Effects of Maternal Pre-Pregnancy BMI on Preterm Infant Microbiome and Fecal Fermentation Profile—A Preliminary Cohort Study
Abstract
1. Introduction
2. Objectives or Purpose
3. Materials and Methods
3.1. Study Design and Subjects
3.2. Stool Collection and Microbiome and Short Chain Fatty Acid Analysis
3.3. Metagenomic Analysis
3.4. Statistical Analysis
4. Results
4.1. Subject Demographics
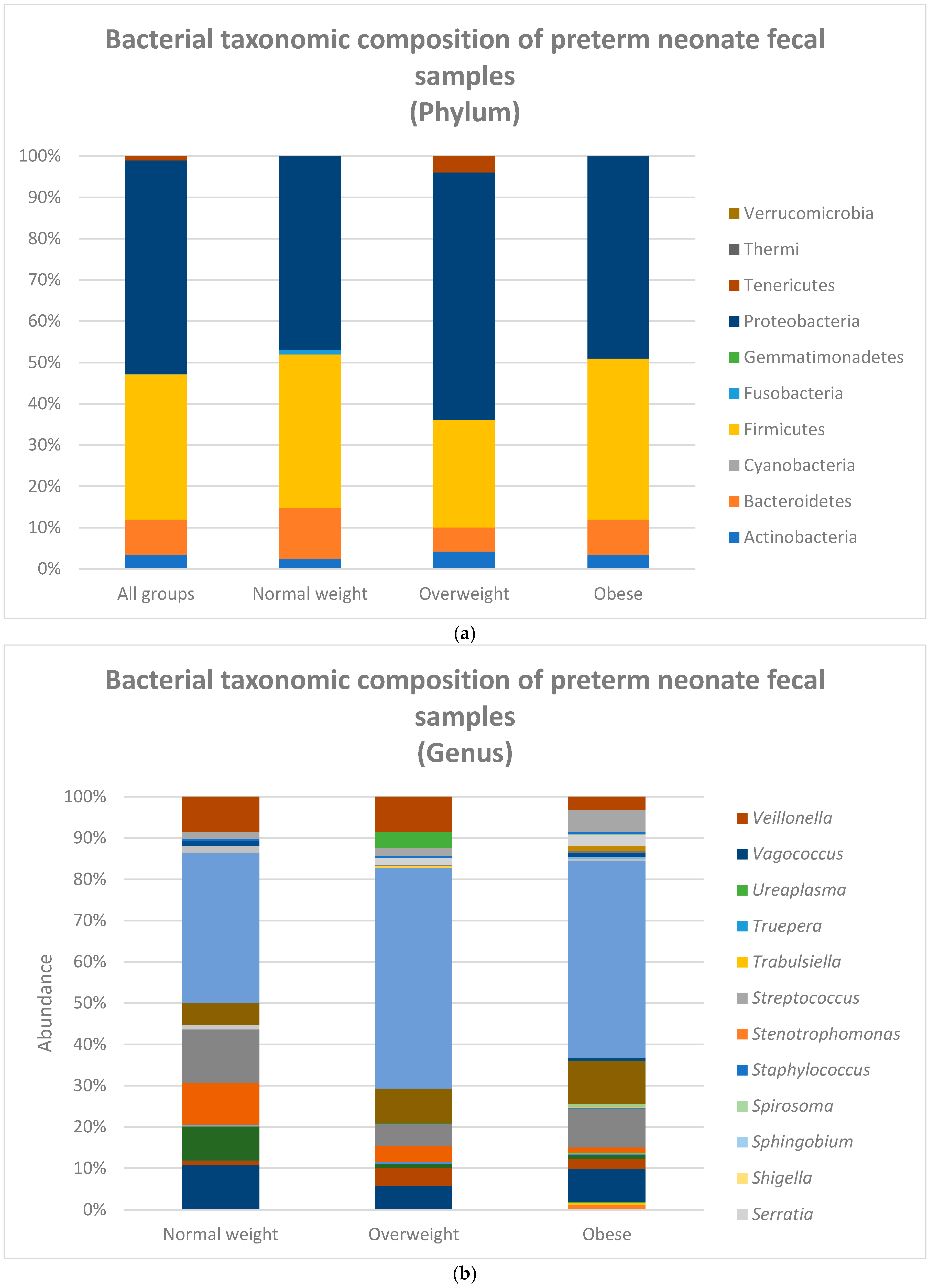
4.2. Metagenomic Analysis Results
4.3. Fecal Fermentation Profile (FFP)
4.4. Other Factors That May Impact Microbiome
5. Discussion
Summary of Findings
6. Conclusions
Suggestions for Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casirati, A.; Somaschini, A.; Perrone, M.; Vandoni, G.; Sebastiani, F.; Montagna, E.; Somaschini, M.; Caccialanza, R. Preterm birth and metabolic implications on later life: A narrative review focused on body composition. Front. Nutr. 2022, 9, 978271. [Google Scholar] [CrossRef]
- The 2022 March of Dimes Report Card: Starkand Unacceptable Disparities Persist Alongside a Troubling Rise in Preterm Birth Rates. 2022. Available online: https://www.marchofdimes.org/sites/default/files/2022-11/March-of-Dimes-2022-Full-Report-Card.pdf (accessed on 31 August 2023).
- De Costa, A.; Moller, A.B.; Blencowe, H.; Johansson, E.W.; Hussain-Alkhateeb, L.; Ohuma, E.O.; Okwaraji, Y.B.; Cresswell, J.; Requejo, J.H.; Bahl, R.; et al. Study protocol for WHO and UNICEF estimates of global, regional, and national preterm birth rates for 2010 to 2019. PLoS ONE 2021, 16, e0258751. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, J.; Li, Z.; Wan, W. The Change of Cytokines and Gut Microbiome in Preterm Infants for Bronchopulmonary Dysplasia. Front. Microbiol. 2022, 13, 804887. [Google Scholar] [CrossRef]
- Clark, R.H.; Wagner, C.L.; Merritt, R.J.; Bloom, B.T.; Neu, J.; Young, T.E.; Clark, D.A. Nutrition in the neonatal intensive care unit: How do we reduce the incidence of extrauterine growth restriction? J. Perinatol. 2003, 23, 337–344. [Google Scholar] [CrossRef]
- Koroglu, O.A.; Onay, H.; Cakmak, B.; Bilgin, B.; Yalaz, M.; Tunc, S.; Ozkinay, F.; Kultursay, N. Association of vitamin D receptor gene polymorphisms and bronchopulmonary dysplasia. Pediatr. Res. 2014, 76, 171–176. [Google Scholar] [CrossRef]
- March of Dimes. Nowhere to Go: Maternity Care Deserts Across the U.S. 2022. Available online: https://www.marchofdimes.org/maternity-care-deserts-report (accessed on 31 August 2023).
- Maternal Vulnerability in the US—A Shameful Problem for One of the World’s Wealthiest Countries. 2022. Available online: https://mvi.surgoventures.org/ (accessed on 31 August 2023).
- Sen, S.; Penfield-Cyr, A.; Hollis, B.W.; Wagner, C.L. Maternal Obesity, 25-Hydroxy Vitamin D Concentration, and Bone Density in Breastfeeding Dyads. J. Pediatr. 2017, 187, 147–152.e1. [Google Scholar] [CrossRef]
- Enstad, S.; Cheema, S.; Thomas, R.; Fichorova, R.N.; Martin, C.R.; O’tierney-Ginn, P.; Wagner, C.L.; Sen, S. The impact of maternal obesity and breast milk inflammation on developmental programming of infant growth. Eur. J. Clin. Nutr. 2021, 75, 180–188. [Google Scholar] [CrossRef]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal. Med. 2016, 21, 74–79. [Google Scholar] [CrossRef]
- Callaghan, W.M.; MacDorman, M.F.; Rasmussen, S.A.; Qin, C.; Lackritz, E.M. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 2006, 118, 1566–1573. [Google Scholar] [CrossRef]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, M.J.; Dias, T.; Damus, K.; Russell, R.; Bettegowda, V.R.; Dolan, S.; Schwarz, R.H.; Green, N.S.; Petrini, J. Changes in the gestational age distribution among U.S. singleton births: Impact on rates of late preterm birth, 1992 to 2002. Semin. Perinatol. 2006, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lapidaire, W.; Lucas, A.; Clayden, J.D.; Clark, C.; Fewtrell, M.S. Human milk feeding and cognitive outcome in preterm infants: The role of infection and NEC reduction. Pediatr. Res. 2022, 91, 1207–1214. [Google Scholar] [CrossRef]
- Taylor, S.N.; Basile, L.A.; Ebeling, M.; Wagner, C.L. Intestinal permeability in preterm infants by feeding type: Mother’s milk versus formula. Breastfeed. Med. 2009, 4, 11–15. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e1. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1. [Google Scholar] [CrossRef]
- Ford, S.L.; Lohmann, P.; Preidis, G.A.; Gordon, P.S.; O’Donnell, A.; Hagan, J.; Venkatachalam, A.; Balderas, M.; Luna, R.A.; Hair, A.B. Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother’s own milk compared with donor breast milk. Am. J. Clin. Nutr. 2019, 109, 1088–1097. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsu, Y.C.; Lin, M.C.; Chen, C.H.; Wang, T.M. The association of macronutrients in human milk with the growth of preterm infants. PLoS ONE 2020, 15, e0230800. [Google Scholar] [CrossRef]
- Hair, A.B.; Hawthorne, K.M.; Chetta, K.E.; Abrams, S.A. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res. Notes 2013, 6, 459. [Google Scholar] [CrossRef]
- Chetta, K.E.; Schulz, E.V.; Wagner, C.L. Outcomes improved with human milk intake in preterm and full-term infants. Semin. Perinatol. 2021, 45, 151384. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. Br. Heart J. 1993, 69, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Martyn, C.N.; Osmond, C.; Hales, C.N.; Fall, C.H. Growth in utero and serum cholesterol concentrations in adult life. BMJ 1993, 307, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Maternal and fetal origins of coronary heart disease. J. R. Coll. Physicians Lond. 1994, 28, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Forsén, T.; Eriksson, J.G.; Tuomilehto, J.; Osmond, C.; Barker, D.J. Growth in utero and during childhood among women who develop coronary heart disease: Longitudinal study. BMJ 1999, 319, 1403–1407. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Kajantie, E.; Thornburg, K.L.; Osmond, C.; Barker, D.J. Mother’s body size and placental size predict coronary heart disease in men. Eur. Heart J. 2011, 32, 2297–2303. [Google Scholar] [CrossRef]
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: A prospective case-control study. Lancet 2016, 387, 1928–1936. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 2010, 92, 574–584. [Google Scholar] [CrossRef]
- Pinheiro, T.V.; Goldani, M.Z.; IVAPSA Group. Maternal pre-pregnancy overweight/obesity and gestational diabetes interaction on delayed breastfeeding initiation. PLoS ONE 2018, 13, e0194879. [Google Scholar] [CrossRef]
- Ley, S.H.; Hanley, A.J.; Stone, D.; O’Connor, D.L. Effects of pasteurization on adiponectin and insulin concentrations in donor human milk. Pediatr. Res. 2011, 70, 278–281. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E.; et al. Effect of Supplemental Donor Human Milk Compared with Preterm Formula on Neurodevelopment of Very Low-Birth-Weight Infants at 18 Months: A Randomized Clinical Trial. JAMA 2016, 316, 1897–1905. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, A.K.; Gregory, E.C.W. Increases in Prepregnancy Obesity: United States, 2016–2019. NCHS Data Brief 2020, 392, 1–8. [Google Scholar]
- Tsuduki, T.; Kitano, Y.; Honma, T.; Kijima, R.; Ikeda, I. High dietary fat intake during lactation promotes development of diet-induced obesity in male offspring of mice. J. Nutr. Sci. Vitaminol. 2013, 59, 384–392. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef]
- Isganaitis, E. Milky ways: Effects of maternal obesity on human milk composition and childhood obesity risk. Am. J. Clin. Nutr. 2021, 113, 772–774, Erratum in Am. J. Clin. Nutr. 2021, 113, 1715. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Sobieraj, P.; Szostak-Węgierek, D.; Wesołowska, A. Impact of Infant and Maternal Factors on Energy and Macronutrient Composition of Human Milk. Nutrients 2020, 12, 2591. [Google Scholar] [CrossRef]
- De Luca, A.; Frasquet-Darrieux, M.; Gaud, M.A.; Christin, P.; Boquien, C.-Y.; Millet, C.; Herviou, M.; Darmaun, D.; Robins, R.J.; Ingrand, P.; et al. Higher Leptin but Not Human Milk Macronutrient Concentration Distinguishes Normal-Weight from Obese Mothers at 1-Month Postpartum. PLoS ONE 2016, 11, e0168568. [Google Scholar] [CrossRef]
- Zierle-Ghosh, A.; Jan, A. Physiology, Body Mass Index. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Wei, P.L.; Hung, C.S.; Kao, Y.W.; Lin, Y.-C.; Lee, C.-Y.; Chang, T.-H.; Shia, B.-C.; Lin, J.-C. Characterization of Fecal Microbiota with Clinical Specimen Using Long-Read and Short-Read Sequencing Platform. Int. J. Mol. Sci. 2020, 21, 7110. [Google Scholar] [CrossRef]
- Morgan, X.C.; Huttenhower, C. Chapter 12: Human microbiome analysis. PLoS Comput. Biol. 2012, 8, e1002808. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Preciado, A.; Torres, A.G.; Merino, E.; Bonomi, H.R.; Goldbaum, F.A.; García-Angulo, V.A. Extensive Identification of Bacterial Riboflavin Transporters and Their Distribution across Bacterial Species. PLoS ONE 2015, 10, e0126124. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169, Erratum in Nature 2015, 520, 388. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Williams, C.M. Prebiotics and lipid metabolism. Curr. Opin. Lipidol. 2002, 13, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ichim, T.E.; Patel, A.N.; Shafer, K.A. Experimental support for the effects of a probiotic/digestive enzyme supplement on serum cholesterol concentrations and the intestinal microbiome. J. Transl. Med. 2016, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Spadafora, P.; Eshuis, H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Payne, A.N.; Chassard, C.; Zimmermann, M.; Müller, P.; Stinca, S.; Lacroix, C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr. Diabetes 2011, 1, e12. [Google Scholar] [CrossRef]

| Min–Max | Mean (SD) | N (%) | |
|---|---|---|---|
| Maternal Factor | |||
| Pre-pregnancy BMI | 18–46.7 | 30.75 (6.92) | |
| Normal weight | 10 (19%) | ||
| Overweight | 15 (27%) | ||
| Obese | 29 (54%) | ||
| Race/ethnicity | |||
| Black | 25 (46%) | ||
| White | 20 (37%) | ||
| Hispanic | 7 (13%) | ||
| Other | 2 (4%) | ||
| Maternal diabetes | |||
| No | 39 (72%) | ||
| Yes | 15 (28%) | ||
| Infant Factor | |||
| Gestational age | 169–242 days | 208 (19.09) | |
| Extremely preterm (22–27 weeks) | 14 (26%) | ||
| Very preterm (28–31 weeks) | 25 (46%) | ||
| Moderately preterm (32–37 weeks) | 15 (28%) | ||
| Birth Weight | 620 g–2350 g | 1263 (504.41) | |
| Micro-preemie (<800 g) | 9 (16.5%) | ||
| ELBW (<1000 g) | 9 (16.5%) | ||
| VLBW (<1500 g) | 15 (28%) | ||
| LBW (<2500 g) | 21 (39%) | ||
| NBW (>2500 g) | 0 (0%) | ||
| Fenton Growth % | |||
| Birth weight | 0.1–93.3% | 32.94 (26.63) | |
| SGA (<10%) | 5 (9%) | ||
| AGA (10–90%) | 48 (89%) | ||
| LGA (>90%) | 1 (2%) | ||
| Birth length | 0.2–99.1% | 29.54 (31.61) | |
| SGA (<10%) | 7 (13%) | ||
| AGA (10–90%) | 39 (72%) | ||
| LGA (>90%) | 8 (15%) | ||
| OFC (occipital frontal circumference) | 0.2–92% | 23.53 (23.99) | |
| SGA (<10%) | 9 (17%) | ||
| AGA (10–90%) | 44 (81%) | ||
| LGA (>90%) | 1 (2%) | ||
| Delivery Mode | |||
| Vaginal | 15 (28%) | ||
| Cesarean | 39 (72%) | ||
| Fecal sample collection | 2–95 days | 15.63 (19.24) | |
| Week 1 | 9 (17%) | ||
| Week 2 | 15 (28%) | ||
| Week 3 | 15 (28%) | ||
| Week 4 | 5 (9%) | ||
| Week 5 + | 10 (18%) | ||
| Feeding milk type | |||
| Mom only | 5 (9%) | ||
| Donor only | 5 (9%) | ||
| Combination | 44 (81%) |
| Gestation (Days) | Birth Weight (g) | Fenton Growth (Birth Weight) | Fenton Growth (Birth Length) | Fenton Growth (Birth OFC) | Day of Life at Sample Collection | Race ETHNICITY (BLACK) | Maternal Diabetes (Yes) | C-Section | Feeding Type (Mom Only Milk) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal BMI (<25 kg/m2) (n = 10) | 216.02 (14.27) | 1408.15 (429.56) | 35.44 (16.76) | 37.80 (20.54) | 22.96 (15.07) | 12.72 (9.37) | 3 (30%) | 2 (20%) | 6 (60%) | 1 (10%) |
| Overweight BMI (25–29.9 kg/m2) (n = 15) | 209.86 (20.28) | 1346.17 (549.69) | 43.53 (22.93) | 50.17 (31.37) | 32.11 (23.28) | 24.10 (22.07) | 7 (33%) | 3 (14%) | 13 (62%) | 5 (25%) |
| Obese BMI (≥30 kg/m2) (n = 29) | 207.49 (19.24) | 1330.59 (510.11) | 49.32 (28.98) | 51.24 (31.47) | 39.40 (26.04) | 20.59 (19.30) | 22 (55%) | 14 (35%) | 27 (66%) | 4 (10%) |
| p-value | 0.3 | 0.3 | 0.3 | 0.7 | 0.2 | 0.5 | 0.3 | 0.2 | 0.9 | 0.4 |
| Model | Beta | SE | p-Value | |
|---|---|---|---|---|
| Acetic acid * | Pre-pregnancy BMI one unit increase | −0.5137 | 0.257 | 0.051 |
| Butyric acid * | Pre-pregnancy BMI one unit increase | 0.02122 | 0.069 | 0.76 |
| Propionic acid * | Pre-pregnancy BMI one unit increase | 0.5914 | 0.248 | 0.021 |
| APB acids combined * | Pre-pregnancy BMI one unit increase | 0.09892 | 0.126 | 0.44 |
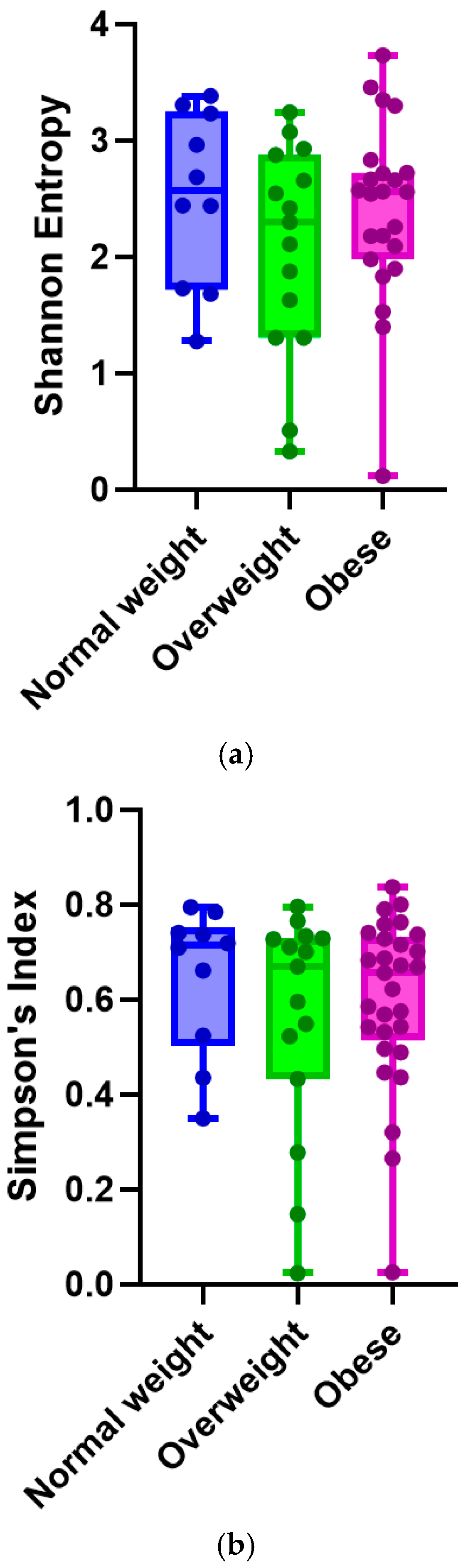
| Shannon diversity index (alpha diversity) * | Pre-pregnancy BMI one unit increase | 0.004639 | 0.014 | 0.74 |
| Alpha Diversity | Beta Diversity | |||
|---|---|---|---|---|
| Kruskal–Wallis | Shannon Diversity | Bray–Curtis | Jaccard | |
| Infant weeks of life | 0.1 | 0.55 | 0.14 | 0.20 |
| Gestational age | 0.9 | 0.46 | >0.05 | 0.06 |
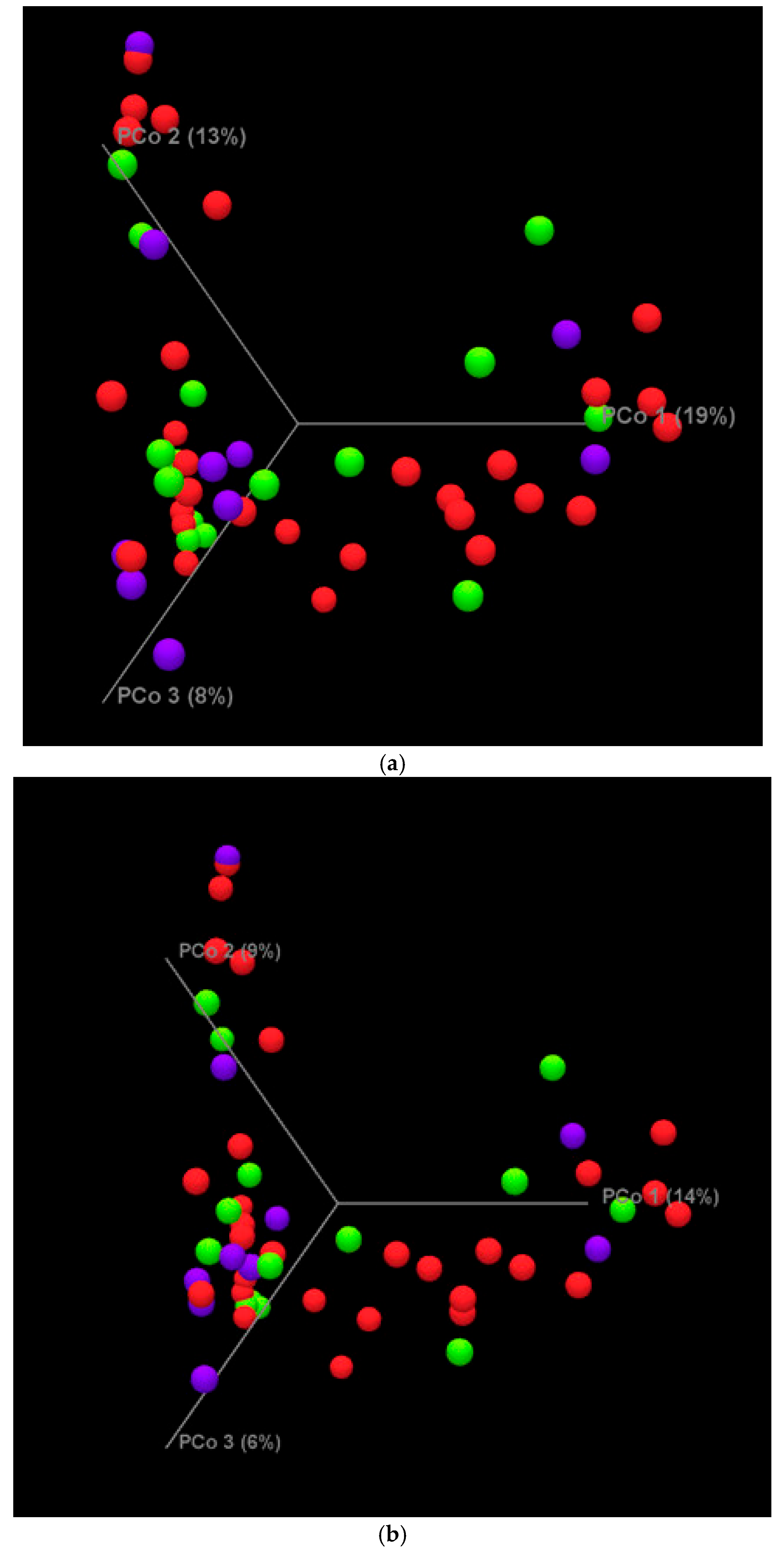
| Delivery mode | 0.2 | 0.79 | 0.02 | 0.03 |
| Race/ethnicity | 0.3 | 0.30 | 0.99 | 0.99 |
| Feeding type | 0.1 | 0.63 | 0.48 | 0.50 |
| MoM dose | 0.1 | 0.74 | 0.66 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, K.L.; Wahlquist, A.E.; James, D.; Clark, W.A.; Wagner, C.L. Effects of Maternal Pre-Pregnancy BMI on Preterm Infant Microbiome and Fecal Fermentation Profile—A Preliminary Cohort Study. Nutrients 2025, 17, 987. https://doi.org/10.3390/nu17060987
Thomas KL, Wahlquist AE, James D, Clark WA, Wagner CL. Effects of Maternal Pre-Pregnancy BMI on Preterm Infant Microbiome and Fecal Fermentation Profile—A Preliminary Cohort Study. Nutrients. 2025; 17(6):987. https://doi.org/10.3390/nu17060987
Chicago/Turabian StyleThomas, Kristy L., Amy E. Wahlquist, Dalton James, William Andrew Clark, and Carol L. Wagner. 2025. "Effects of Maternal Pre-Pregnancy BMI on Preterm Infant Microbiome and Fecal Fermentation Profile—A Preliminary Cohort Study" Nutrients 17, no. 6: 987. https://doi.org/10.3390/nu17060987
APA StyleThomas, K. L., Wahlquist, A. E., James, D., Clark, W. A., & Wagner, C. L. (2025). Effects of Maternal Pre-Pregnancy BMI on Preterm Infant Microbiome and Fecal Fermentation Profile—A Preliminary Cohort Study. Nutrients, 17(6), 987. https://doi.org/10.3390/nu17060987







