Effects of Vitamin E Intake and Voluntary Wheel Running on Whole-Body and Skeletal Muscle Metabolism in Ovariectomized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Ovariectomy Surgeries
2.4. Vitamin E Intake
2.5. Metabolic Monitoring
2.6. Glucose Tolerance Test
2.7. Mitochondrial Function
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
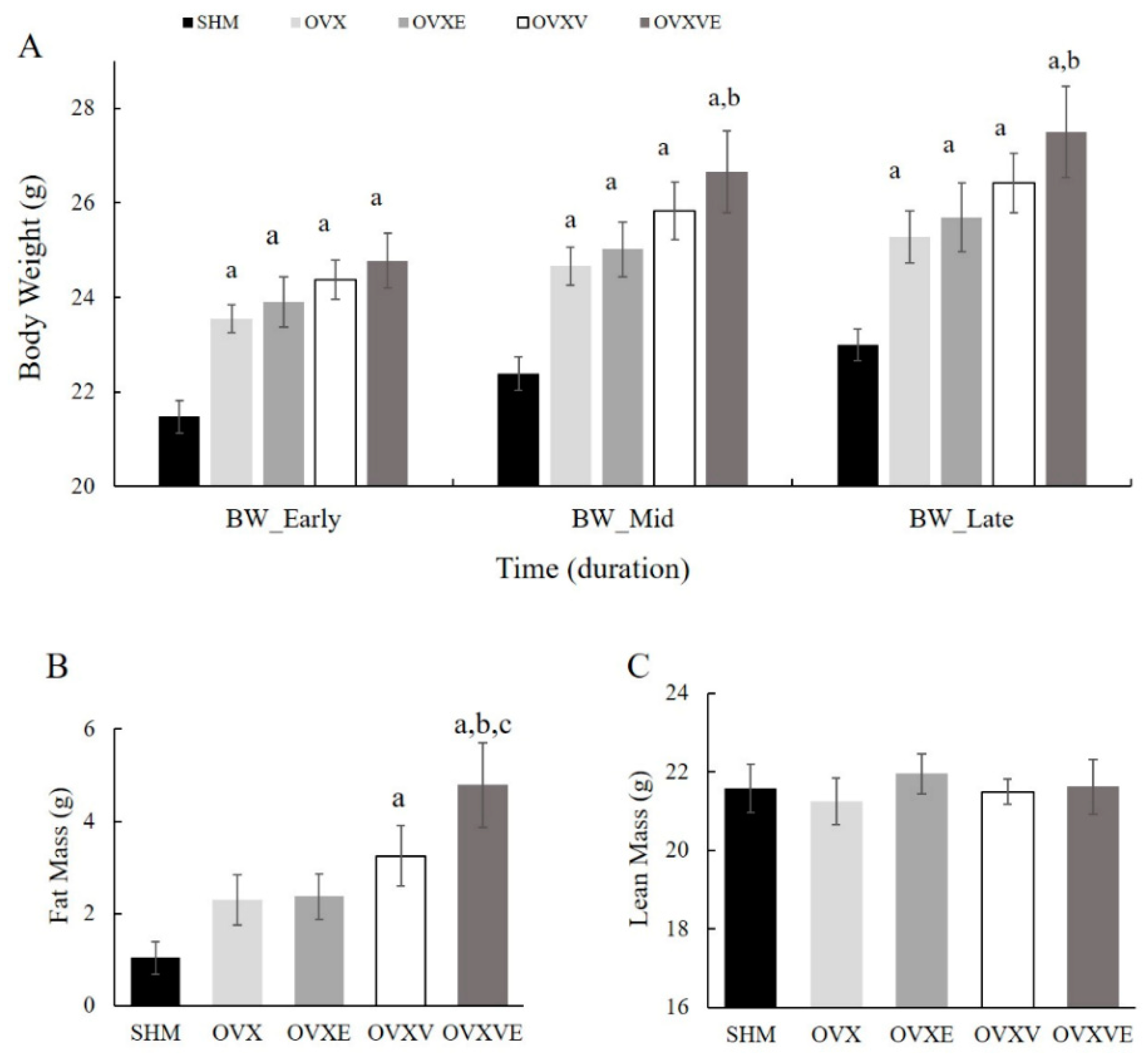
3.1. Body Weight
3.2. Fat and Lean Mass
3.3. Food Intake and Wheel Running Distance
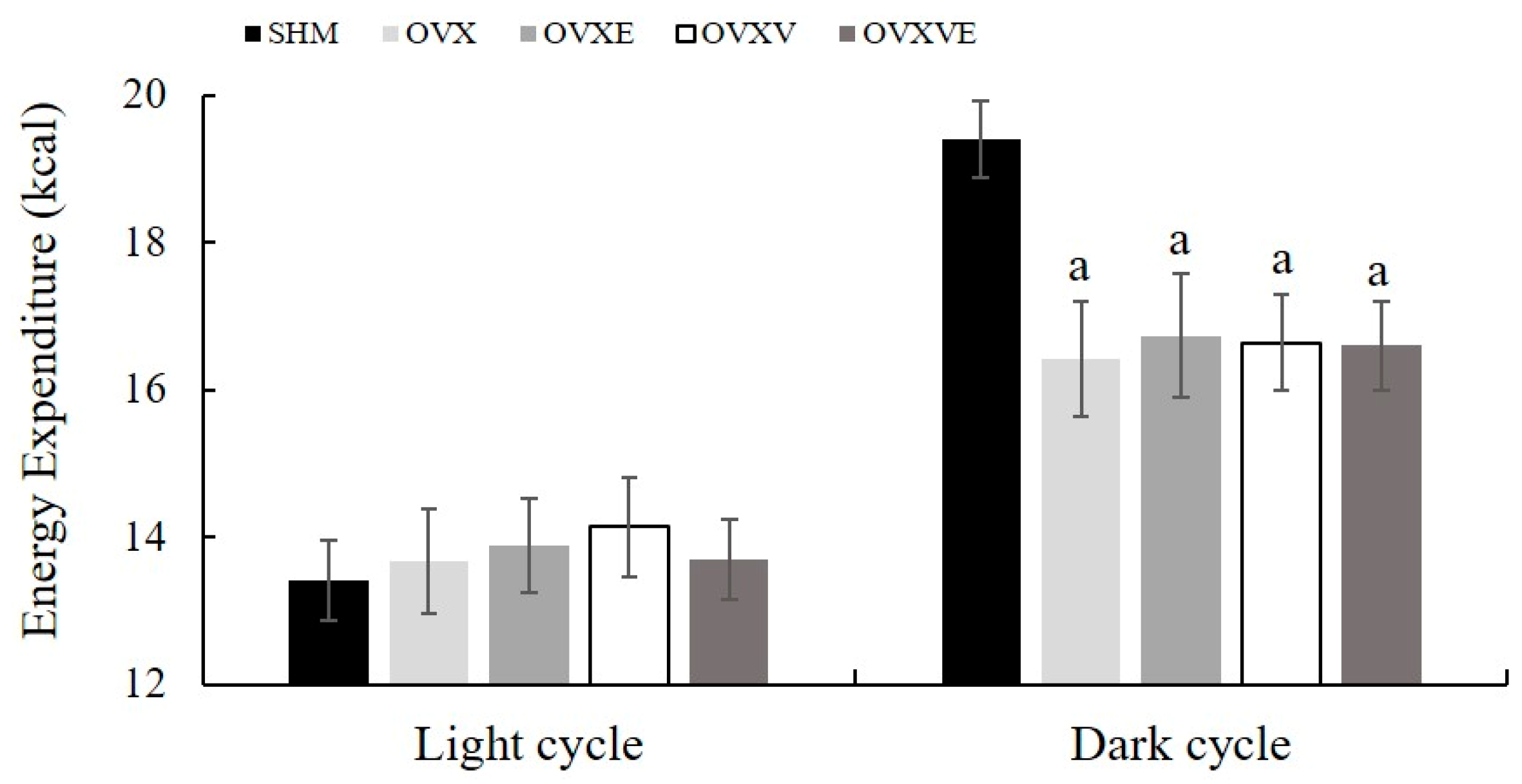
3.4. Resting Metabolic Rate
3.5. Glucose Tolerance Test
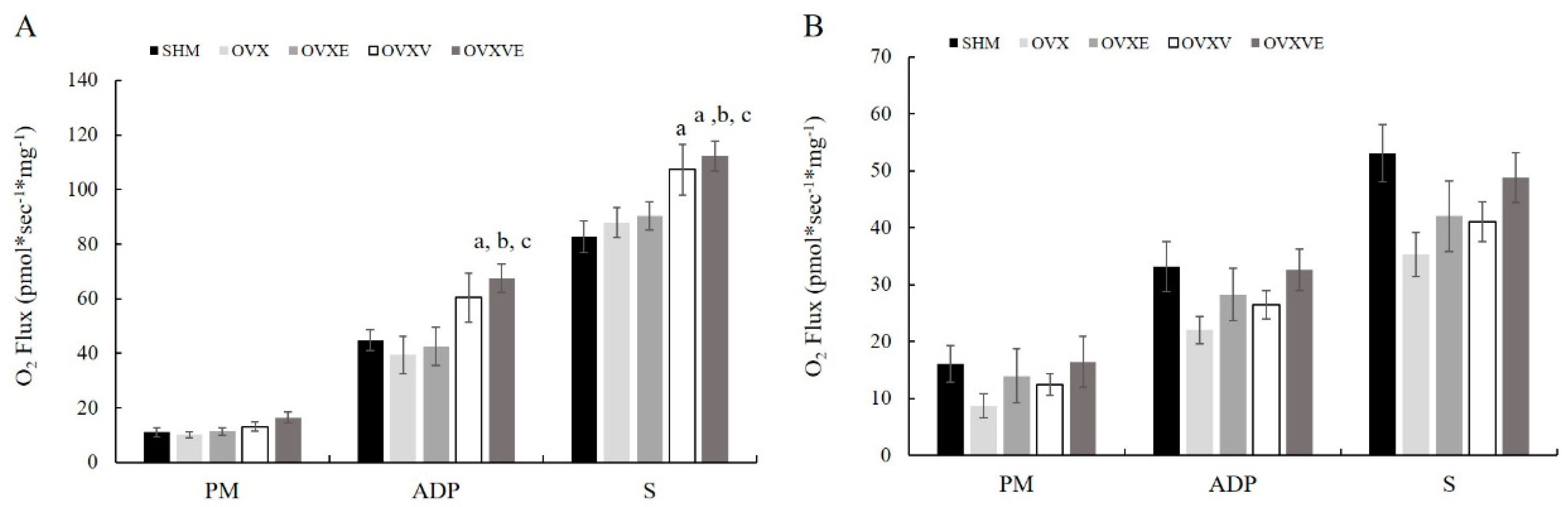
3.6. Mitochondrial Function
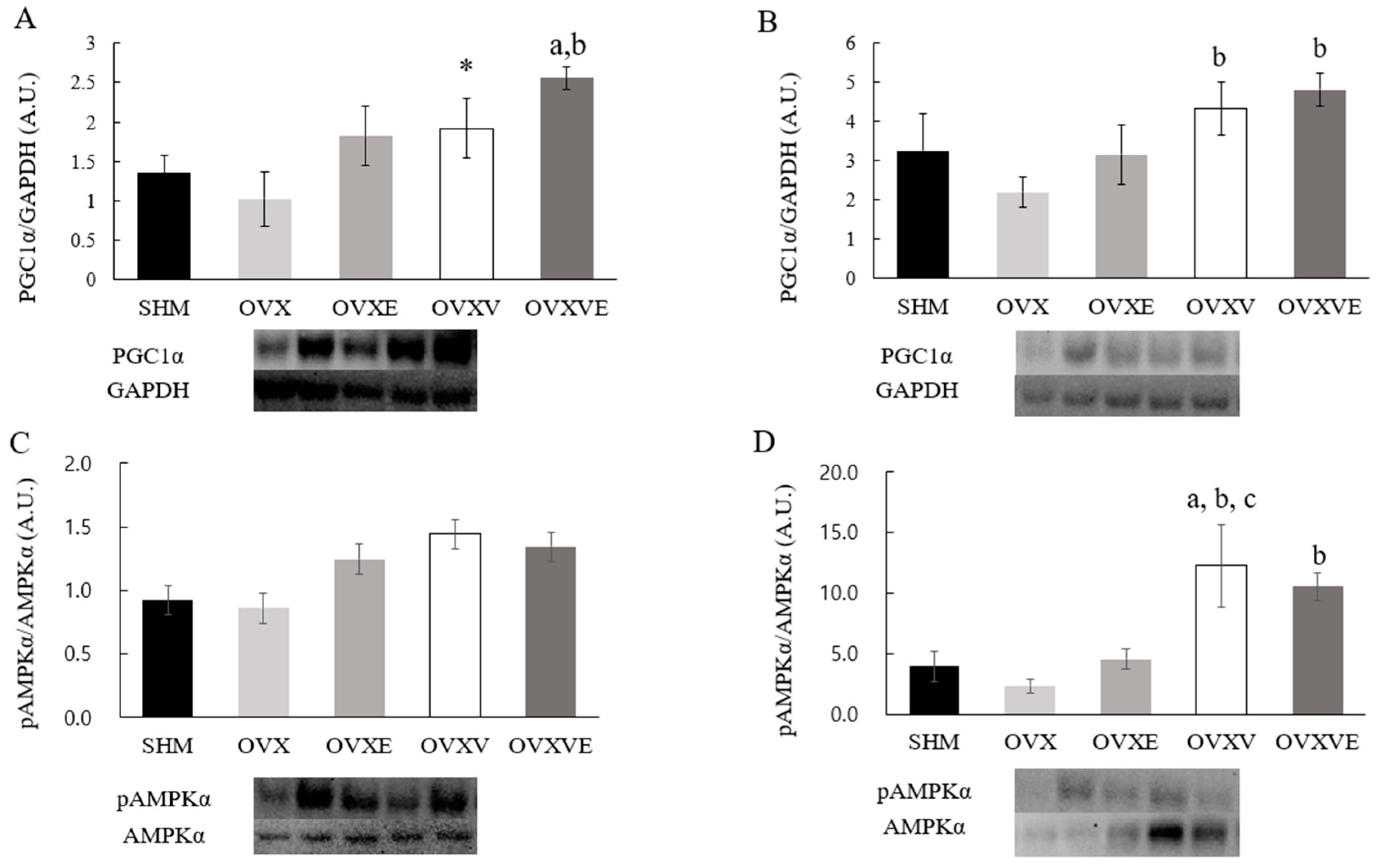
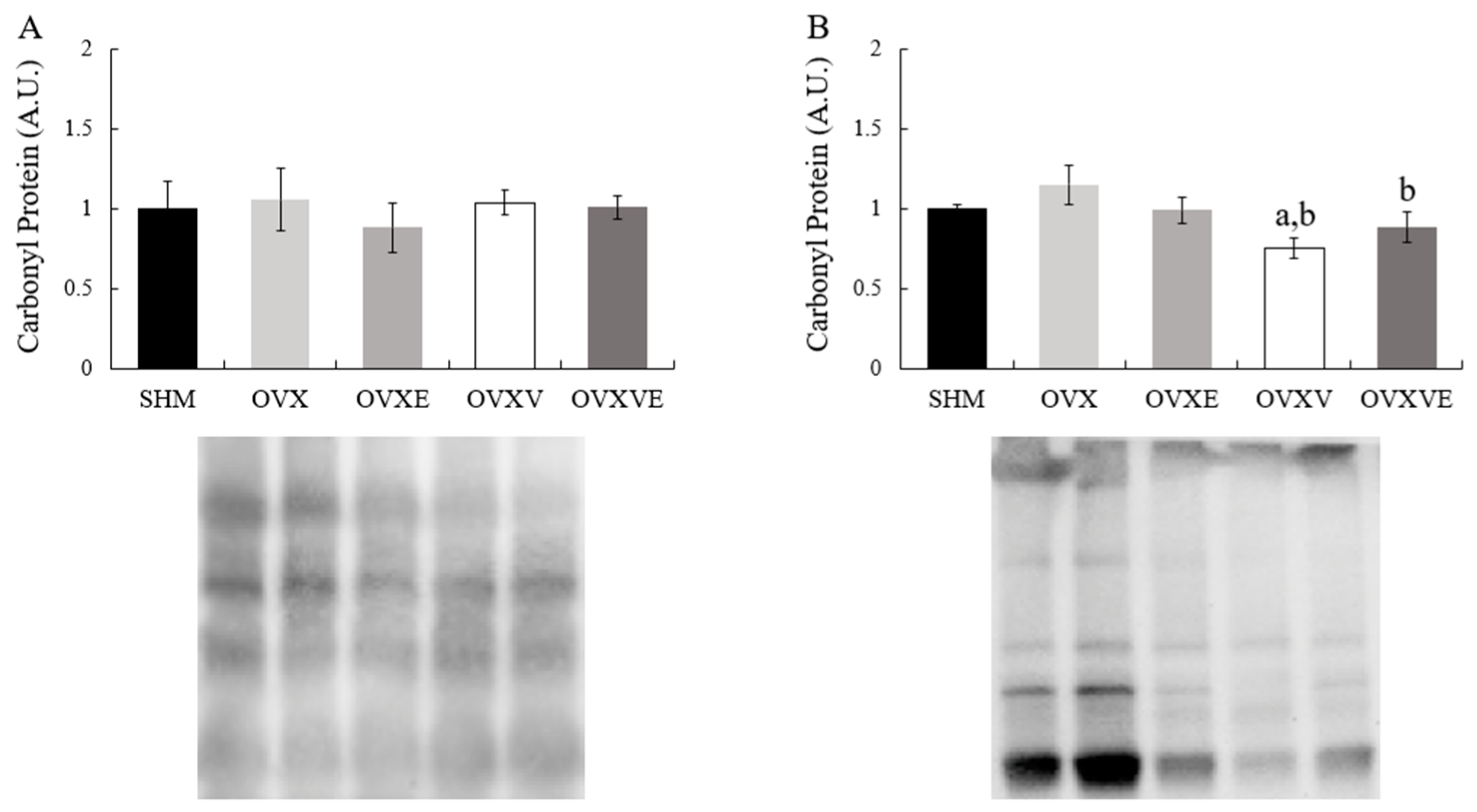
3.7. Protein Contents of Skeletal Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, C.N.; Taylor, V. Effects and management of the menopausal transition in women with depression and bipolar disorder. J. Clin. Psychiatry 2007, 68 (Suppl. S9), 16–21. [Google Scholar] [PubMed]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers 2015, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gohlke-Bärwolf, C. Coronary artery disease–is menopause a risk factor? Basic Res. Cardiol. 2000, 95 (Suppl. S1), I77–I83. [Google Scholar] [CrossRef]
- Wespes, E.; Schulman, C.C. Male andropause: Myth, reality, and treatment. Int. J. Impot. Res. 2002, 14 (Suppl. S1), S93–S98. [Google Scholar] [CrossRef]
- Talaulikar, V. Menopause transition: Physiology and symptoms. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef]
- McNeill, A.M.; Rosamond, W.D.; Girman, C.J.; Golden, S.H.; Schmidt, M.I.; East, H.E.; Ballantyne, C.M.; Heiss, G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 2005, 28, 385–390. [Google Scholar] [CrossRef]
- Tandon, V.R.; Mahajan, A.; Sharma, S.; Sharma, A. Prevalence of cardiovascular risk factors in postmenopausal women: A rural study. J. Midlife Health 2010, 1, 26–29. [Google Scholar] [CrossRef]
- Després, J.P. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 1993, 9, 452–459. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [CrossRef] [PubMed]
- Iyer, T.K.; Manson, J.E. Recent trends in menopausal hormone therapy use in the US: Insights, disparities, and implications for practice. JAMA Health Forum 2024, 5, e243135. [Google Scholar] [CrossRef]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Benussi, C.; De Simone, L.; Genazzani, A.R. Climacteric modifications in body weight and fat tissue distribution. Climacteric 1999, 2, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.; Pedersen, S.B.; Vestergaard, P.; Mosekilde, L.; Richelsen, B. Hormone replacement therapy affects body composition and leptin differently in obese and non-obese postmenopausal women. J. Endocrinol. 1999, 163, 55–62. [Google Scholar] [CrossRef]
- Sørensen, M.B.; Rosenfalck, A.M.; Højgaard, L.; Ottesen, B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes. Res. 2001, 9, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.M.; Reboussin, D.M.; Brosnihan, K.B.; Sharp, P.C.; Shumaker, S.A.; Snyder, T.E.; Furberg, C.D.; Kowalchuk, G.J.; Stuckey, T.D.; Rogers, W.J.; et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N. Engl. J. Med. 2000, 343, 522–529. [Google Scholar] [CrossRef]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Duckles, S.P.; Krause, D.N.; Stirone, C.; Procaccio, V. Estrogen and mitochondria: A new paradigm for vascular protection? Mol. Interv. 2006, 6, 26–35. [Google Scholar] [CrossRef]
- Wang, J.; Green, P.S.; Simpkins, J.W. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J. Neurochem. 2001, 77, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Cammarata, P.R.; Baines, C.P.; Yager, J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta 2009, 1793, 1540–1570. [Google Scholar] [CrossRef]
- O’Lone, R.; Knorr, K.; Jaffe, I.Z.; Schaffer, M.E.; Martini, P.G.; Karas, R.H.; Bienkowska, J.; Mendelsohn, M.E.; Hansen, U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol. Endocrinol. 2007, 21, 1281–1296. [Google Scholar] [CrossRef]
- Karolkiewicz, J.; Michalak, E.; Pospieszna, B.; Deskur-Smielecka, E.; Nowak, A.; Pilaczyńska-Szcześniak, Ł. Response of oxidative stress markers and antioxidant parameters to an 8-week aerobic physical activity program in healthy, postmenopausal women. Arch. Gerontol. Geriatr. 2009, 49, e67–e71. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Fortepiani, L.A. Novel mechanisms responsible for postmenopausal hypertension. Hypertension 2004, 43, 918–923. [Google Scholar] [CrossRef]
- Rousseau, A.S.; Margaritis, I.; Arnaud, J.; Faure, H.; Roussel, A.M. Physical activity alters antioxidant status in exercising elderly subjects. J. Nutr. Biochem. 2006, 17, 463–470. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Halliwell, B. The chemistry of free radicals. Toxicol. Ind. Health 1993, 9, 1–21. [Google Scholar] [CrossRef]
- Loft, S.; Poulsen, H.E. Cancer risk and oxidative DNA damage in man. J. Mol. Med. 1996, 74, 297–312. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant defense: Vitamins E and C and carotenoids. Diabetes 1997, 46 (Suppl. S2), S14–S18. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Ferrándiz, M.L.; De Juan, E.; Sevila, I.; Martínez, M. N-acetylcysteine protects against age-related decline of oxidative phosphorylation in liver mitochondria. Eur. J. Pharmacol. 1995, 292, 333–335. [Google Scholar] [CrossRef]
- Sokol, R.J.; McKim, J.M., Jr.; Goff, M.C.; Ruyle, S.Z.; Devereaux, M.W.; Han, D.; Packer, L.; Everson, G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology 1998, 114, 164–174. [Google Scholar] [CrossRef]
- Polidori, M.C.; Mecocci, P.; Cherubini, A.; Senin, U. Physical activity and oxidative stress during aging. Int. J. Sports Med. 2000, 21, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Viguie, C.; Stanley, W.C.; Brooks, G.A.; Packer, L. Blood glutathione oxidation during human exercise. J. Appl. Physiol. 1988, 64, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, A.T. Effects of physical exercise and/or vitamin E on tissue oxidative metabolism. Biochem. Soc. Trans. 1984, 12, 403–404. [Google Scholar] [CrossRef]
- Robertson, J.D.; Maughan, R.J.; Duthie, G.G.; Morrice, P.C. Increased blood antioxidant systems of runners in response to training load. Clin. Sci. 1991, 80, 611–618. [Google Scholar] [CrossRef]
- Bendich, A. Exercise and free radicals: Effects of antioxidant vitamins. Med. Sport Sci. 1991, 32, 59–78. [Google Scholar] [CrossRef]
- Clarkson, P.M. Micronutrients and exercise: Anti-oxidants and minerals. J. Sports Sci. 1995, 13, S11–S24. [Google Scholar] [CrossRef]
- Kanter, M.M. Free radicals, exercise, and antioxidant supplementation. Int. J. Sport Nutr. 1994, 4, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Evans, Z.P.; Ellett, J.D.; Fariss, M.W.; Schnellmann, R.G.; Schmidt, M.G.; Chavin, K. Vitamin E succinate reduces ischemia/reperfusion injury in steatotic livers. Transpl. Proc. 2008, 40, 3327–3329. [Google Scholar] [CrossRef]
- Vieira-Potter, V.J.; Padilla, J.; Park, Y.M.; Welly, R.J.; Scroggins, R.J.; Britton, S.L.; Koch, L.G.; Jenkins, N.T.; Crissey, J.M.; Zidon, T.; et al. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R530–R542. [Google Scholar] [CrossRef] [PubMed]
- Eshima, H.; Siripoksup, P.; Mahmassani, Z.S.; Johnson, J.M.; Ferrara, P.J.; Verkerke, A.R.P.; Salcedo, A.; Drummond, M.J.; Funai, K. Neutralizing mitochondrial ROS does not rescue muscle atrophy induced by hindlimb unloading in female mice. J. Appl. Physiol. 2020, 129, 124–132. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Rector, R.S.; Uptergrove, G.M.; Borengasser, S.J.; Morris, E.M.; Wei, Y.; Laye, M.J.; Burant, C.F.; Qi, N.R.; Ridenhour, S.E.; et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J. Physiol. 2009, 587, 1805–1816. [Google Scholar] [CrossRef]
- Boldarine, V.T.; Pedroso, A.P.; Neto, N.I.P.; Dornellas, A.P.S.; Nascimento, C.M.O.; Oyama, L.M.; Ribeiro, E.B. High-fat diet intake induces depressive-like behavior in ovariectomized rats. Sci. Rep. 2019, 9, 10551. [Google Scholar] [CrossRef]
- McElroy, J.F.; Wade, G.N. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol. Behav. 1987, 39, 361–365. [Google Scholar] [CrossRef]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; De Simone, L.; Orlandi, R.; Genazzani, A.R. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas 2001, 39, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Azman, A.; Khalid, B.; Ima-Nirwana, S. The effects of vitamin E on bodyweight and fat mass in intact and ovariectomized female rats. Med. J. Islamic. World Acad. Sci. 2001, 14, 125–138. [Google Scholar]
- Bjørneboe, A.; Bjørneboe, G.E.; Drevon, C.A. Absorption, transport and distribution of vitamin E. J. Nutr. 1990, 120, 233–242. [Google Scholar] [CrossRef]
- Machlin, L.J. Vitamin E. A Comprehensive Treatise; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Meydani, M.; Fielding, R.A.; Cannon, J.G.; Blumberg, J.B.; Evans, W.J. Muscle uptake of vitamin E and its association with muscle fiber type. J. Nutr. Biochem. 1997, 8, 74–78. [Google Scholar] [CrossRef]
- Lizcano, F.; Guzmán, G. Estrogen deficiency and the origin of obesity during menopause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, R.E.; Jones, P.P.; Davy, K.P.; DeSouza, C.A.; Tanaka, H.; Davy, B.M.; Seals, D.R. Regular exercise and the age-related decline in resting metabolic rate in women. J. Clin. Endocrinol. Metab. 1997, 82, 3208–3212. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Holmäng, A.; Björntorp, P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta. Physiol. Scand. 1993, 149, 91–97. [Google Scholar] [CrossRef]
- Puah, J.A.; Bailey, C.J. Effect of ovarian hormones on glucose metabolism in mouse soleus muscle. Endocrinology 1985, 117, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P.; De Jounge, K.; Sjöström, L.; Sullivan, L. The effect of physical training on insulin production in obesity. Metabolism 1970, 19, 631–638. [Google Scholar] [CrossRef]
- Heath, G.W.; Gavin, J.R., 3rd; Hinderliter, J.M.; Hagberg, J.M.; Bloomfield, S.A.; Holloszy, J.O. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 512–517. [Google Scholar] [CrossRef]
- Park, Y.-M.; Padilla, J.; Kanaley, J.A.; Zidon, T.; Welly, R.J.; Britton, S.L.; Koch, L.G.; Thyfault, J.P.; Booth, F.W.; Vieira-Potter, V.J. Voluntary running attenuates metabolic dysfunction in ovariectomized low-fit rats. Med. Sci. Sports Exerc. 2017, 49, 254. [Google Scholar] [CrossRef]
- LeBlanc, J.; Nadeau, A.; Richard, D.; Tremblay, A. Studies on the sparing effect of exercise on insulin requirements in human subjects. Metabolism 1981, 30, 1119–1124. [Google Scholar] [CrossRef]
- Hill, J.O.; Saris, W.; Levine, J.A. Energy expenditure in physical activity. In Handbook of Obesity; CRC Press: Boca Raton, FL, USA, 2003; pp. 647–670. [Google Scholar]
- Carroll, A.M.; Palmer, A.A.; Lionikas, A. QTL Analysis of type I and type IIA fibers in soleus muscle in a cross between LG/J and SM/J mouse strains. Front. Genet. 2011, 2, 99. [Google Scholar] [CrossRef]
- Mao, G.; Kraus, G.A.; Kim, I.; Spurlock, M.E.; Bailey, T.B.; Zhang, Q.; Beitz, D.C. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. J. Nutr. 2010, 140, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, inflammation, and oxidative stress: An integrative view in metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]

| Ingredient | CON | VIT |
|---|---|---|
| Ground Whole Wheat (%) | 34.90 | 34.55 |
| Ground No.2 Yellow Corn (%) | 21.00 | 20.79 |
| Ground Whole Oats (%) | 10.00 | 9.9 |
| Wheat Middlings (%) | 10.00 | 9.9 |
| Fish Meal (60% protein) | 9.00 | 8.91 |
| Soybean Meal (47.5%) | 5.00 | 4.95 |
| Alfalfa (17% protein) | 2.00 | 1.98 |
| Corn Gluten meal (60% protein) | 2.00 | 1.98 |
| Major Nutrient | CON | VIT |
| Alpha-Tocopherol (IU/g) | 0.045 | 10.045 |
| Energy (kcal/g) | 3.11 | 3.08 |
| Crude Protein (%) | 18.0 | 18.0 |
| Crude Fat (%) | 5.0 | 5.0 |
| Crude Fiber (%) | 5.0 | 5.0 |
| Ash (%) | 8.0 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Yoon, H.-J.; Park, K.-W.; Lee, H.; Tan, Y.; Ryu, B.-J.; Lee, S.-M.; Cho, C.-E.; Kim, J.-G.; Kim, N.-A.; et al. Effects of Vitamin E Intake and Voluntary Wheel Running on Whole-Body and Skeletal Muscle Metabolism in Ovariectomized Mice. Nutrients 2025, 17, 991. https://doi.org/10.3390/nu17060991
Jin Y, Yoon H-J, Park K-W, Lee H, Tan Y, Ryu B-J, Lee S-M, Cho C-E, Kim J-G, Kim N-A, et al. Effects of Vitamin E Intake and Voluntary Wheel Running on Whole-Body and Skeletal Muscle Metabolism in Ovariectomized Mice. Nutrients. 2025; 17(6):991. https://doi.org/10.3390/nu17060991
Chicago/Turabian StyleJin, Youngyun, Hee-Jung Yoon, Ki-Woong Park, Hanall Lee, Yuan Tan, Byung-Jun Ryu, Seung-Min Lee, Chae-Eun Cho, Jae-Geun Kim, Nam-Ah Kim, and et al. 2025. "Effects of Vitamin E Intake and Voluntary Wheel Running on Whole-Body and Skeletal Muscle Metabolism in Ovariectomized Mice" Nutrients 17, no. 6: 991. https://doi.org/10.3390/nu17060991
APA StyleJin, Y., Yoon, H.-J., Park, K.-W., Lee, H., Tan, Y., Ryu, B.-J., Lee, S.-M., Cho, C.-E., Kim, J.-G., Kim, N.-A., & Park, Y.-M. (2025). Effects of Vitamin E Intake and Voluntary Wheel Running on Whole-Body and Skeletal Muscle Metabolism in Ovariectomized Mice. Nutrients, 17(6), 991. https://doi.org/10.3390/nu17060991






