Sex Differences in the Association Between Masticatory Function and Sarcopenia Components: The Shizuoka Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement of Objective and Subjective Masticatory Function
2.3. Assessment of Skeletal Muscle Mass and Physical Performance
2.4. Dietary Intake Assessment
2.5. Basic Clinical Parameters
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMI | skeletal muscle mass index |
| BMI | body mass index |
References
- Locker, D. Measuring oral health: A conceptual framework. Community Dent Health 1988, 5, 3–18. [Google Scholar] [PubMed]
- Minakuchi, S.; Tsuga, K.; Ikebe, K.; Ueda, T.; Tamura, F.; Nagao, K.; Furuya, J.; Matsuo, K.; Yamamoto, K.; Kanazawa, M.; et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology 2018, 35, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Miura, H. Systematic review of the association of mastication with food and nutrient intake in the independent elderly. Arch. Gerontol. Geriatr. 2014, 59, 497–505. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Fan, Y.; Shu, X.; Leung, K.C.M.; Man, E.C. Associations of general health conditions with masticatory performance and maximum bite force in older adults: A systematic review of cross-sectional studies. J. Dent. 2022, 123, 104186. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tsuji, T.; Akishita, M.; Iijima, K. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Obuchi, S.; Kawai, H.; Fujiwara, Y.; et al. Relationship between oral hypofunction and sarcopenia in community-dwelling older adults: The Otassha Study. Int. J. Environ. Res. Public. Health 2021, 18, 6666. [Google Scholar] [CrossRef]
- Takata, Y.; Ansai, T.; Awano, S.; Hamasaki, T.; Yoshitake, Y.; Kimura, Y.; Sonoki, K.; Wakisaka, M.; Fukuhara, M.; Takehara, T. Relationship of physical fitness to chewing in an 80-year-old population. Oral. Dis. 2004, 10, 44–49. [Google Scholar] [CrossRef]
- Moriya, S.; Muramatsu, T.; Tei, K.; Nakamura, K.; Muramatsu, M.; Notani, K.; Inoue, N. Relationships between oral conditions and physical performance in a rural elderly population in Japan. Int. Dent. J. 2009, 59, 369–375. [Google Scholar]
- Moriya, S.; Tei, K.; Yamazaki, Y.; Hata, H.; Shinkai, S.; Yoshida, H.; Muramatsu, M.; Kitagawa, Y.; Inoue, N.; Yamada, H.; et al. Relationships between perceived chewing ability and muscle strength of the body among the elderly. J. Oral. Rehabil. 2011, 38, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Notani, K.; Murata, A.; Inoue, N.; Miura, H. Analysis of moment structures for assessing relationships among perceived chewing ability, dentition status, muscle strength, and balance in community-dwelling older adults. Gerodontology 2014, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Mihara, Y.; Matsuda, K.I.; Ikebe, K.; Hatta, K.; Fukutake, M.; Enoki, K.; Ogawa, T.; Takeshita, H.; Inomata, C.; Gondo, Y.; et al. Association of handgrip strength with various oral functions in 82- to 84-year-old community-dwelling Japanese. Gerodontology 2018, 35, 214–220. [Google Scholar] [CrossRef]
- Abe, T.; Tominaga, K.; Ando, Y.; Toyama, Y.; Takeda, M.; Yamasaki, M.; Okuyama, K.; Hamano, T.; Isomura, M.; Nabika, T.; et al. Number of teeth and masticatory function are associated with sarcopenia and diabetes mellitus status among community-dwelling older adults: A Shimane CoHRE study. PLoS ONE 2021, 16, e0252625. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Notani, K.; Miura, H.; Inoue, N. Relationship between masticatory ability and physical performance in community-dwelling edentulous older adults wearing complete dentures. Gerodontology 2012, 31, 251–259. [Google Scholar] [CrossRef]
- Takagi, D.; Watanabe, Y.; Edahiro, A.; Ohara, Y.; Murakami, M.; Murakami, K.; Murakami, K.; Hironaka, S.; Taniguchi, Y.; Kitamura, A.; et al. Factors affecting masticatory function of community-dwelling older people: Investigation of the differences in the relevant factors for subjective and objective assessment. Gerodontology 2017, 34, 357–364. [Google Scholar] [CrossRef]
- Kamdem, B.; Seematter-Bagnoud, L.; Botrugno, F.; Santos-Eggimann, B. Relationship between oral health and Fried’s frailty criteria in community- dwelling older persons. BMC Geriatr. 2017, 17, 174. [Google Scholar] [CrossRef]
- Nishi, T.; Ohta, M.; Takano, T.; Ogami, K.; Ueda, T.; Sakurai, K. Oral function is associated with the body and muscle mass indices of middle-aged dental patients. Clin. Exp. Dent. Res. 2022, 8, 217–224. [Google Scholar] [CrossRef]
- Maughan, R.; Watson, J.S.; Weir, J. Strength and cross-sectional area of human skeletal muscle. J. Physiol. 1983, 338, 37–49. [Google Scholar] [CrossRef]
- Nagasawa, T.; Yanbin, X.; Tsuga, K.; Abe, Y. Sex difference of electromyogram of masticatory muscles and mandibular movement during chewing of food. J. Oral. Rehabil. 1997, 24, 605–609. [Google Scholar] [CrossRef]
- Kim, M.J. Food consumption frequency of Korean adults based on whether or not having chewing difficulty using 2013-2016 KNHANES by sex-stratified comparative analysis. Nutr. Res. Pr. 2020, 14, 637–653. [Google Scholar] [CrossRef]
- UHA Mikakuto Co., Ltd. “Soshaku No Ohanashi”. Available online: https://www.uha-mikakuto.co.jp/sosyaku/ (accessed on 15 January 2025).
- Nokubi, T.; Yoshimuta, Y.; Nokubi, F.; Yasui, S.; Kusunoki, C.; Ono, T.; Maeda, Y.; Yokota, K. Validity and reliability of a visual scoring method for masticatory ability using test gummy jelly. Gerodontology 2013, 30, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Department of Social and Preventive Epidemiology, School of Public Health, The University of Tokyo. BDHQ. Available online: http://www.nutrepi.m.u-tokyo.ac.jp/dhq/summary.html (accessed on 15 January 2025).
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, K.; Morii, K.; Matsuda, K.; Hazeyama, T.; Nokubi, T. Reproducibility and precision in measuring masticatory performance using test gummy jelly. Prosthodont. Res. Pr. 2005, 4, 9–15. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Fusco, D.; Sisto, A.; Ortolani, E.; Savera, G.; Salini, S.; Marzetti, E. Age-related variations of muscle mass, strength, and physical performance in community-dwellers: Results from the Milan EXPO survey. J. Am. Med. Dir. Assoc. 2017, 18, e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Lee, S.; Lee, S.C.; Harada, K.; Hotta, R.; Nakakubo, S.; Bae, S.; et al. Age-dependent changes in physical performance and body composition in community-dwelling Japanese older adults. J. Cachexia Sarcopenia Muscle 2017, 8, 607–614. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Shimokata, H.; Ando, F.; Yuki, A.; Otsuka, R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: A 12-year longitudinal study. Geriatr. Gerontol. Int. 2014, 14, 85–92. [Google Scholar] [CrossRef]
- N’gom, P.I.; Woda, A. Influence of impaired mastication on nutrition. J. Prosthet. Dent. 2002, 87, 667–673. [Google Scholar] [CrossRef] [PubMed]
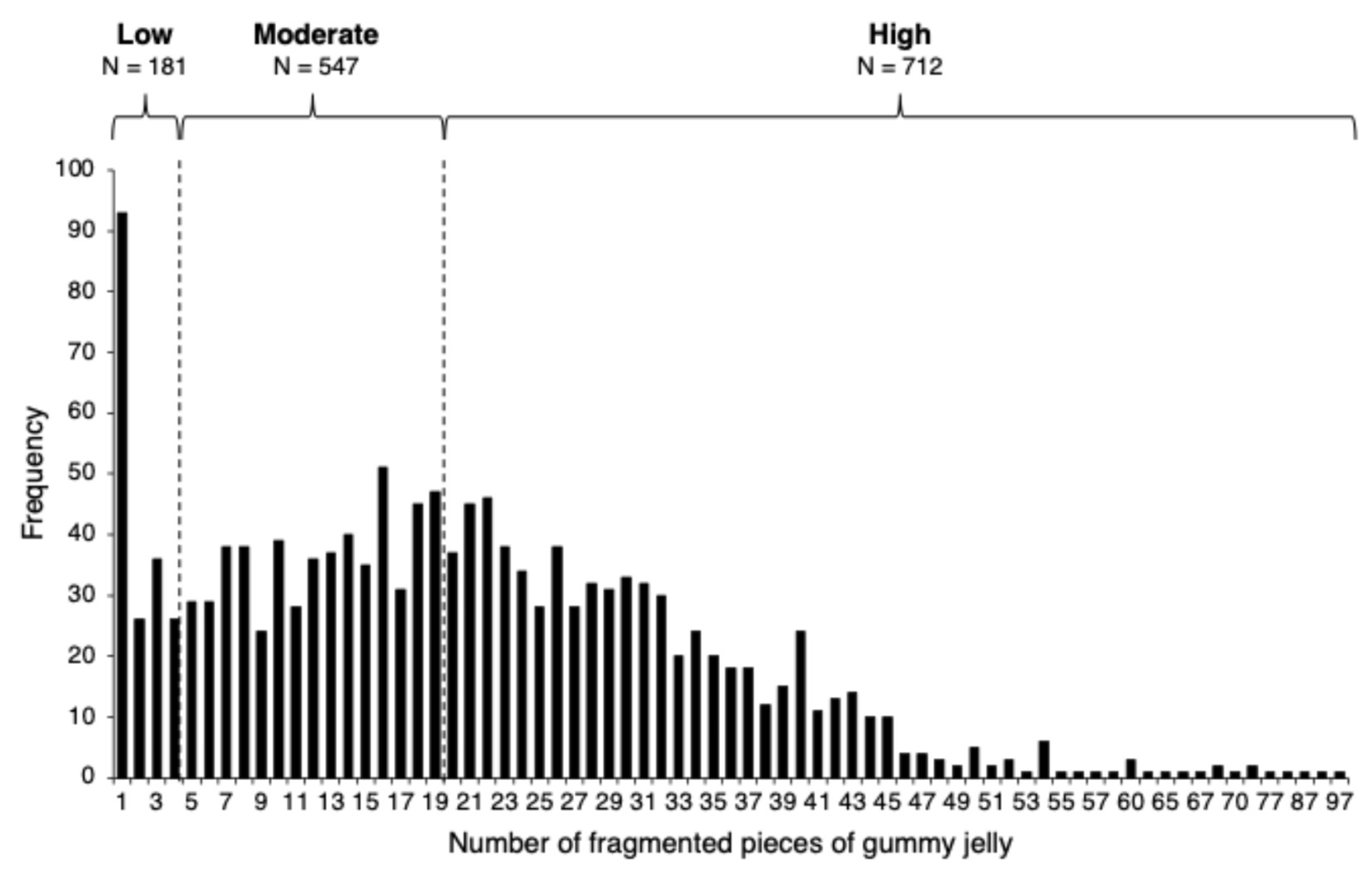
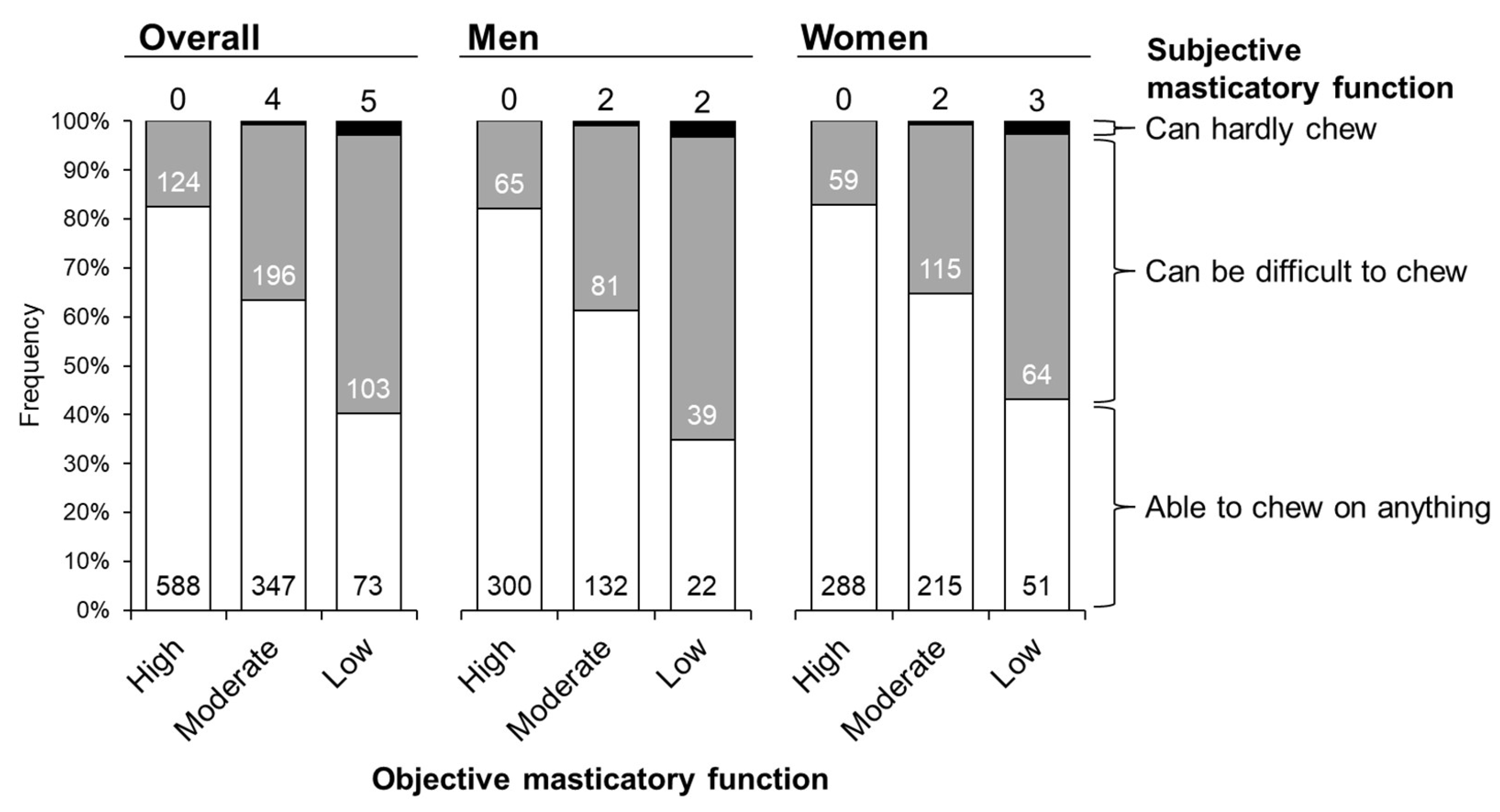
| Overall | Men | Women | |
|---|---|---|---|
| n | 1440 | 643 | 797 |
| Age, years | 73.9 (4.6) | 74.1 (4.5) | 73.8 (4.6) |
| Body mass index, kg/m2 | 22.6 (3.2) | 23.2 (2.9) | 22.1 (3.4) |
| Waist circumference, cm | 82.7 (9.3) | 84.9 (8.7) | 80.9 (9.4) |
| Sarcopenia components | |||
| Skeletal muscle mass index, kg/m2 | 7.0 (0.9) | 7.7 (0.7) | 6.4 (0.5) |
| Handgrip strength, kg | 27.9 (8.3) | 34.7 (6.6) | 22.4 (4.4) |
| Gait speed, m/s | 1.4 (0.2) | 1.4 (0.2) | 1.4 (0.2) |
| Chair-stand test, s | 7.4 (2.1) | 7.4 (2.2) | 7.4 (2.0) |
| Dietary intake | |||
| Total energy, kcal | 1843 (523) | 2019 (543) | 1701 (461) |
| Protein, g | 77 (28) | 80 (29) | 74 (27) |
| Fat, g | 56 (20) | 59 (21) | 54 (19) |
| Carbohydrate, g | 240 (70) | 261 (72) | 224 (63) |
| Masticatory function | |||
| Fragmented pieces of gummy jelly, n | 20.6 (13.8) | 23.0 (14.4) | 18.7 (12.9) |
| Self-assessment, n | |||
| Able to chew on anything | 1008 | 454 | 554 |
| Can be difficult to chew | 423 | 185 | 238 |
| Can hardly chew | 9 | 4 | 5 |
| Masticatory Function | n | Age, Years Old | Body Mass Index, kg/m2 | Waist Circumference, cm | Skeletal Muscle Mass Index, kg/m2 | Handgrip Strength, kg | Gait Speed, m/s | Five-Time Chair-Stand Test Time, s | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Objective | Low | 63 | 75.4 (0.6) | 23.0 (0.4) | 84.4 (1.1) | 7.4 (0.09) | 31.8 (0.8) | 1.3 (0.03) | 7.7 (0.3) |
| Moderate | 215 | 74.3 (0.3) | 23.4 (0.2) | 84.9 (0.6) | 7.7 (0.05) | 34.2 (0.4) | 1.4 (0.02) | 7.5 (0.2) | ||
| High | 365 | 73.7 (0.2) | 23.2 (0.2) | 84.9 (0.5) | 7.7 (0.04) | 35.5 (0.3) | 1.4 (0.01) | 7.3 (0.1) | ||
| p | 0.014 | 0.630 | 0.904 | 0.005 | <0.001 | 0.003 | 0.337 | |||
| Subjective | Can hardly chew | 4 | 68.8 (2.2) | 24.8 (1.5) | 89.0 (4.3) | 7.7 (0.37) | 32.2 (3.3) | 1.2 (0.11) | 7.5 (1.1) | |
| Can be difficult to chew | 185 | 74.1 (0.3) | 23.4 (0.2) | 85.2 (0.6) | 7.7 (0.05) | 34.9 (0.5) | 1.4 (0.02) | 7.6 (0.2) | ||
| Able to chew on anything | 454 | 74.1 (0.2) | 23.2 (0.1) | 84.7 (0.4) | 7.7 (0.03) | 34.7 (0.3) | 1.4 (0.01) | 7.4 (0.1) | ||
| p | 0.061 | 0.466 | 0.536 | 0.974 | 0.714 | 0.021 | 0.701 | |||
| Women | Objective | Low | 118 | 75.0 (0.4) | 22.9 (0.3) | 82.9 (0.9) | 6.4 (0.04) | 21.9 (0.4) | 1.4 (0.02) | 7.9 (0.2) |
| Moderate | 332 | 74.1 (0.3) | 22.0 (0.2) | 80.8 (0.5) | 6.3 (0.03) | 21.8 (0.2) | 1.4 (0.01) | 7.6 (0.1) | ||
| High | 347 | 73.1 (0.2) | 21.9 (0.2) | 80.4 (0.5) | 6.4 (0.03) | 23.0 (0.2) | 1.5 (0.01) | 7.1 (0.1) | ||
| p | <0.001 | 0.028 | 0.041 | 0.129 | 0.001 | 0.004 | <0.001 | |||
| Subjective | Can hardly chew | 5 | 74.6 (2.1) | 23.1 (1.5) | 85.4 (4.2) | 6.5 (0.22) | 21.4 (2.0) | 1.5 (0.10) | 6.8 (0.9) | |
| Can be difficult to chew | 238 | 74.2 (0.3) | 22.0 (0.2) | 80.6 (0.6) | 6.3 (0.03) | 22.0 (0.3) | 1.4 (0.01) | 7.6 (0.1) | ||
| Able to chew on anything | 554 | 73.6 (0.2) | 22.1 (0.1) | 81.0 (0.4) | 6.4 (0.02) | 22.5 (0.2) | 1.5 (0.01) | 7.4 (0.1) | ||
| p | 0.197 | 0.757 | 0.494 | 0.312 | 0.204 | 0.056 | 0.137 | |||
| Masticatory Function | Body Mass Index 1 | Waist Circumference 1 | Skeletal Muscle Mass Index 2 | Handgrip Strength 3 | Gait Speed 3 | Five-Time Chair-Stand Test Time 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | ||
| Men | Objective | ||||||||||||
| Low | −0.151 | 0.561 | −0.274 | 0.724 | −0.126 | 0.013 | −0.982 | 0.049 | −0.047 | 0.020 | 0.054 | 0.783 | |
| Moderate | 0.170 | 0.366 | 0.160 | 0.775 | 0.047 | 0.196 | 0.058 | 0.871 | 0.011 | 0.458 | 0.023 | 0.873 | |
| High | reference | reference | reference | reference | reference | reference | |||||||
| Subjective | |||||||||||||
| Can hardly chew | 0.824 | 0.401 | 2.455 | 0.401 | −0.223 | 0.243 | −2.879 | 0.126 | −0.141 | 0.061 | 0.188 | 0.800 | |
| Can be difficult to chew | −0.327 | 0.522 | −1.012 | 0.506 | 0.099 | 0.321 | 1.536 | 0.117 | 0.053 | 0.175 | −0.017 | 0.965 | |
| Able to chew on anything | reference | reference | reference | reference | reference | reference | |||||||
| Women | Objective | ||||||||||||
| Low | 0.610 | 0.007 | 1.584 | 0.012 | −0.029 | 0.308 | −0.085 | 0.747 | −0.018 | 0.192 | 0.239 | 0.060 | |
| Moderate | −0.253 | 0.140 | −0.618 | 0.199 | −0.012 | 0.567 | −0.306 | 0.125 | −0.002 | 0.812 | 0.063 | 0.513 | |
| High | reference | reference | reference | reference | reference | reference | |||||||
| Subjective | |||||||||||||
| Can hardly chew | 0.795 | 0.432 | 3.231 | 0.253 | 0.032 | 0.796 | −0.694 | 0.554 | 0.055 | 0.369 | −0.522 | 0.358 | |
| Can be difficult to chew | −0.426 | 0.418 | −1.789 | 0.224 | −0.033 | 0.618 | 0.209 | 0.732 | −0.043 | 0.179 | 0.368 | 0.213 | |
| Able to chew on anything | reference | reference | reference | reference | reference | reference | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagao-Sato, S.; Kushida, O.; Kurita, Y.; Ozaki, E.; Kuriyama, N.; Kato, M.; Akamatsu, R.; Goda, T.; Tabara, Y. Sex Differences in the Association Between Masticatory Function and Sarcopenia Components: The Shizuoka Study. Nutrients 2025, 17, 968. https://doi.org/10.3390/nu17060968
Nagao-Sato S, Kushida O, Kurita Y, Ozaki E, Kuriyama N, Kato M, Akamatsu R, Goda T, Tabara Y. Sex Differences in the Association Between Masticatory Function and Sarcopenia Components: The Shizuoka Study. Nutrients. 2025; 17(6):968. https://doi.org/10.3390/nu17060968
Chicago/Turabian StyleNagao-Sato, Sayaka, Osamu Kushida, Yasunari Kurita, Etsuko Ozaki, Nagato Kuriyama, Michitaka Kato, Rie Akamatsu, Toshinao Goda, and Yasuharu Tabara. 2025. "Sex Differences in the Association Between Masticatory Function and Sarcopenia Components: The Shizuoka Study" Nutrients 17, no. 6: 968. https://doi.org/10.3390/nu17060968
APA StyleNagao-Sato, S., Kushida, O., Kurita, Y., Ozaki, E., Kuriyama, N., Kato, M., Akamatsu, R., Goda, T., & Tabara, Y. (2025). Sex Differences in the Association Between Masticatory Function and Sarcopenia Components: The Shizuoka Study. Nutrients, 17(6), 968. https://doi.org/10.3390/nu17060968






