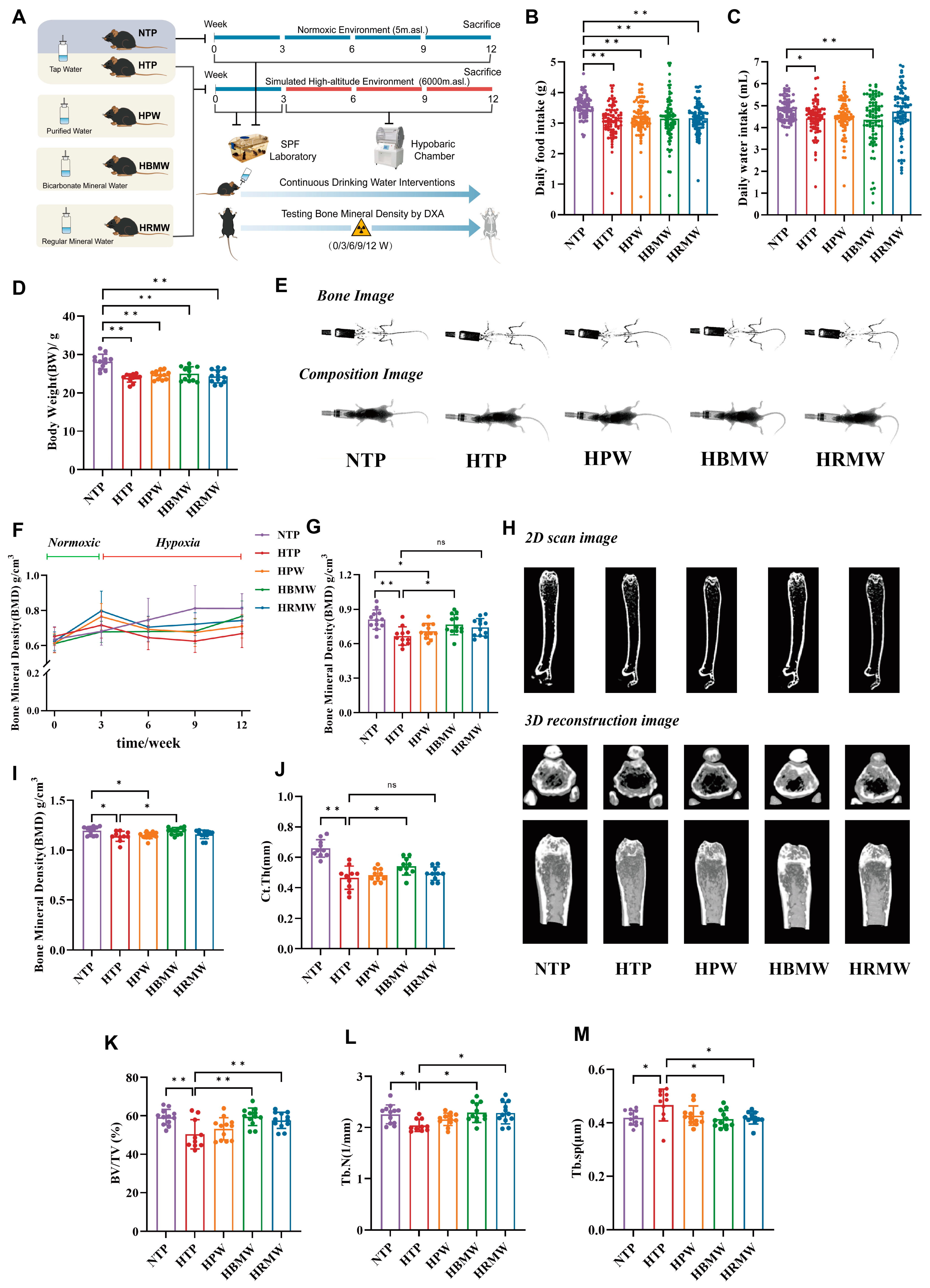
3.2. Bodyweight, Diet, and Water Consumption
3.3. Protective Effect of BMW Against High-Altitude Hypoxia-Induced Osteoporosis
3.3.1. BMW Maintains Bone Mineral Density Under High Altitude Hypoxia Exposure
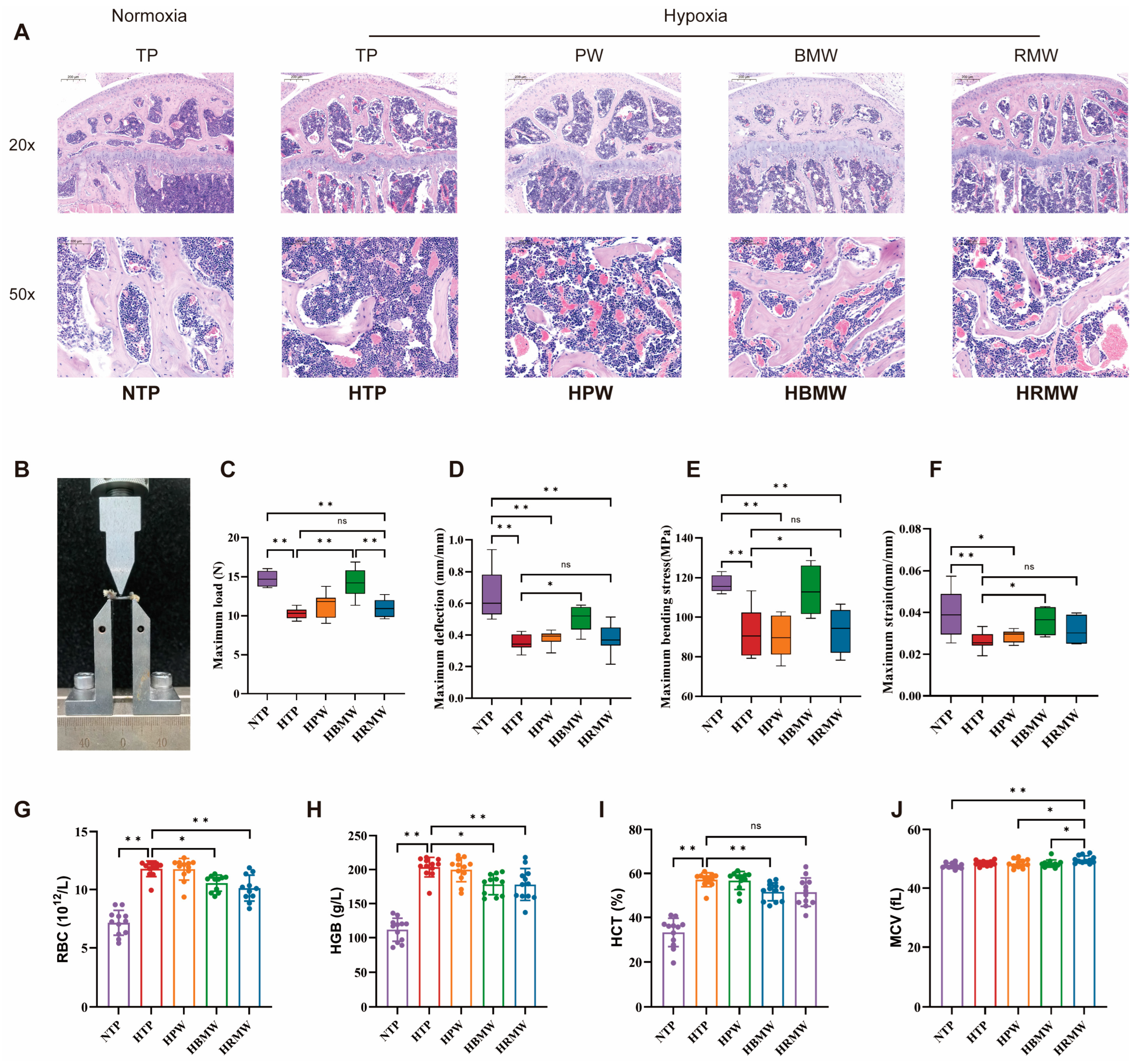
3.3.2. BMW Improves Bone Resorption and Formation Related Indicators Under High-Altitude Hypoxia Exposure
3.3.3. BMW Improves Bone Support and Hematopoiesis Under High Altitude Hypoxia Exposure
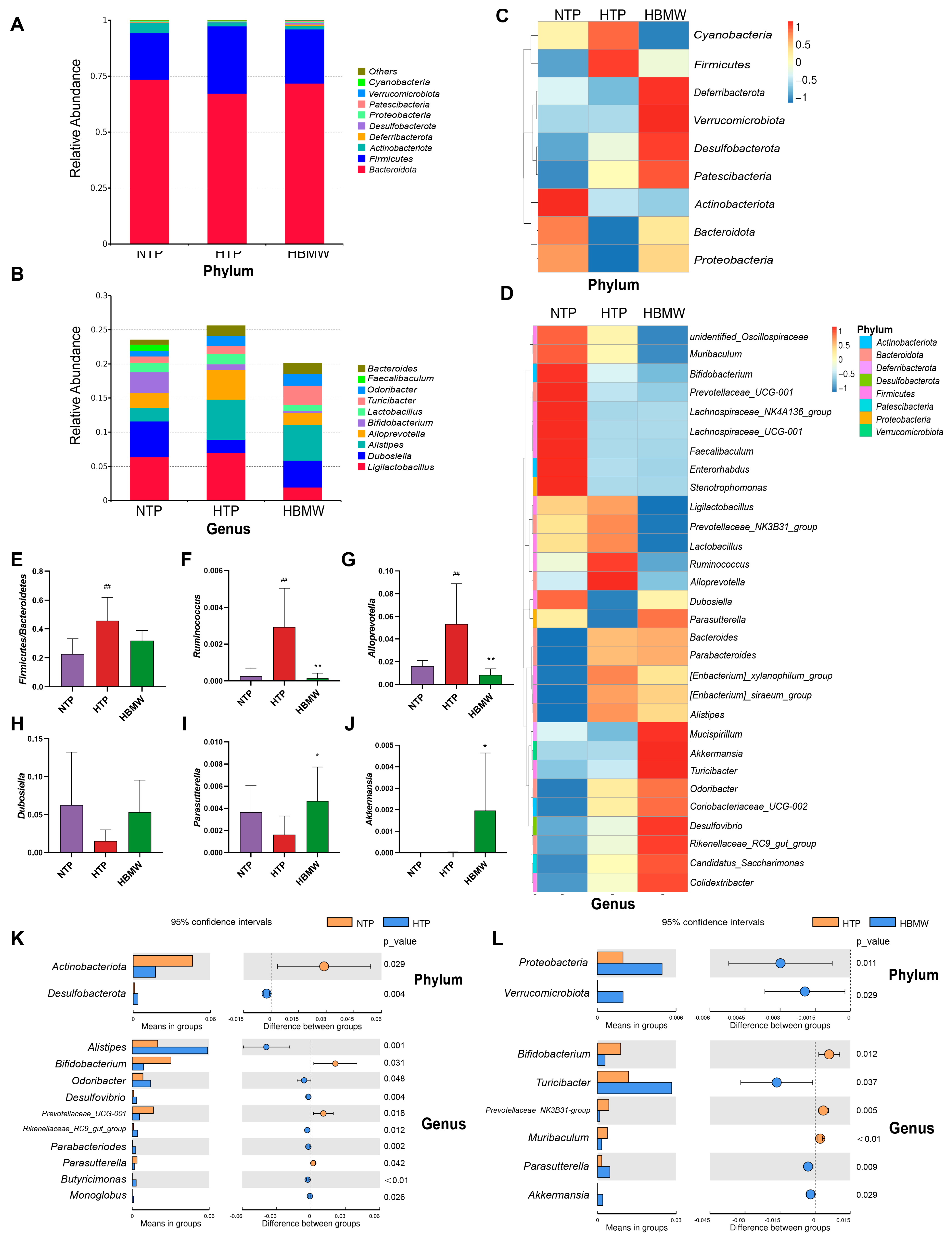
3.4. BMW Alters the Gut Microbiota Structure of Mice Under High-Altitude Hypoxia Exposure
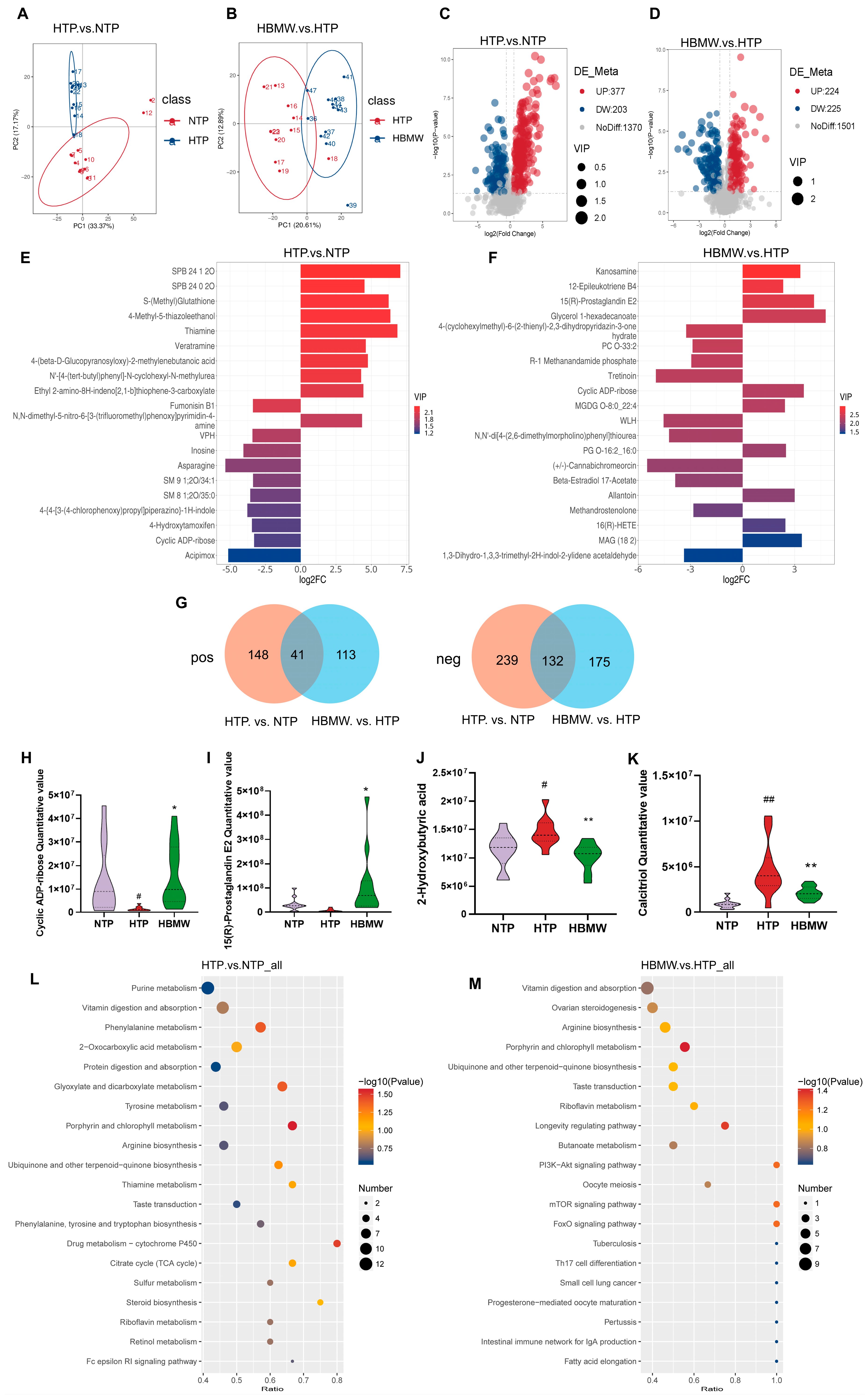
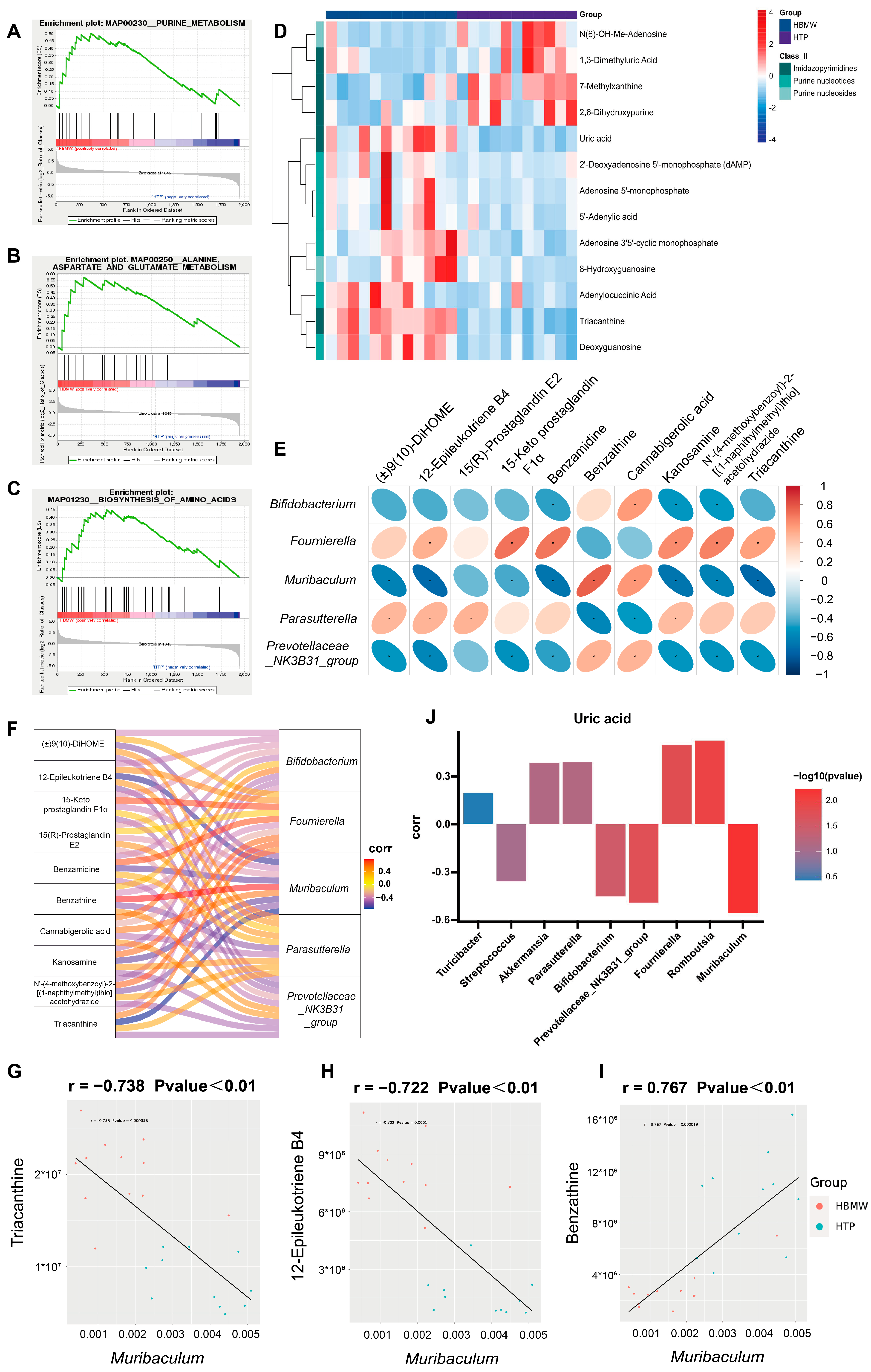
3.5. BMW Induces Changes in the Metabolite Profiles in Fecal Samples of Mice Under High-Altitude Hypoxia Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DXA | Dual-energy X-ray Absorptiometry |
| UHPLC-MS/MS | Ultra-High Performance Liquid Chromatography coupled with Tandem Mass Spectrometry |
| PAM | PI3K/AKT/mTOR |
| BMD | Bone Mineral Density |
| MSCs | Mesenchymal Stem Cells |
| SPF | Specific Pathogen-Free |
| BV/TV | bone volume fraction |
| Tb.Th | trabecular thickness |
| Tb.N | trabecular number |
| Tb.Sp | trabecular bone spacing |
| SMI | structure model index |
| EUTM | Electronic Universal Testing Machine |
| Fmax | maximum load |
| dmax | maximum deflection |
| CTX I | Cross Linked C-Telopeptide of Type I Collagen |
| BALP | Bone Alkaline Phosphatase |
| PINP | Propeptide of Type I Procollagen |
| PTH | Parathyroid Hormone |
| BGP | Bone Gla-protein |
| CT | Calcitonin |
| CTAB | Cetyltrimethylammonium Ammonium Bromide |
| TRAP | Tartrate-resistant Acid Phosphatase |
| HAPC | High-altitude Polycythemia |
| cADPR | cyclic ADP-ribose |
| GSEA | Gene Set Enrichment Analysis |
| dAMP | deoxyadenosine monophosphate |
| PGE2 | Prostaglandin E2 |
| EP4 | Prostaglandin E2 receptor 4 |
References
- World Health Organization. Nutrients in Drinking Water; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Marque, S.; Jacqmin-Gadda, H.; Dartigues, J.F.; Commenges, D. Cardiovascular mortality and calcium and magnesium in drinking water: An ecological study in elderly people. Eur. J. Epidemiol. 2003, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Theisen, C.F.; Wodschow, K.; Hansen, B.; Schullehner, J.; Gislason, G.; Ersbøll, B.K.; Ersbøll, A.K. Drinking water magnesium and cardiovascular mortality: A cohort study in Denmark, 2005–2016. Environ. Int. 2022, 164, 107277. [Google Scholar] [CrossRef]
- Ramsay, L.; Petersen, M.M.; Hansen, B.; Schullehner, J.; van der Wens, P.; Voutchkova, D.; Kristiansen, S.M. Drinking Water Criteria for Arsenic in High-Income, Low-Dose Countries: The Effect of Legislation on Public Health. Environ. Sci. Technol. 2021, 55, 3483–3493. [Google Scholar] [CrossRef]
- Peterson, A.K.; Moody, M.; Nakashima, I.; Abraham, R.; Schmidt, T.A.; Rowe, D.; Deymier, A. Effects of acidosis on the structure, composition, and function of adult murine femurs. Acta Biomater. 2021, 121, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Melamed, M.L.; Abramowitz, M.K. Serum bicarbonate and bone mineral density in US adults. Am. J. Kidney Dis. 2015, 65, 240–248. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Palermo, N.J.; Gilhooly, C.H.; Shea, M.K.; Fielding, R.A.; Ceglia, L. Potassium Bicarbonate Supplementation Lowers Bone Turnover and Calcium Excretion in Older Men and Women: A Randomized Dose-Finding Trial. J. Bone Miner. Res. 2015, 30, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Katz, R.; Larive, B.; Kendrick, C.; Isakova, T.; Sprague, S.; Wolf, M.; Raj, D.S.; Fried, L.F.; Gassman, J.; et al. Oral Sodium Bicarbonate and Bone Turnover in CKD: A Secondary Analysis of the BASE Pilot Trial. J. Am. Soc. Nephrol. 2024, 35, 57–65. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wu, Y.; Liu, X.F.; Chen, X.; Su, J.C. Targeting the gut microbiota-related metabolites for osteoporosis: The inextricable connection of gut-bone axis. Ageing Res. Rev. 2024, 94, 102196. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Krieger, N.S. Effects of acid on bone. Kidney Int. 2022, 101, 1160–1170. [Google Scholar] [CrossRef]
- Wang, L.; Yu, W.; Yin, X.; Cui, L.; Tang, S.; Jiang, N.; Cui, L.; Zhao, N.; Lin, Q.; Chen, L.; et al. Prevalence of Osteoporosis and Fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw. Open 2021, 4, e2121106. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Cenzer, I.; Nguyen, B.; Lee, S.J. Time to Benefit of Bisphosphonate Therapy for the Prevention of Fractures Among Postmenopausal Women With Osteoporosis: A Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Händel, M.N.; Cardoso, I.; von Bülow, C.; Rohde, J.F.; Ussing, A.; Nielsen, S.M.; Christensen, R.; Body, J.J.; Brandi, M.L.; Diez-Perez, A.; et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: Systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ 2023, 381, e068033. [Google Scholar] [CrossRef]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef]
- Bozec, A.; Bakiri, L.; Hoebertz, A.; Eferl, R.; Schilling, A.F.; Komnenovic, V.; Scheuch, H.; Priemel, M.; Stewart, C.L.; Amling, M.; et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 2008, 454, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Otsuka, Y.; Aoki, H.; Goto, Y.; Kawaguchi, Y.; Waguri-Nagaya, Y.; Miyazawa, K.; Goto, S.; Aoyama, M. The Inducible Nitric Oxide Synthase Pathway Promotes Osteoclastogenesis under Hypoxic Culture Conditions. Am. J. Pathol. 2021, 191, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, S.M.; Song, S.U.; Park, S.G.; Kim, W.S.; Park, I.G.; Lee, J.; Sung, J.H. Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation. Int. J. Mol. Sci. 2016, 17, 1389. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, K.; Zha, X.; Du, X.; Li, Y.; Chen, S.; Wu, Y.; Li, S.; Lu, Y.; Zhang, Y.; et al. Opportunistic Screening Using Low-Dose CT and the Prevalence of Osteoporosis in China: A Nationwide, Multicenter Study. J. Bone Miner. Res. 2021, 36, 427–435. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.; Li, Y.; Ge, J.; Pan, J.; Li, G.; He, Y.; Zhong, H.; Wang, B.; Huang, Y.; et al. Gut microbiota facilitates adaptation of the plateau zokor (Myospalax baileyi) to the plateau living environment. Front. Microbiol. 2023, 14, 1136845. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Waldbaum, J.D.H.; Xhumari, J.; Akinsuyi, O.S.; Arjmandi, B.; Anton, S.; Roesch, L.F.W. Association between Dysbiosis in the Gut Microbiota of Primary Osteoporosis Patients and Bone Loss. Aging Dis. 2023, 14, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.R.; Ward, W.E.; Comelli, E.M. Gut microbiota-bone axis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, B.C.; Dai, X.Y.; Xu, Y.R.; Kang, J.X.; Li, J.L. Drinking alkaline mineral water confers diarrhea resistance in maternally separated piglets by maintaining intestinal epithelial regeneration via the brain-microbe-gut axis. J. Adv. Res. 2023, 52, 29–43. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Deng, Z.; Xiang, T.; Ni, Q.; Xu, J.; Sun, D.; Luo, F. Gut microbially produced tryptophan metabolite melatonin ameliorates osteoporosis via modulating SCFA and TMAO metabolism. J. Pineal Res. 2024, 76, e12954. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kumar, V.; Rohilla, A. Understanding Osteoporosis: Human Bone Density, Genetic Mechanisms, Gut Microbiota, and Future Prospects. Probiotics Antimicrob. Proteins 2023, 16, 875–883. [Google Scholar] [CrossRef]
- GB 5749-2022; Chinese National Drinking Water Standard. National Standardization Administration: Beijing, China, 2022.
- GB 14924.3-2010; Laboratory Animals—Nutrients for Formula Feed. National Standardization Administration: Beijing, China, 2010.
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Sellick, C.A.; Hansen, R.; Stephens, G.M.; Goodacre, R.; Dickson, A.J. Metabolite extraction from suspension-cultured mammalian cells for global metabolite profiling. Nat. Protoc. 2011, 6, 1241–1249. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Wynn, E.; Krieg, M.A.; Aeschlimann, J.M.; Burckhardt, P. Alkaline mineral water lowers bone resorption even in calcium sufficiency: Alkaline mineral water and bone metabolism. Bone 2009, 44, 120–124. [Google Scholar] [CrossRef]
- Burckhardt, P. The effect of the alkali load of mineral water on bone metabolism: Interventional studies. J. Nutr. 2008, 138, 435S–437S. [Google Scholar] [CrossRef] [PubMed]
- Lossdörfer, S.; Götz, W.; Jäger, A. PTH(1-34)-induced changes in RANKL and OPG expression by human PDL cells modify osteoclast biology in a co-culture model with RAW 264.7 cells. Clin. Oral. Investig. 2011, 15, 941–952. [Google Scholar] [CrossRef]
- Jilka, R.L.; O’Brien, C.A.; Bartell, S.M.; Weinstein, R.S.; Manolagas, S.C. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J. Bone Miner. Res. 2010, 25, 2427–2437. [Google Scholar] [CrossRef]
- Lan, D.; Ji, W.; Lin, B.; Chen, Y.; Huang, C.; Xiong, X.; Fu, M.; Mipam, T.D.; Ai, Y.; Zeng, B.; et al. Correlations between gut microbiota community structures of Tibetans and geography. Sci. Rep. 2017, 7, 16982. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yao, Y.; Li, D.; Xu, H.; Wu, J.; Wen, A.; Xie, M.; Ni, Q.; Zhang, M.; Peng, G.; et al. Characterization of the Gut Microbiota in Six Geographical Populations of Chinese Rhesus Macaques (Macaca mulatta), Implying an Adaptation to High-Altitude Environment. Microb. Ecol. 2018, 76, 565–577. [Google Scholar] [CrossRef]
- Bai, X.; Liu, G.; Yang, J.; Zhu, J.; Wang, Q.; Zhou, Y.; Gu, W.; La, L.; Li, X. Changes in the Gut Microbiota of Rats in High-Altitude Hypoxic Environments. Microbiol. Spectr. 2022, 10, e0162622. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Bifari, F.; Ruocco, C.; Decimo, I.; Fumagalli, G.; Valerio, A.; Nisoli, E. Amino acid supplements and metabolic health: A potential interplay between intestinal microbiota and systems control. Genes. Nutr. 2017, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Tian, X.; Zhao, M.; Mu, Y.; Yi, H.; Zhang, Z.; Zhang, L. Bifidobacterium improves oestrogen-deficiency-induced osteoporosis in mice by modulating intestinal immunity. Food Funct. 2024, 15, 1840–1851. [Google Scholar] [CrossRef]
- Coskun, M.; Babayeva, A.; Barlas, T.; Muhittin Yalcin, M.; Akturk, M.; Balos Toruner, F.; Ayhan Karakoc, M.; Karakan, T.; Cindoruk, M.; Yetkin, I.; et al. Relationship between gut microbiome and bone deficits in primary hyperparathyroidism: A proof-of-concept pilot study. J. Investig. Med. 2024, 72, 541–552. [Google Scholar] [CrossRef]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.; Gu, X.; Su, Y.; Zheng, Q.; Yuan, X.; Bao, Z.; Lu, J.; Li, L. Health and Disease: Akkermansia muciniphila, the Shining Star of the Gut Flora. Research 2023, 6, 0107. [Google Scholar] [CrossRef]
- Liu, T.H.; Zhao, L.; Zhang, C.Y.; Li, X.Y.; Wu, T.L.; Dai, Y.Y.; Sheng, Y.Y.; Ren, Y.L.; Xue, Y.Z. Gut microbial evidence chain in high-salt diet exacerbates intestinal aging process. Front. Nutr. 2022, 9, 1046833. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Si, J.; Kang, H.; You, H.J.; Ko, G. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Yan, H.; Li, T.; Gao, X.; Wang, Y.; Li, A.; Wang, S.; et al. The Relationship Between Osteoporosis and Intestinal Microbes in the Henan Province of China. Front. Cell Dev. Biol. 2021, 9, 752990. [Google Scholar] [CrossRef]
- Zhao, Z.; Cai, Z.; Chen, A.; Cai, M.; Yang, K. Application of metabolomics in osteoporosis research. Front. Endocrinol. 2022, 13, 993253. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Lee, H.C.; Laher, I. Emerging role of cyclic ADP-ribose (cADPR) in smooth muscle. Pharmacol. Ther. 2005, 105, 189–207. [Google Scholar] [CrossRef]
- Manik, M.K.; Shi, Y.; Li, S.; Zaydman, M.A.; Damaraju, N.; Eastman, S.; Smith, T.G.; Gu, W.; Masic, V.; Mosaiab, T.; et al. Cyclic ADP ribose isomers: Production, chemical structures, and immune signaling. Science 2022, 377, eadc8969. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Park, Y.J.; Hwang, B.; Park, S.L.; Han, S.W.; Park, S.S.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Triacanthine exerts antitumor effects on bladder cancer in vitro and in vivo. Phytomedicine 2019, 64, 153069. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Ermis, E.; Nargis, T.; Webster, K.; Tersey, S.A.; Anderson, R.M.; Mirmira, R.G. Leukotriene B4 receptor 2 governs macrophage migration during tissue inflammation. J. Biol. Chem. 2024, 300, 105561. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Tian, L.Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef]
- Ahmad, I.; Hoque, M.; Alam, S.S.M.; Zughaibi, T.A.; Tabrez, S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/mTOR Pathway: A Prospective Role in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6651. [Google Scholar] [CrossRef]
- Ge, X.; Zhou, G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am. J. Transl. Res. 2021, 13, 6330–6341. [Google Scholar] [PubMed]
- Yuan, H.; Chen, F.; Chang-Jen, H.; Chen, Z. LCN2 Suppressed Inflammation through PI3K/AKT/MTOR Signaling Pathway. J. Hepatol. 2016, 64, S530. [Google Scholar] [CrossRef]
- Ying, Z.; Xiangyang, C.; Peifeng, L.; Yanan, F.; Leilei, Z.; Wuyin, L.; Youwen, L. PSMC6 promotes osteoblast apoptosis through inhibiting PI3K/AKT signaling pathway activation in ovariectomy-induced osteoporosis mouse model. J. Cell. Physiol. 2020, 235, 5511–5524. [Google Scholar] [CrossRef]
- Jia, T.; Jiaxin, L.; Zhaoyang, R.; Jinlong, W.; Danyang, L.; Bojun, C.; Liang, D.; Xiaoping, L.; Wenbo, J.; Kai, X.; et al. Accelerated fracture healing by osteogenic Ti45Nb implants through the PI3K–Akt signaling pathway. Bio-Des. Manuf. 2023, 6, 718–734. [Google Scholar] [CrossRef]
- Ziquan, L.; Wenbiao, S.; Qian, Z. Role of Essential Amino Acids in Age-Induced Bone Loss. Int. J. Mol. Sci. 2022, 23, 11281. [Google Scholar] [CrossRef]
- Xin, L.; Yifei, W.; Mengting, G.; Beihua, B.; Yudan, C.; Fang, C.; Li, Z.; Zhipeng, L.; Jinjun, S.; Wei-Feng, Y. Metabolomics-driven of relationships among kidney, bone marrow and bone of rats with postmenopausal osteoporosis. Bone 2022, 156, 116306. [Google Scholar] [CrossRef]
- Ziheng, W.; Fei, G.; Yonglu, C.; Si, W.; Xin, D.; Dianwen, S. Metabolomics Coupled with Pathway Analysis Provides Insights into Sarco-Osteoporosis Metabolic Alterations and Estrogen Therapeutic Effects in Mice. Biomolecules 2021, 12, 41. [Google Scholar] [CrossRef]
- Sujin, K.; Seulki, L.; Hyuktae, K. Association between serum uric acid level and bone mineral density in men more than 50 years of age. Front. Endocrinol. 2023, 14, 1259077. [Google Scholar] [CrossRef]
- Karina, R.-R.; Anna, D.A.-P.; Alberto, H.B.; Amado, D.Q.-S.; Guadalupe, L.-R.; Yvonne, N.F.; Jorge, S.; Rafael, V.C.; Berenice, R.P. Uric Acid Levels Are Associated with Bone Mineral Density in Mexican Populations: A Longitudinal Study. Nutrients 2022, 14, 4245. [Google Scholar] [CrossRef]
- Luiz Eduardo Baggio, S.; Raíssa, L.-A.; Vinícius Santos, A.; Robson, C.S.; Ângela, T.S.W. Purinergic signaling in the modulation of redox biology. Redox Biol. 2021, 47, 102137. [Google Scholar] [CrossRef]
- Kun-Mo, L.; Cheng Wei, L.; Kuo-Chin, H.; Pei-Chen, W.; Chi-Feng, P.; Chih-Jen, W.; Ren-Si, S.; Jin-Shuen, C.; Po-Jen, H.; Kuo-Cheng, L. The Paradoxical Role of Uric Acid in Osteoporosis. Nutrients 2019, 11, 2111. [Google Scholar] [CrossRef] [PubMed]
- Kazuyuki, K.; Robert, L.K.; Qi-Jun, Z.; Meenakshi, P.; Margarete, M.; Aldons, J.L.; Göran, B.; Fredrik, B.; Federico, E.R. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053. [Google Scholar] [CrossRef]

| TP | PW | BMW | RMW | |
|---|---|---|---|---|
| pH | 7.1 | 6.3 | 6.7 | 6.1 |
| Total dissolved solids (mg/L) | 198 | 50 | 1626 | 1967 |
| Ca (mg/L) | 39.6 | 1.6 | 370.6 | 376.6 |
| K (mg/L) | 1.97 | <0.05 | 13.88 | 13.54 |
| Na (mg/L) | 5.91 | 0.46 | 82.96 | 92.43 |
| Mg (mg/L) | 11.2 | 1.9 | 89.2 | 74.9 |
| Mn (mg/L) | <0.01 | <0.01 | <0.01 | 0.001 |
| Fe (mg/L) | 0.12 | <0.01 | 0.07 | 0.07 |
| Cu (mg/L) | <0.01 | <0.01 | <0.01 | 0.01 |
| Zn (mg/L) | <0.01 | <0.01 | <0.01 | 0.01 |
| Li (mg/L) | 0.036 | 0.033 | 0.216 | 0.216 |
| Sr (mg/L) | 0.14 | 0.03 | 1.31 | 1.31 |
| F− (mg/L) | 0.107 | <0.1 | <0.1 | <0.1 |
| Cl− (mg/L) | 16.5 | 0.37 | 45 | 42.5 |
| SiO32− (mg/L) | 1.19 | <0.1 | 22.42 | 20.36 |
| SO42− (mg/L) | 21.6 | 0.9 | 54 | 472 |
| CO32− (mg/L) | N.D. | N.D. | 3.0 | 1.2 |
| HCO3− (mg/L) | 18.65 | 6.36 | 1651.5 | 335.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Liu, W.; Zhang, X.; Xue, B.; Yang, X.; Zhao, C.; Li, C.; Wang, S.; Qiu, Z.; Li, C.; et al. Bicarbonate-Rich Mineral Water Mitigates Hypoxia-Induced Osteoporosis in Mice via Gut Microbiota and Metabolic Pathway Regulation. Nutrients 2025, 17, 998. https://doi.org/10.3390/nu17060998
Ding Y, Liu W, Zhang X, Xue B, Yang X, Zhao C, Li C, Wang S, Qiu Z, Li C, et al. Bicarbonate-Rich Mineral Water Mitigates Hypoxia-Induced Osteoporosis in Mice via Gut Microbiota and Metabolic Pathway Regulation. Nutrients. 2025; 17(6):998. https://doi.org/10.3390/nu17060998
Chicago/Turabian StyleDing, Yufan, Weili Liu, Xi Zhang, Bin Xue, Xiaobo Yang, Chen Zhao, Chenyu Li, Shang Wang, Zhigang Qiu, Chao Li, and et al. 2025. "Bicarbonate-Rich Mineral Water Mitigates Hypoxia-Induced Osteoporosis in Mice via Gut Microbiota and Metabolic Pathway Regulation" Nutrients 17, no. 6: 998. https://doi.org/10.3390/nu17060998
APA StyleDing, Y., Liu, W., Zhang, X., Xue, B., Yang, X., Zhao, C., Li, C., Wang, S., Qiu, Z., Li, C., Wang, J., & Shen, Z. (2025). Bicarbonate-Rich Mineral Water Mitigates Hypoxia-Induced Osteoporosis in Mice via Gut Microbiota and Metabolic Pathway Regulation. Nutrients, 17(6), 998. https://doi.org/10.3390/nu17060998






