Greater Numbers of Chews and Bites and Slow External Rhythmic Stimulation Prolong Meal Duration in Healthy Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement of Meal Duration, Numbers of Chews and Bites, and Eating Tempo
2.3. BDHQ (Brief-Type Self-Administered Diet History Questionnaire)
2.4. Handgrip Strength and Five-Times Sit-to-Stand Test
2.5. Experiments
2.6. Statistics
3. Results
3.1. Background of the Subjects in This Study
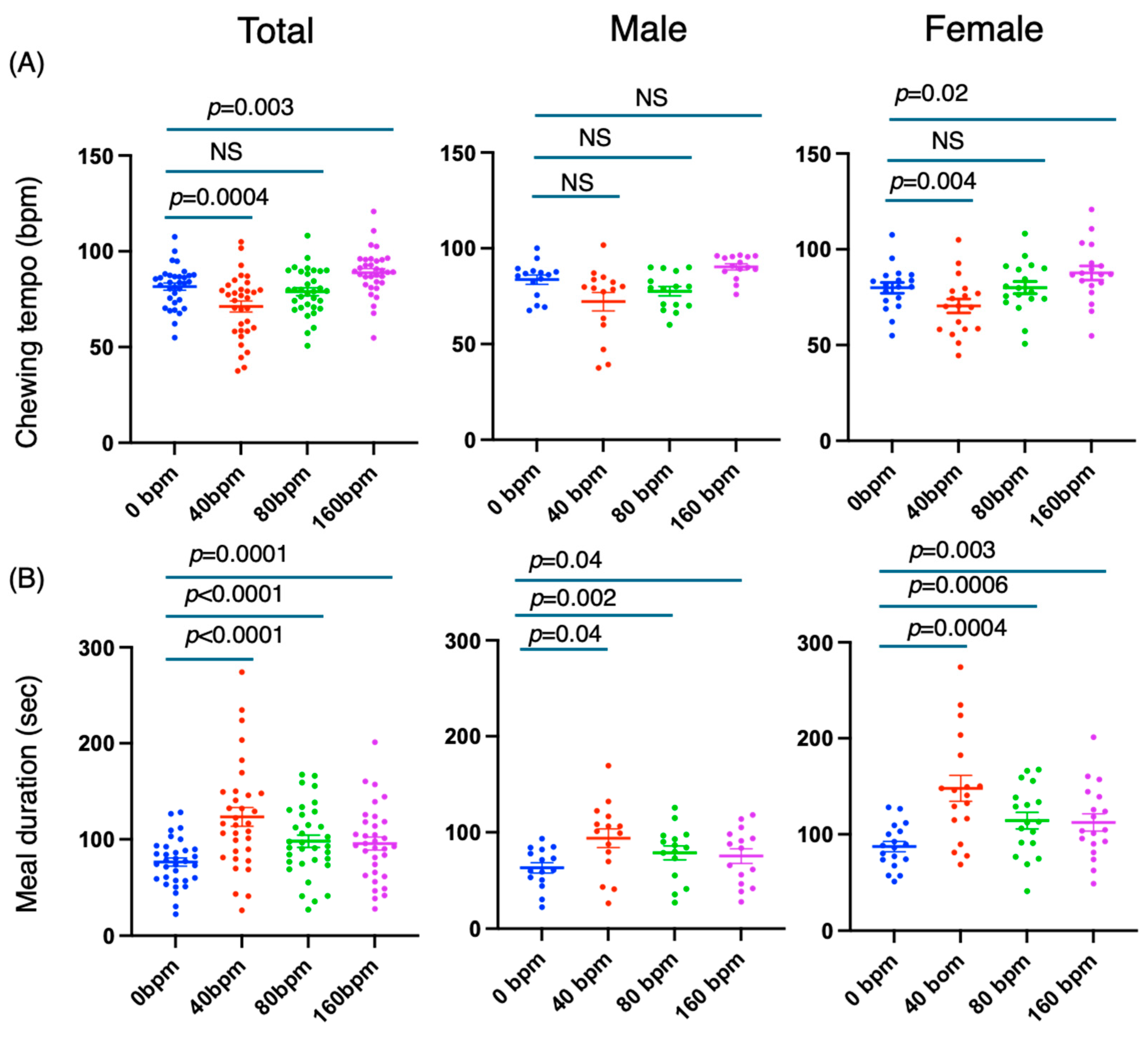
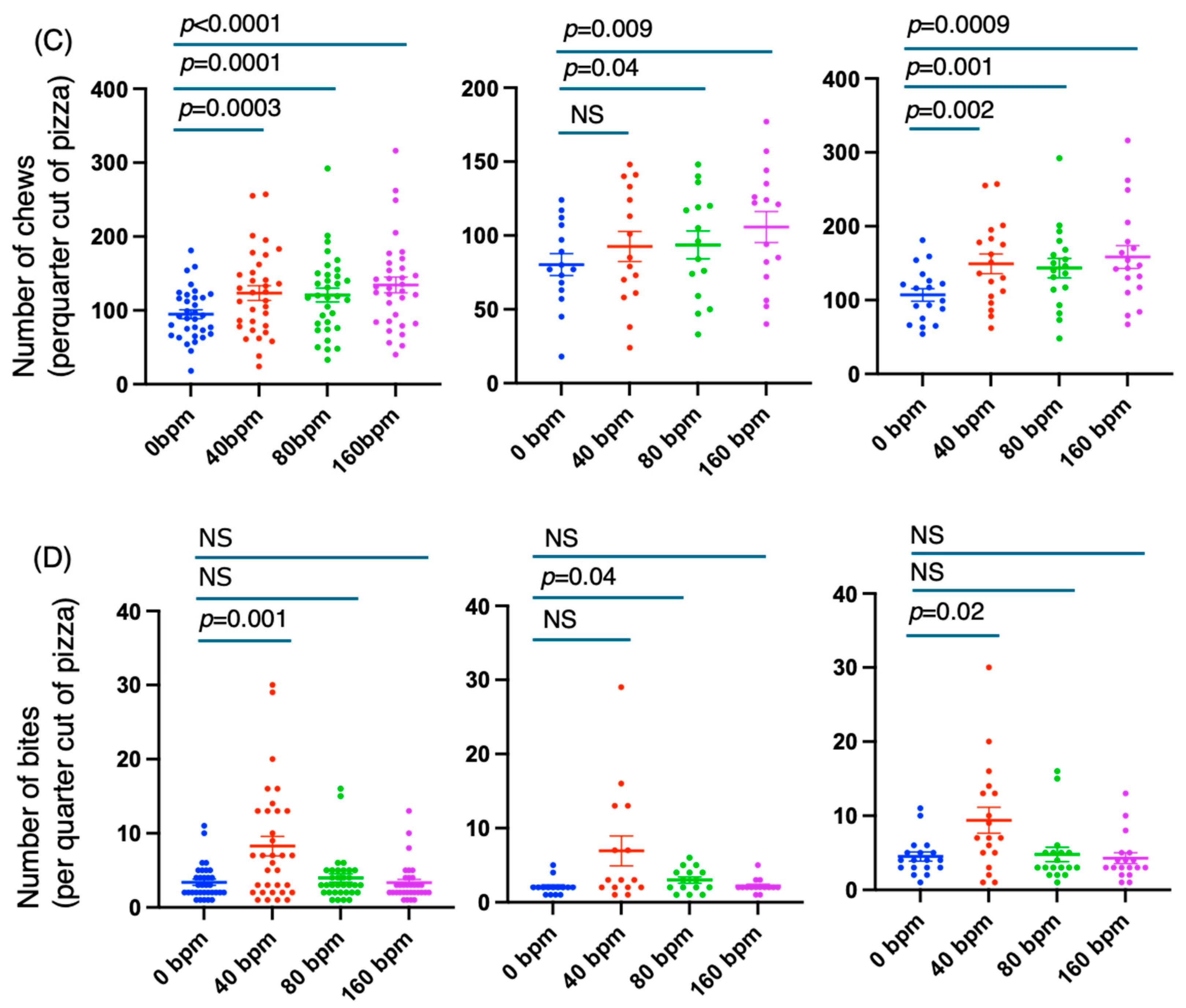
3.2. The Meal Duration Was Associated with the Numbers of Chews and Bites but Not the Average Eating Tempo
3.3. The Slow Rhythm Produced by the Metronome More Potently Prolonged the Meal Duration and Increased the Numbers of Chews and Bites by Decreasing the Chewing Tempo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Amin, T.; Mercer, J.G. Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake. Curr. Obes. Rep. 2016, 5, 106–112. [Google Scholar] [CrossRef]
- Nour, T.Y.; Altintaş, K.H. Obesity after Natural disasters and Associated Risk Factors: A Systematic Review. Disaster Med. Public Health Prep. 2025, 19, e8. [Google Scholar] [CrossRef]
- Kim, A.-R.; Kim, J.-W.; Kim, N.-Y.; Lee, H.-S. Listening to Music Can Reduce Calorie Intake and Increase Satiety: A Systematic literature Review and Additional Pilot Study. Exerc. Sci. 2024, 33, 423–435. [Google Scholar] [CrossRef]
- Sáenz-Pardo-Reyes, E.; Housni, F.E.; López-Espinoza, A.; Martínez Moreno, A.G.; Padilla Galindo, M.D.R.; Velázquez Saucedo, G. Efecto de las técnicas y estrategias de modificación de la velocidad al comer sobre la ingesta de alimentos o energía: Revisión sistemática y metaanálisis [Effect of eating speed modification techniques and strategies on food or energy intake: A systematic review and meta-analysis]. Nutr. Hosp. 2021, 38, 631–644. (In Spanish) [Google Scholar] [CrossRef]
- Cui, T.; Xi, J.; Tang, C.; Song, J.; He, J.; Brytek-Matera, A. The Relationship between Music and Food Intake: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2571. [Google Scholar] [CrossRef]
- Martínez-López, M.F.; López-Gil, J.F. Meal Duration and Obesity-Related Indicators among Adolescents: Insights from the EHDLA Study. Nutrients 2024, 16, 2769. [Google Scholar] [CrossRef]
- Smit, H.J.; Kemsley, E.K.; Tapp, H.S.; Henry, C.J. Does prolonged chewing reduce food intake? Fletcherism revisited. Appetite 2011, 57, 295–298. [Google Scholar] [CrossRef]
- Garcidueñas-Fimbres, T.E.; Paz-Graniel, I.; Nishi, S.K.; Salas-Salvadó, J.; Babio, N. Eating Speed, Eating Frequency, and Their Relationships with Diet Quality, Adiposity, and Metabolic Syndrome, or Its Components. Nutrients 2021, 13, 1687. [Google Scholar] [CrossRef]
- Tao, L.; Yang, K.; Huang, F.; Liu, X.; Li, X.; Luo, Y.; Wu, L.; Guo, X. Association between self-reported eating speed and metabolic syndrome in a Beijing adult population: A cross-sectional study. BMC Public Health 2018, 18, 855. [Google Scholar] [CrossRef]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Wada, K.; Matsushita, K.; OuYang, P.; Hotta, Y.; Takefuji, S.; Mitsuhashi, H.; Sugiura, K.; et al. Eating Fast Leads to Insulin Resistance: Findings in Middle-Aged Japanese Men and Women. Prev. Med. 2008, 46, 154–159. [Google Scholar] [CrossRef]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Murata, C.; Sekiya, A.; Wada, K.; Zhang, H.M.; Matsushita, K.; Sugiura, K.; Takefuji, S.; et al. Eating fast leads to obesity: Findings based on self-administered questionnaires among middle-aged Japanese men and women. J. Epidemiol. 2006, 16, 117–124. [Google Scholar] [CrossRef]
- Nagahama, S.; Kurotani, K.; Pham, N.M.; Nanri, A.; Kuwahara, K.; Dan, M.; Nishiwaki, Y.; Mizoue, T. Self-Reported Eating Rate and Metabolic Syndrome in Japanese People: Cross-Sectional Study. BMJ Open 2014, 4, e005241. [Google Scholar] [CrossRef]
- Jiménez-Ten Hoevel, C.; Llauradó, E.; Valls, R.M.; Besora-Moreno, M.; Queral, J.; Solà, R.; Pedret, A. Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review. Nutrients 2025, 17, 435. [Google Scholar] [CrossRef] [PubMed]
- Food Education for Everyone. Available online: https://www.maff.go.jp/j/syokuiku/minna_navi/topics/topics4_02.html (accessed on 22 January 2025).
- Morquette, P.; Lavoie, R.; Fhima, M.D.; Lamoureux, X.; Verdier, D.; Kolta, A. Generation of the masticatory central pattern and its modulation by sensory feedback. Prog. Neurobiol. 2012, 96, 340–355. [Google Scholar] [CrossRef]
- Lund, J.P.; Kolta, A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 2006, 21, 167–174. [Google Scholar] [CrossRef]
- Valenstein, E.S.; Kakolewski, J.W.; Cox, V.C. Sex differences in taste preference for glucose and saccharin solutions. Science 1967, 156, 942–943. [Google Scholar] [CrossRef]
- Iizuka, K.; Yanagi, K.; Deguchi, K.; Ushiroda, C.; Yamamoto-Wada, R.; Kobae, K.; Yamada, Y.; Naruse, H. Sex and Age Differences in the Effects of Food Frequency on Metabolic Parameters in Japanese Adults. Nutrients 2024, 16, 2931. [Google Scholar] [CrossRef]
- Westenhoefer, J. Age and gender dependent profile of food choice. Forum Nutr. 2005, 57, 44–51. [Google Scholar] [CrossRef]
- Grzymisławska, M.; Puch, E.A.; Zawada, A.; Grzymisławski, M. Do nutritional behaviors depend on biological sex and cultural gender? Adv. Clin. Exp. Med. 2020, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K. Sex differences in brain responses to food stimuli: A meta-analysis on neuroimaging studies. Obes. Rev. 2018, 19, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Uehara, F.; Yamaga, Y.; Yoshimura, S.; Okawa, J.; Tanimura, M.; Ono, T. Reliability of a novel wearable device to measure chewing frequency. J. Prosthodont. Res. 2021, 65, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Hori, K.; Yoshimura, S.; Uehara, F.; Sato, N.; Hasegawa, Y.; Akazawa, K.; Ono, T. Masticatory Behavior Change with a Wearable Chewing Counter: A Randomized Controlled Trial. J. Dent. Res. 2023, 102, 21–27. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Fukushima, A.; Hagiwara, H.; Fujioka, H.; Kimura, F.; Akema, T.; Funabashi, T. Sex differences in feeding behavior in rats: The relationship with neuronal activation in the hypothalamus. Front. Neurosci. 2015, 9, 88. [Google Scholar] [CrossRef]
- Funabashi, T.; Hagiwara, H.; Mogi, K.; Mitsushima, D.; Shinohara, K.; Kimura, F. Sex differences in the responses of orexin neurons in the lateral hypothalamic area and feeding behavior to fasting. Neurosci. Lett. 2009, 463, 31–34. [Google Scholar] [CrossRef]
- Kakolewski, J.W.; Cox, V.C.; Valenstein, E.S. Sex differences in body-weight change following gonadectomy of rats. Psychol. Rep. 1968, 22, 547–554. [Google Scholar] [CrossRef]
- Czaja, J.A. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol. Behav. 1984, 33, 553–558. [Google Scholar] [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas 2012, 71, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Report of the 2009 National Health and Nutrition Survey. Available online: https://www.mhlw.go.jp/bunya/kenkou/eiyou/h21-houkoku.html (accessed on 22 January 2025).
- Tamura, K.; Shiga, H. Gender differences in masticatory movement path and rhythm in dentate adults. J. Prosthodont. Res. 2014, 58, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Paphangkorakit, J.; Kanpittaya, K.; Pawanja, N.; Pitiphat, W. Effect of chewing rate on meal intake. Eur. J. Oral. Sci. 2019, 127, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, T.A.; Kaplan, J.M.; Tomassini, A.; Stellar, E. Bite size, ingestion rate, and meal size in lean and obese women. Appetite 1993, 21, 131–145. [Google Scholar] [CrossRef]
- Maruyama, K.; Sato, S.; Ohira, T.; Maeda, K.; Noda, H.; Kubota, Y.; Nishimura, S.; Kitamura, A.; Kiyama, M.; Okada, T.; et al. The joint impact on being overweight of self reported behaviors of eating quickly and eating until full: Cross sectional survey. BMJ 2008, 337, a2002. [Google Scholar] [CrossRef]
- Leong, S.L.; Madden, C.; Gray, A.; Waters, D.; Horwath, C. Faster self-reported speed of eating is related to higher body mass index in a nationwide survey of middle-aged women. J. Am. Diet. Assoc. 2011, 111, 1192–1197. [Google Scholar] [CrossRef]

| Total (n = 33) | Male (n = 15) | Female (n = 18) | p | |
|---|---|---|---|---|
| Age (years) | 37.2 (11.1) | 37.1 (11.3) | 37.3 (11.2) | 0.95 |
| BMI (kg/m2) | 23.0 (3.2) | 24.1 (3.1) | 22.0 (3.0) | 0.07 |
| Handgrip strength (kg) | 33.5 (8.5) | 41.0 (5.2) | 27.2 (5.0) | <0.001 |
| Five-times sit-to-stand test (s) | 7.0 (1.1) | 6.9 (1.2) | 7.0 (1.0) | 0.83 |
| Meal duration (s) | 76.3 (24.8) | 63.1 (20.7) | 87.4 (22.8) | 0.003 |
| Number of chews | 94.8 (35.2) | 80.3 (28.7) | 107.0 (36.1) | 0.02 |
| Chewing Tempo (bpm) | 81.6 (10.8) | 83.6 (9.3) | 79.9 (11.9) | 0.32 |
| Number of bites | 3.4 (2.4) | 2.1 (1.1) | 4.5 (2.6) | 0.001 |
| Total energy (kcal) | 1504.0 (427.4) | 1626.7 (508.3) | 1401.7 (342.2) | 0.14 |
| Protein intake (g) | 54.1 (17.8) | 55.8 (15.9) | 52.7 (19.9) | 0.63 |
| Fat intake (g) | 47.1 (15.22) | 47.5 (16.1) | 46.7 (15.4) | 0.88 |
| Carbohydrate intake (g) | 194.8 (63.0) | 215.2 (81.9) | 177.8 (38.7) | 0.095 |
| Dietary fiber intake (g) | 8.3 (3.2) | 8.5 (3.3) | 8.1 (3.3) | 0.76 |
| Dependent Value | Meal Duration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
| Independent Value | β [95% CI] | p | β [95% CI] | p | β [95% CI] | p | β [95% CI] | p | β [95% CI] | p |
| Number of chews | 0.6 [0.4, 0.7] | <0.001 | ||||||||
| Eating tempo (bpm) | −0.002 [−0.8, 0.8] | 0.99 | ||||||||
| Number of bites | 5.8 [2.5, 9.2] | 0.001 | ||||||||
| BMI (kg/m2) | −0.9 [−3.5, 1.8] | 0.52 | ||||||||
| Five-times sit-to-stand test (s) | 3.4 [−4.0, 10.8] | 0.36 | ||||||||
| Sex (M: 1, F: 0) | −9.3 [−18.5, −0.1] | 0.047 | −24.3 [−40.4, −8.2] | 0.004 | −10.1 [−25.6, 5.5] | 0.2 | −22.5 [−39.2, −5.9] | 0.01 | −24.0 [−39.6, −8.3] | 0.004 |
| Dependent Value | Meal Duration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
| Independent Value | β [95% CI] | p | β [95% CI] | p | β [95% CI] | p | β [95% CI] | p | β [95% CI] | p |
| Energy (kcal) | 0.01 [−0.007, 0.03] | 0.21 | ||||||||
| Protein (g) | 0.4 [−0.05, 0.8] | 0.08 | ||||||||
| Fat (g) | 0.4 [−0.08, 0.9] | 0.1 | ||||||||
| Carbo-hydrate (g) | 0.06 [−0.07, 0.2] | 0.36 | ||||||||
| Dietary fiber (g) | 1.4 [−1.0, 3.9] | 0.24 | ||||||||
| Sex (M: 1, F: 0) | 26.9 [10.9, 42.9] | 0.002 | 25.4 [10.3, 40.6] | 0.002 | 24.6 [9.5, 39.8] | 0 | 26.4 [10.1, 42.8] | 0.002 | 24.8 [9.3, 40.3] | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoshima, M.; Deguchi, K.; Yamamoto-Wada, R.; Ushiroda, C.; Hiraiwa, E.; Yokoi, M.; Ono, C.; Yoshida, M.; Iizuka, K. Greater Numbers of Chews and Bites and Slow External Rhythmic Stimulation Prolong Meal Duration in Healthy Subjects. Nutrients 2025, 17, 962. https://doi.org/10.3390/nu17060962
Aoshima M, Deguchi K, Yamamoto-Wada R, Ushiroda C, Hiraiwa E, Yokoi M, Ono C, Yoshida M, Iizuka K. Greater Numbers of Chews and Bites and Slow External Rhythmic Stimulation Prolong Meal Duration in Healthy Subjects. Nutrients. 2025; 17(6):962. https://doi.org/10.3390/nu17060962
Chicago/Turabian StyleAoshima, Megumi, Kanako Deguchi, Risako Yamamoto-Wada, Chihiro Ushiroda, Eri Hiraiwa, Miyuki Yokoi, Chisato Ono, Mitsuyoshi Yoshida, and Katsumi Iizuka. 2025. "Greater Numbers of Chews and Bites and Slow External Rhythmic Stimulation Prolong Meal Duration in Healthy Subjects" Nutrients 17, no. 6: 962. https://doi.org/10.3390/nu17060962
APA StyleAoshima, M., Deguchi, K., Yamamoto-Wada, R., Ushiroda, C., Hiraiwa, E., Yokoi, M., Ono, C., Yoshida, M., & Iizuka, K. (2025). Greater Numbers of Chews and Bites and Slow External Rhythmic Stimulation Prolong Meal Duration in Healthy Subjects. Nutrients, 17(6), 962. https://doi.org/10.3390/nu17060962







