Prolonged Parenteral Nutrition Increases the Risk of Comorbidities in Very-Low-Birth-Weight Infants: A Prospective National Cohort Study in South Korea
Highlights
- Prolonged parenteral nutrition exceeding 28 days is associated with significant increases in the risks of periventricular leukomalacia and bronchopulmonary dysplasia in very-low-birth-weight infants, even after adjusting for gestational age and birth weight.
- An intermediate duration of parenteral nutrition is associated with a higher risk of the retinopathy of prematurity in male infants, while a prolonged duration correlates with an increased severity of bronchopulmonary dysplasia, suggesting a critical role of nutritional management in preventing neonatal comorbidities.
- Predictive models developed in this study demonstrate strong explanatory power in estimating the risk of periventricular leukomalacia, bronchopulmonary dysplasia, and the retinopathy of prematurity based on the duration of parenteral nutrition.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
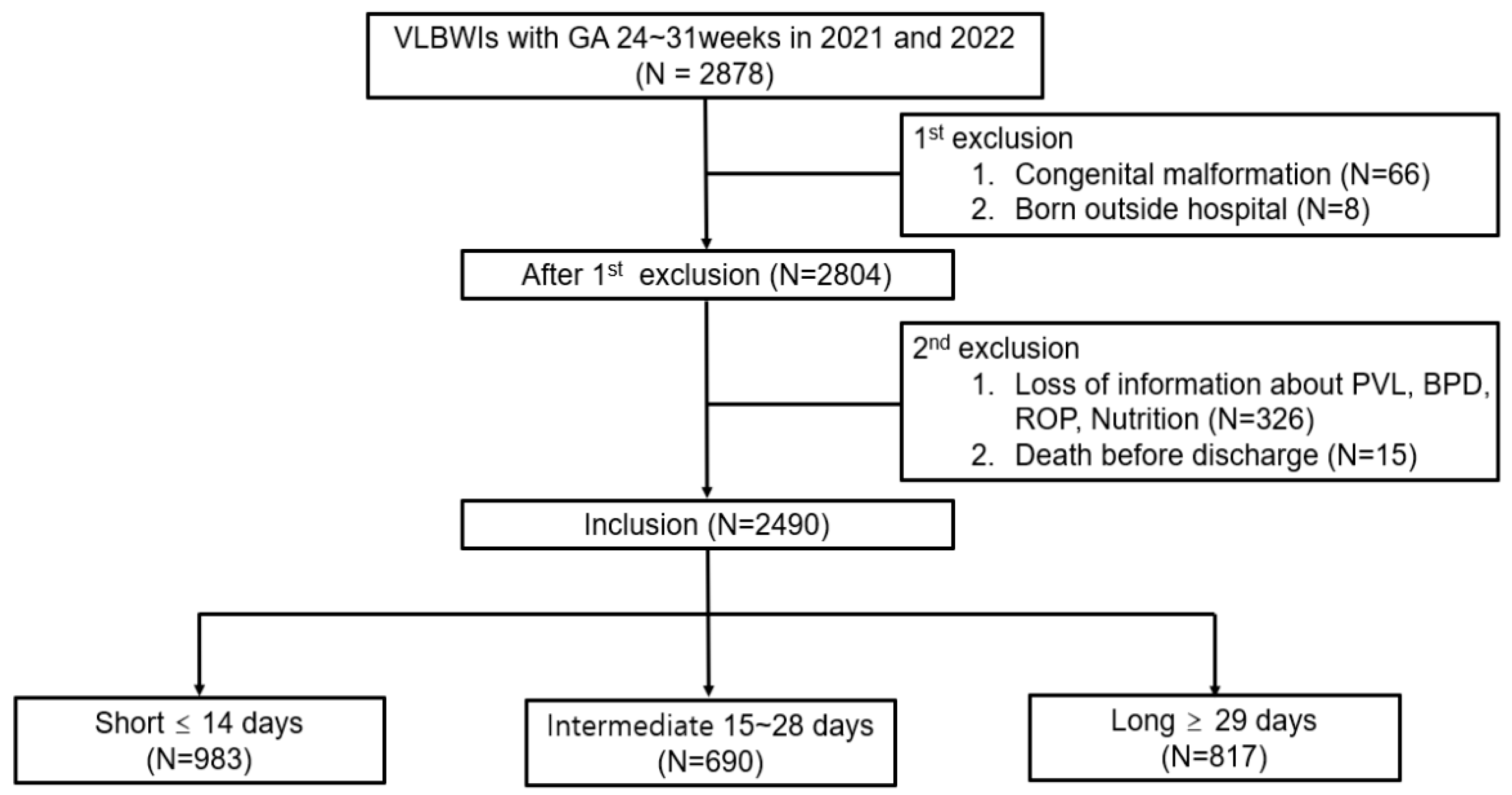
2.2. Study Population
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Cohort and Their Comorbidities
3.2. Comparison of Confounder by Duration of Parenteral Nutrition
3.3. Duration of Parenteral Nutrition and the Risk of PVL
3.4. Duration of Parenteral Nutrition and the Risk of BPD
3.5. Duration of Parenteral Nutrition and the Risk of ROP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VLBWI | Very-Low-Birth-Weight Infant |
| PVL | Periventricular Leukomalacia |
| BPD | Bronchopulmonary Dysplasia |
| ROP | Retinopathy of Prematurity |
| IGF-1 | Insulin-like Growth Factor 1 |
| KNN | Korean Neonatal Network |
| NICU | Neonatal Intensive Care Unit |
| GA | Gestational Age |
| PMA | Postmenstrual Age |
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.W.; Lee, J.H.; Oh, M.; Chang, Y.S. Serial Short-Term Outcomes of Very-Low-Birth-Weight Infants in the Korean Neonatal Network From 2013 to 2020. J. Korean Med. Sci. 2022, 37, e229. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Heras, A.; Chambers, R.; Solomon, Z.; Blatt, L.; Martin, C.R. Nutrition-based implications and therapeutics in the development and recovery of bronchopulmonary dysplasia. Semin. Perinatol. 2023, 47, 151818. [Google Scholar] [CrossRef]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- Hellström, A.; Kermorvant-Duchemin, E.; Johnson, M.; Sáenz de Pipaón, M.; Smith, L.E.; Hård, A.L. Nutritional interventions to prevent retinopathy of prematurity. Pediatr. Res. 2024, 96, 905–911. [Google Scholar] [CrossRef]
- Lee, H.H.; Jung, J.M.; Nam, S.H.; Lim, G.; Chung, M.L. Risk factor analysis of parenteral nutrition-associated cholestasis in extremely low birth weight infants. Acta Paediatr. 2016, 105, e313–e319. [Google Scholar] [CrossRef]
- de Sousa, J.C.S.; de Carvalho, A.V.D.; Monte de Prada, L.C.; Marinho, A.P.; de Lima, K.F.; Macedo, S.K.O.; Santos, C.D.P.; da Câmara, S.M.A.; Barreto, A.; Pereira, S.A. Nutritional Factors Associated with Late-Onset Sepsis in Very Low Birth Weight Newborns. Nutrients 2021, 14, 196. [Google Scholar] [CrossRef]
- Pivodic, A.; Holmström, G.; Smith, L.E.H.; Hård, A.L.; Löfqvist, C.; Al-Hawasi, A.; Larsson, E.; Lundgren, P.; Gränse, L.; Tornqvist, K.; et al. Prognostic Value of Parenteral Nutrition Duration on Risk of Retinopathy of Prematurity: Development and Validation of the Revised DIGIROP Clinical Decision Support Tool. JAMA Ophthalmol. 2023, 141, 716–724. [Google Scholar] [CrossRef]
- Kong, M.; Shin, D.H.; Kim, S.J.; Ham, D.I.; Kang, S.W.; Chang, Y.S.; Park, W.S. Retinopathy of prematurity in infants born before 25 weeks gestation in a Korean single neonatal intensive care unit: Incidence, natural history and risk factors. J. Korean Med. Sci. 2012, 27, 1556–1562. [Google Scholar] [CrossRef]
- Alja’nini, Z.; Merlino-Barr, S.; Brumfiel, A.; McNelis, K.; Viswanathan, S.; Collin, M.; Groh-Wargo, S. Effect of parenteral nutrition duration on patterns of growth and body composition in very low-birth-weight premature infants. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Fenin, A.; Newman, J.C.; Taylor, S.N. Very low birth weight infants receive full enteral nutrition within 2 postnatal weeks. J. Perinatol. 2020, 40, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.C.; Procianoy, R.S.; Dill, J.C.; da Costa, C.S. Periventricular leukomalacia in very low birth weight preterm neonates with high risk for neonatal sepsis. J. Pediatr. 2008, 84, 211–216. [Google Scholar] [CrossRef][Green Version]
- Park, J.M.; Choi, B.S.; Sohn, I.A.; Seol, I.J.; Kim, C.R.; Park, H.K.; Lee, H.J. Risk Factors for Cystic Periventricular Leukomalacia in Very Low Birth Weight Infants. Neonatal Med. 2014, 21, 172–178. [Google Scholar] [CrossRef]
- Yoon, S.A.; Lee, M.H.; Chang, Y.S. Impact of time to full enteral feeding on long-term neurodevelopment without mediating by postnatal growth failure in very-low-birth-weight-infants. Sci. Rep. 2023, 13, 2990. [Google Scholar] [CrossRef]
- Abiramalatha, T.; Bandyopadhyay, T.; Ramaswamy, V.V.; Shaik, N.B.; Thanigainathan, S.; Pullattayil, A.K.; Amboiram, P. Risk Factors for Periventricular Leukomalacia in Preterm Infants: A Systematic Review, Meta-analysis, and GRADE-Based Assessment of Certainty of Evidence. Pediatr. Neurol. 2021, 124, 51–71. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, L.; Liu, Y.; Shen, T.; Yang, Z.; Wang, S.; Ma, Y. Development and verification of a risk prediction model for bronchopulmonary dysplasia in very low birth weight infants. Transl. Pediatr. 2021, 10, 2533–2543. [Google Scholar] [CrossRef]
- Pan, J.J.; Zou, Y.S.; Tong, M.L.; Wang, J.; Zhou, X.Y.; Cheng, R.; Yang, Y. Dose pulmonary hemorrhage increase the risk of bronchopulmonary dysplasia in very low birth weight infants? J. Matern. Fetal Neonatal Med. 2023, 36, 2206941. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, T.T.; Zhao, J. Association between red blood cell transfusion and bronchopulmonary dysplasia: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1095889. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Hong, K.E.; Yum, H.R.; Lee, J.H.; Kim, K.S.; Youn, Y.A.; Park, S.H. Characteristic clinical features associated with aggressive posterior retinopathy of prematurity. Eye 2017, 31, 924–930. [Google Scholar] [CrossRef]
- Mitra, S.; Aune, D.; Speer, C.P.; Saugstad, O.D. Chorioamnionitis as a risk factor for retinopathy of prematurity: A systematic review and meta-analysis. Neonatology 2014, 105, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Hutchinson, J.L.; Henderson-Smart, D.J.; Donoghue, D.A.; Simpson, J.M.; Evans, N.J. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics 2005, 115, 990–996. [Google Scholar] [CrossRef]
- Wikstrand, M.H.; Hård, A.L.; Niklasson, A.; Smith, L.; Löfqvist, C.; Hellström, A. Maternal and neonatal factors associated with poor early weight gain and later retinopathy of prematurity. Acta Paediatr. 2011, 100, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Farina, D.; Maestri, A.; Giovannozzi, C.; Leonessa, M.L.; Arisio, R.; Gomirato, G. Mode of delivery and threshold retinopathy of prematurity in pre-term ELBW neonates. Acta Paediatr. 2007, 96, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Löfqvist, C.; van Marter, L.; van Weissenbruch, M.; Ramenghi, L.A.; Beardsall, K.; Dunger, D.; et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016, 105, 576–586. [Google Scholar] [CrossRef]
- Hansen-Pupp, I.; Löfqvist, C.; Polberger, S.; Niklasson, A.; Fellman, V.; Hellström, A.; Ley, D. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr. Res. 2011, 69, 448–453. [Google Scholar] [CrossRef]
- Kurtoğlu, S.; Kondolot, M.; Mazicioğlu, M.M.; Hatipoğlu, N.; Akin, M.A.; Akyildiz, B. Growth hormone, insulin like growth factor-1, and insulin-like growth factor-binding protein-3 levels in the neonatal period: A preliminary study. J. Pediatr. Endocrinol. Metab. 2010, 23, 885–889. [Google Scholar] [CrossRef]
- Jacobo, S.M.; Kazlauskas, A. Insulin-like growth factor 1 (IGF-1) stabilizes nascent blood vessels. J. Biol. Chem. 2015, 290, 6349–6360. [Google Scholar] [CrossRef]
- Pang, Y.; Zheng, B.; Fan, L.W.; Rhodes, P.G.; Cai, Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia 2007, 55, 1099–1107. [Google Scholar] [CrossRef]
- Kim, D.J.; Cho, S.Y.; Kim, S.U.; Jo, D.W.; Hwang, H.I.; Shin, H.K.; Jun, Y.H. IGF-1 Protects Neurons in the Cortex and Subventricular Zone in a Periventricular Leucomalacia Model. In Vivo 2021, 35, 307–312. [Google Scholar] [CrossRef]
- Hellström, W.; Hortensius, L.M.; Löfqvist, C.; Hellgren, G.; Tataranno, M.L.; Ley, D.; Benders, M.; Hellström, A.; Björkman-Burtscher, I.M.; Heckemann, R.A.; et al. Postnatal serum IGF-1 levels associate with brain volumes at term in extremely preterm infants. Pediatr. Res. 2023, 93, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Pupp, I.; Hövel, H.; Hellström, A.; Hellström-Westas, L.; Löfqvist, C.; Larsson, E.M.; Lazeyras, F.; Fellman, V.; Hüppi, P.S.; Ley, D. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J. Clin. Endocrinol. Metab. 2011, 96, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Guo, Q.; Wang, Y.; Ma, L.; Zhang, X. Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases. Biomed. Res. Int. 2018, 2018, 6057589. [Google Scholar] [CrossRef] [PubMed]
- Yumani, D.F.J.; Walschot, F.H.; Lafeber, H.N.; van Weissenbruch, M.M. Associations between Bronchopulmonary Dysplasia, Insulin-like Growth Factor I and Nutrition. Nutrients 2024, 16, 957. [Google Scholar] [CrossRef]
- Löfqvist, C.; Hellgren, G.; Niklasson, A.; Engström, E.; Ley, D.; Hansen-Pupp, I. Low postnatal serum IGF-I levels are associated with bronchopulmonary dysplasia (BPD). Acta Paediatr. 2012, 101, 1211–1216. [Google Scholar] [CrossRef]
- Ley, D.; Hallberg, B.; Hansen-Pupp, I.; Dani, C.; Ramenghi, L.A.; Marlow, N.; Beardsall, K.; Bhatti, F.; Dunger, D.; Higginson, J.D.; et al. rhIGF-1/rhIGFBP-3 in Preterm Infants: A Phase 2 Randomized Controlled Trial. J. Pediatr. 2019, 206, 56–65.e58. [Google Scholar] [CrossRef]
- Hellstrom, A.; Perruzzi, C.; Ju, M.; Engstrom, E.; Hard, A.L.; Liu, J.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Sjodell, L.; et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: Direct correlation with clinical retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2001, 98, 5804–5808. [Google Scholar] [CrossRef]
- Smith, L.E. IGF-1 and retinopathy of prematurity in the preterm infant. Biol. Neonate 2005, 88, 237–244. [Google Scholar] [CrossRef]
- Engström, E.; Niklasson, A.; Wikland, K.A.; Ewald, U.; Hellström, A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr. Res. 2005, 57, 605–610. [Google Scholar] [CrossRef]
- Yumani, D.F.J.; Calor, A.K.; van Weissenbruch, M.M. The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients 2020, 12, 675. [Google Scholar] [CrossRef]
- Paulsen, M.E.; Marka, N.; Nagel, E.M.; Gonzalez Villamizar, J.D.; Nathan, B.M.; Ramel, S.E. An exploratory study of clinical factors associated with IGF-1 and IGFBP-3 in preterm infants. Pediatr. Res. 2024, 96, 402–408. [Google Scholar] [CrossRef]
| Variable | Total (N = 2490) | Short (N = 983) | Intermediate (N = 690) | Long (N = 817) | p-Value |
|---|---|---|---|---|---|
| GA (week) | 28.1 ± 2.0 | 29.2 ± 1.5 | 28.2 ± 1.8 | 26.7 ± 1.9 | 0.000 * |
| Birth weight (gm) | 1080 ± 257 | 1222 ± 192 | 1085 ± 236 | 904 ± 235 | 0.000 * |
| Sex: Female | 1212 (48.7%) | 495 (50.4%) | 343 (49.7%) | 374 (45.8%) | 0.125 |
| Multiple birth | 981 (39.4%) | 434 (44.2%) | 259 (37.5%) | 288 (35.3%) | 0.000 * |
| Maternal DM | 424 (17.0%) | 191 (19.4%) | 104 (15.1%) | 129(15.8%) | 0.034 * |
| Maternal HTN | 614 (24.7%) | 247(25.1%) | 189 (27.4%) | 178 (21.8%) | 0.038 * |
| Chorioamnionitis | 714 (28.7%) | 273 (27.8%) | 184 (26.7%) | 257 (31.5%) | 0.089 |
| PROM | 904 (36.3%) | 379 (38.6%) | 231 (33.5%) | 294 (36.0%) | 0.102 |
| Antenatal steroid | 2299 (92.3%) | 900 (91.6%) | 638 (92.5%) | 761 (93.1%) | 0.446 |
| Antenatal antibiotics | 1683 (67.6%) | 632 (64.3%) | 452 (65.5%) | 599 (73.3%) | 0.000 * |
| Mode of delivery: C/Sec | 2122 (85.2%) | 840 (85.5%) | 598 (86.7%) | 684 (83.7%) | 0.266 |
| 5 min APGAR score | 7.2 ± 1.6 | 7.6 ± 1.5 | 7.1 ± 1.7 | 6.7 ± 1.7 | 0.000 * |
| Chest compression at birth | 59 (2.4%) | 10 (1.0%) | 21 (3.0%) | 28 (3.4%) | 0.001 * |
| Pulmonary hemorrhage | 59 (2.4%) | 7 (0.7%) | 12 (1.7%) | 40 (4.9%) | 0.000 * |
| Air leak syndrome | 73 (2.9%) | 14 (1.4%) | 12 (1.7%) | 47 (5.8%) | 0.000 * |
| RDS | 2061 (82.8%) | 696 (70.8%) | 595 (86.2%) | 770 (94.2%) | 0.000 * |
| IVH severity | 0.000 * | ||||
| Severe | 115 (4.6%) | 8 (0.8%) | 29 (4.2%) | 78 (9.5%) | |
| Mild | 848 (34.1%) | 245 (24.9%) | 221 (32.0%) | 382 (46.8%) | |
| No | 1527 (61.3%) | 730 (74.3%) | 440 (63.8%) | 357 (43.7%) | |
| PDA treatment | 875 (35.1%) | 162 (16.5%) | 256 (37.1%) | 457 (55.9%) | 0.000 * |
| NEC | 117 (4.7%) | 2 (0.2%) | 20 (2.9%) | 95 (11.6%) | 0.000 * |
| Sepsis | 331 (13.3%) | 37 (3.8%) | 78 (11.3%) | 216 (26.4%) | 0.000 * |
| RBC transfusion | 1513 (60.8%) | 330 (33.6%) | 452 (65.5%) | 731 (89.5%) | 0.000 * |
| Variable | OR | Lower.CI | Upper.CI | p-Value |
|---|---|---|---|---|
| Parenteral nutrition | ||||
| Long | 1.71 | 1.11 | 2.64 | 0.002 * |
| Intermediate | 0.89 | 0.63 | 1.25 | 0.505 |
| Short (Reference) | - | - | - | - |
| IVH severity | ||||
| Severe | 7.70 | 5.11 | 11.62 | 0.000 * |
| Mild | 1.66 | 1.20 | 2.30 | 0.002 * |
| No (Reference) | - | - | - | - |
| Multiple birth | 0.61 | 0.39 | 0.95 | 0.029 * |
| 5 min APGAR score | 0.81 | 0.71 | 0.92 | 0.002 * |
| Variable | OR | Lower.CI | Upper.CI | p-Value |
|---|---|---|---|---|
| Parenteral nutrition | ||||
| Long | 1.51 | 1.10 | 2.08 | 0.011 * |
| Intermediate | 1.13 | 0.88 | 1.44 | 0.342 |
| Short (Reference) | - | - | - | - |
| Sex (Female) | 0.70 | 0.54 | 0.91 | 0.007 * |
| Antenatal antibiotics | 0.67 | 0.50 | 0.90 | 0.009 * |
| 5 min APGAR score | 0.79 | 0.71 | 0.88 | 0.000 * |
| RDS | 2.33 | 1.72 | 3.17 | 0.000 * |
| Pulmonary hemorrhage | 7.66 | 1.72 | 34.13 | 0.008 * |
| PDA treatment | 2.62 | 1.91 | 3.58 | 0.000 * |
| Sepsis | 2.89 | 1.67 | 4.99 | 0.000 * |
| RBC transfusion | 5.12 | 3.91 | 6.70 | 0.000 * |
| Variable | OR | Lower.CI | Upper.CI | p-Value |
|---|---|---|---|---|
| Parenteral nutrition | ||||
| Long | 0.88 | 0.69 | 1.12 | 0.303 |
| Intermediate | 1.13 | 0.94 | 1.36 | 0.195 |
| Short (Reference) | - | - | - | - |
| Chorioamnionitis | 1.61 | 1.25 | 2.09 | 0.003 * |
| Mode of delivery: C/Sec | 0.66 | 0.48 | 0.92 | 0.015 * |
| Chest compressionAt birth | 2.03 | 1.04 | 3.96 | 0.039 * |
| RDS | 1.53 | 1.07 | 2.19 | 0.021 * |
| PDA treatment | 1.76 | 1.38 | 2.24 | 0.000 * |
| IVH severity | ||||
| Severe | 1.88 | 1.20 | 2.93 | 0.006 * |
| Mild | 1.22 | 0.90 | 1.64 | 0.196 |
| No (Reference) | - | - | - | - |
| Sepsis | 1.70 | 1.21 | 2.38 | 0.002 * |
| RBC transfusion | 2.29 | 1.75 | 3.02 | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.W.; Suh, Y.-A.; Choi, S.; Park, M.S.; Lee, J.H. Prolonged Parenteral Nutrition Increases the Risk of Comorbidities in Very-Low-Birth-Weight Infants: A Prospective National Cohort Study in South Korea. Nutrients 2025, 17, 996. https://doi.org/10.3390/nu17060996
Kim SW, Suh Y-A, Choi S, Park MS, Lee JH. Prolonged Parenteral Nutrition Increases the Risk of Comorbidities in Very-Low-Birth-Weight Infants: A Prospective National Cohort Study in South Korea. Nutrients. 2025; 17(6):996. https://doi.org/10.3390/nu17060996
Chicago/Turabian StyleKim, Seong Wan, Yoong-A Suh, Seoheui Choi, Moon Sung Park, and Jang Hoon Lee. 2025. "Prolonged Parenteral Nutrition Increases the Risk of Comorbidities in Very-Low-Birth-Weight Infants: A Prospective National Cohort Study in South Korea" Nutrients 17, no. 6: 996. https://doi.org/10.3390/nu17060996
APA StyleKim, S. W., Suh, Y.-A., Choi, S., Park, M. S., & Lee, J. H. (2025). Prolonged Parenteral Nutrition Increases the Risk of Comorbidities in Very-Low-Birth-Weight Infants: A Prospective National Cohort Study in South Korea. Nutrients, 17(6), 996. https://doi.org/10.3390/nu17060996






