Structural Concepts, Definition, Classification, and Macronutrient and Food Composition of Carbohydrate-Restricted Diets for Individuals with Type 2 Diabetes Mellitus: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Literature Search
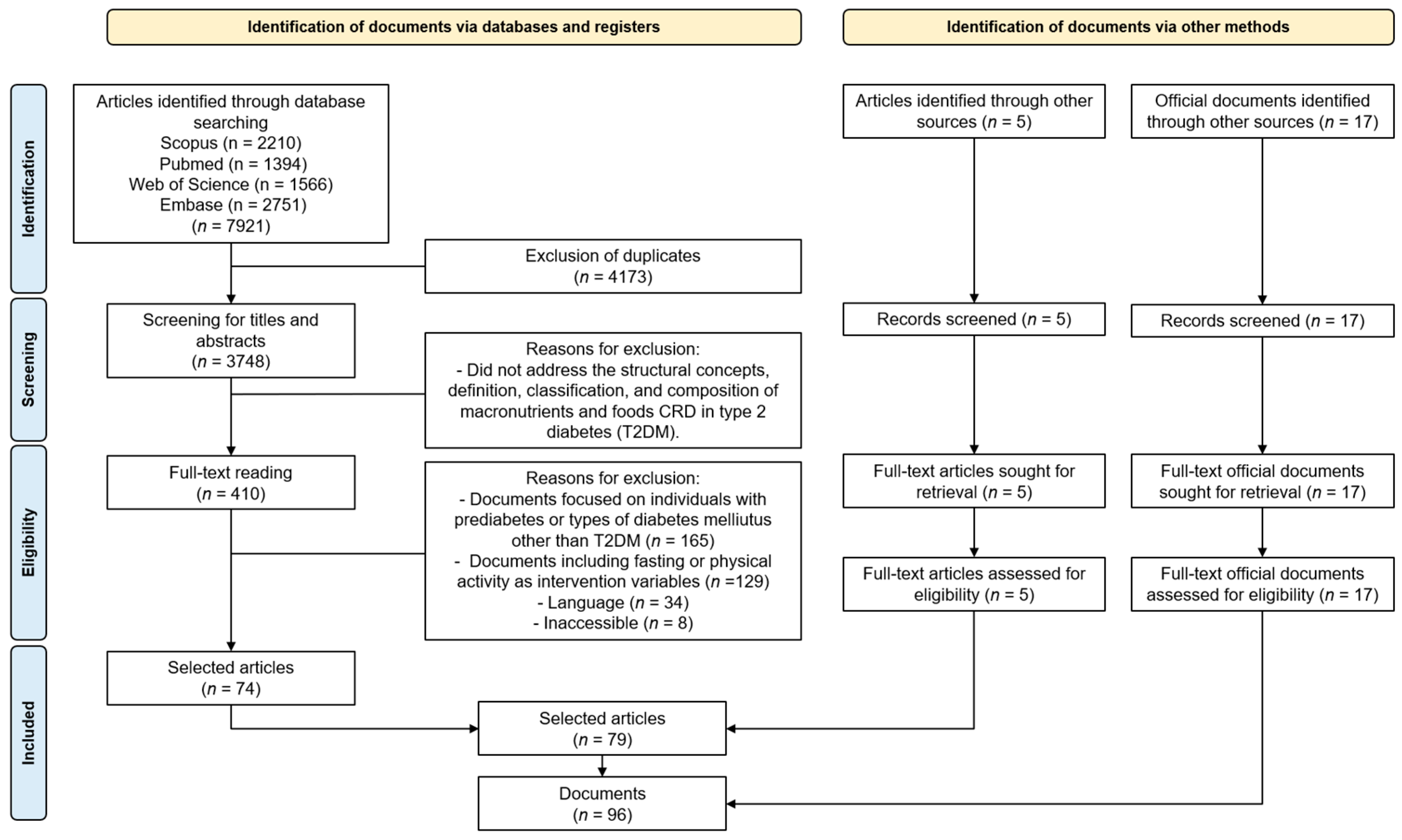
2.4. Study Selection
2.5. Data Extraction and Analysis
3. Results and Discussion
3.1. Structural Concepts Informing Recommendations of CRDs for Individuals with T2DM
3.2. Definition, Classification, and Macronutrient and Food Composition of CRDs
3.3. CRDs in Recommendations for Individuals with T2DM
3.4. Definitions of CRDs and Impact Assessment Studies in Individuals with T2DM
3.5. Food Composition of CRDs for Individuals with T2DM
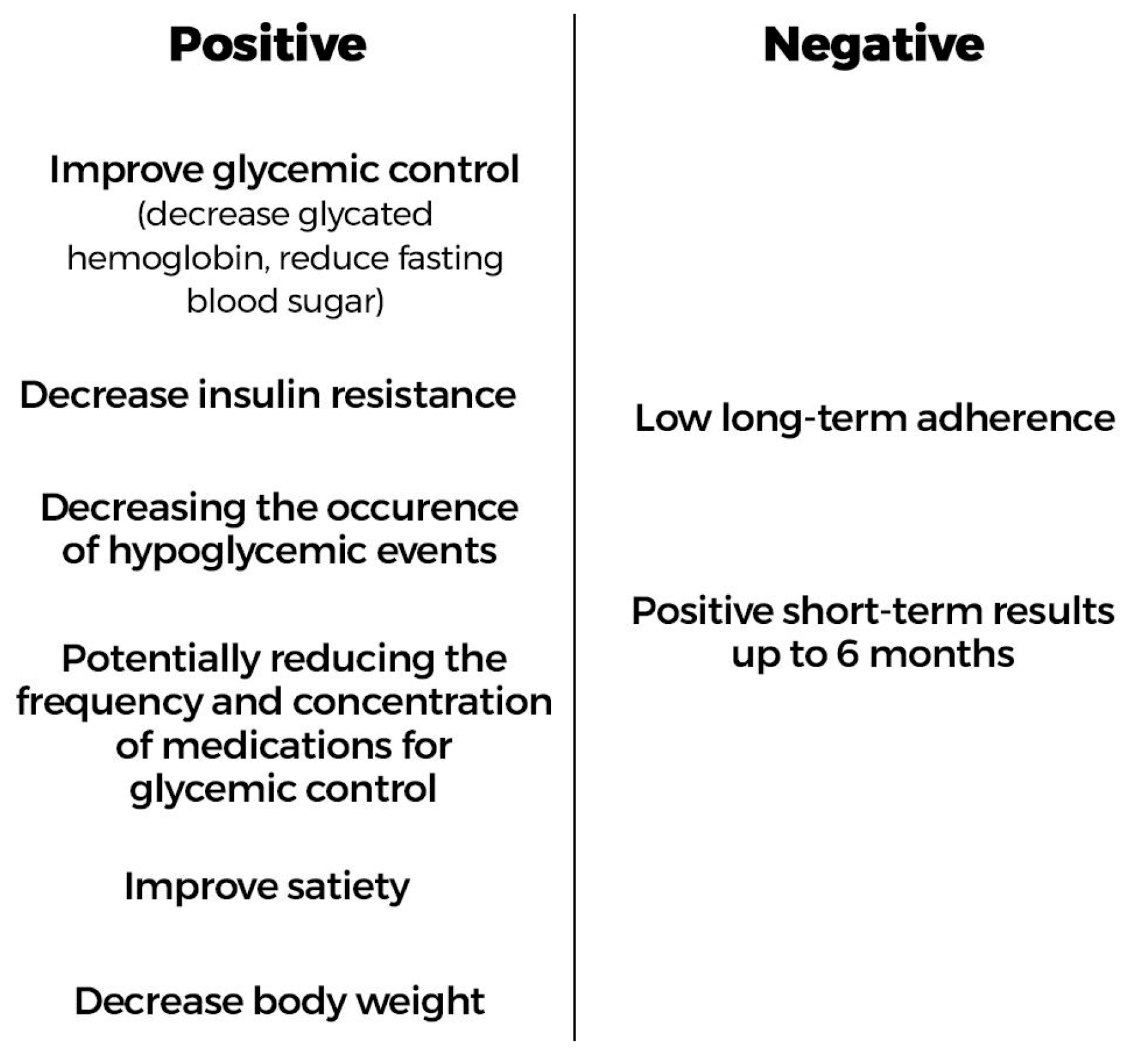
3.6. Evaluation of the Positive and Negative Aspects of CRDs for Individuals with T2DM
3.7. Limitations
3.8. Implications and Recommendations for Future Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S77–S110. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109–119. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- RACGP (Royal Australian College of General Practitioners). General Practice Management of Type 2 Diabetes: 2016-18. East Melbourne, Vic: RACGP. 2016. Available online: https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Diabetes/General-practice-management-of-type-2-diabetes_1.pdf (accessed on 10 January 2024).
- Dyson, P.A.; Twenefour, D.; Breen, C.; Duncan, A.; Elvin, E.; Goff, L.; Hill, A.; Kalsi, P.; Marsland, N.; McArdle, P.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2018, 35, 541–547. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Chan, C.B.; Dworatzek, P.D.; Freeze, C.; Williams, S.L. Nutrition Therapy. Can. J. Diabetes 2018, 42 (Suppl. S1), S64–S79. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Campos, L.F.; Maristela, D.R.B.; Strufaldi, M.; Gomes, D.L.; Guimarães, D.B.; Souto, D.L.; Marques, M.; Sousa, S.S.S.; de Campos, T.F.; et al. Nutritional Therapy in Pre-Diabetes and Type 2 Diabetes Mellitus. Off. Guidel. Braz. Soc. Diabetes Rev. Bras. Diabetes 2023, 17, 77–104. [Google Scholar] [CrossRef]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. Br. Med. J. Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef]
- Meng, Y.; Bai, H.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2017, 131, 124–131. [Google Scholar] [CrossRef]
- Van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: A systematic review including grade assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef]
- Huntriss, R.; Campbell, M.; Bedwell, C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2018, 72, 311–325. [Google Scholar] [CrossRef]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef]
- Korsmo-Haugen, H.K.; Brurberg, K.G.; Mann, J.; Aas, A.M. Carbohydrate quantity in the dietary management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 15–27. [Google Scholar] [CrossRef]
- McArdle, P.D.; Greenfield, S.M.; Rilstone, S.K.; Narendran, P.; Haque, M.S.; Gill, P.S. Carbohydrate restriction for glycaemic control in Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P. Very low carbohydrate ketogenic diets and diabetes. Pract. Diabetes 2020, 37, 121–126. [Google Scholar] [CrossRef]
- Wheatley, S.D.; Deakin, T.A.; Arjomandkhah, N.C.; Hollinrake, P.B.; Reeves, T.E. Low carbohydrate dietary approaches for people with type 2 diabetes—A narrative review. Front. Nutr. 2021, 415, 687658. [Google Scholar] [CrossRef]
- Singh, M.; Hung, E.S.; Cullum, A.; Allen, R.E.; Aggett, P.J.; Dyson, P.; Forouhi, N.G.; Greenwood, D.C.; Pryke, R.; Taylor, R.; et al. Lower carbohydrate diets for adults with type 2 diabetes. Br. J. Nutr. 2021, 127, 1352–1357. [Google Scholar] [CrossRef]
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Shab-Bidar, S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping reviews. JBI Man. Evid. Synth. 2020, 169, 467–473. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Accurso, A.; Bernstein, R.K.; Dahlqvist, A.; Draznin, B.; Feinman, R.D.; Fine, E.J.; Gleed, A.; Jacobs, D.B.; Larson, G.; Lustig, R.H.; et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: Time for a critical appraisal. Nutr. Metab. 2008, 5, 9. [Google Scholar] [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Effect of Dietary Carbohydrate on the Metabolism of Patients with Non-insulin Dependent Diabetes Mellitus. Nutr. Rev. 1986, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Bantle, J.P.; Henry, R.R.; Coulston, A.M.; Griver, K.A.; Raatz, S.K.; Brinkley, L.; Chen, Y.-D.I.; Grundy, S.M.; Huet, B.A.; et al. Effects of Varying Carbohydrate Content of Diet in Patients With Non—Insulin-Dependent Diabetes Mellitus. J. Am. Med. Assoc. 1994, 271, 1421. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Raffray, M.; Roy-Fleming, A.; Blunden, S.; Brazeau, A.S. Ketogenic diet as a normal way of eating in adults with type 1 and type 2 diabetes: A qualitative study. Can. J. Diabetes 2021, 45, 137–143. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C.A. low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Feinman, R.D. Fad Diets in the Treatment of Diabetes. Curr. Diabetes Rep. 2011, 11, 128–135. [Google Scholar] [CrossRef]
- Medeiros, F.L.; Fernandes, A.C.; Padovan, M.; Kraemer, M.V.S.; Bernardo, G.L.; Uggioni, P.L.; Proença, R.P.C. Evolution of dietary recommendations for managing type 2 diabetes mellitus across the ages: A scoping review focusing on carbohydrate-restricted diets. Can. J. Diabetes, 2024; under review. [Google Scholar]
- Sheard, N.F.; Clark, N.G.; Brand-Miller, J.C.; Franz, M.J.; Pi-Sunyer, F.X.; Mayer-Davis, E.; Kulkarni, K.; Geil, P. Dietary Carbohydrate (Amount and Type) in the Prevention and Management of Diabetes: A statement by the American Diabetes Association. Diabetes Care 2004, 27, 2266–2271. [Google Scholar] [CrossRef]
- IOM Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; Available online: https://nap.nationalacademies.org/catalog/10490/dietary-reference-intakes-for-energy-carbohydrate-fiber-fat-fatty-acids-cholesterol-protein-and-amino-acid (accessed on 10 January 2024).
- Kirk, J.K.; Graves, D.E.; Craven, T.E.; Lipkin, E.W.; Austin, M.; Margolis, K.L. Restricted-carbohydrate diets in patients with type 2 diabetes: A meta-analysis. J. Am. Diet. Assoc. 2008, 108, 91–100. [Google Scholar] [CrossRef]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef]
- Halton, T.L.; Liu, S.; Manson, J.E.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [CrossRef]
- Last, A.R.; Wilson, S.A. Low-carbohydrate diets. Am. Fam. Physician 2006, 73, 1942–1948. [Google Scholar]
- Wylie-Rosett, J.; Davis, N.J. Low-carbohydrate diets: An update on current research. Curr. Diabetes Rep. 2009, 9, 396–404. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Ramos Barragán, V.E.; Tamez González, M. Low-carbohydrate diets: A matter of love or hate. Ann. Nutr. Metab. 2011, 58, 320–334. [Google Scholar] [CrossRef]
- Eades, M.R.; Eades, M.D. Protein Power; Bantam Books: New York, NY, USA, 1996. [Google Scholar]
- Atkins, R.C. Dr Atkins’ New Diet Revolution; Avon Books: New York, NY, USA, 2002. [Google Scholar]
- Vernon, M.C.; Eberstein, J.A. Atkins Diabetes Revolution The Groundbreaking Approach to Preventing and Controlling Type 2 Diabetes; William Morrow: New York, NY, USA, 2004. [Google Scholar]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S., Jr.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S., Jr. Macronutrients, food groups, and eating patterns in the management of diabetes: A systematic review of the literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef]
- Yamada, S. Paradigm shifts in nutrition therapy for Type 2 diabetes. Keio J. Med. 2017, 66, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S., Jr. Using a low-carbohydrate diet to treat obesity and type 2 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 255–260. [Google Scholar] [CrossRef]
- Parillo, M.; Rivellese, A.A.; Ciardullo, A.V.; Capaldo, B.; Giacco, A.; Genovese, S.; Riccardi, G. A high-monounsaturated-fat/low-carbohydrate diet improves peripheral insulin sensitivity in non-insulin-dependent diabetic patients. Metabolism 1992, 41, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.J. Fad diets in diabetes. Br. J. Diabetes Vasc. Dis. 2004, 4, 333–337. [Google Scholar] [CrossRef]
- Nielsen, J.V.; Joensson, E. Low-carbohydrate diet in type 2 diabetes: Stable improvement of body weight and glycemic control during 22 months follow-up. Nutr. Metab. 2006, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Worth, J.; Soran, H. Is there a role for low carbohydrate diets in the management of type 2 diabetes? QJM 2007, 100, 659–663. [Google Scholar] [CrossRef]
- Hite, A.H.; Berkowitz, V.G.; Berkowitz, K. Low-carbohydrate diet review: Shifting the paradigm. Nutr. Clin. Pract. 2011, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- He, Y.N.; Feskens, E.; Li, Y.P.; Zhang, J.; Fu, P.; Ma, G.S.; Yang, X.G. Association between high fat-low carbohydrate diet score and newly diagnosed type 2 diabetes in Chinese population. Biomed. Environ. Sci. 2012, 25, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Fernemark, H.; Jaredsson, C.; Bunjaku, B.; Rosenqvist, U.; Nystrom, F.H.; Guldbrand, H. A randomized cross-over trial of the postprandial effects of three different diets in patients with type 2 diabetes. PLoS ONE 2013, 8, e79324. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Haimoto, H.; Sasakabe, T.; Kawamura, T.; Umegaki, H.; Komeda, M.; Wakai, K. Three-graded stratification of carbohydrate restriction by level of baseline hemoglobin A1c for type 2 diabetes patients with a moderate low-carbohydrate diet. Nutr. Metab. 2014, 11, 33. [Google Scholar] [CrossRef]
- Saslow, L.R.; Kim, S.; Daubenmier, J.J.; Moskowitz, J.T.; Phinney, S.D.; Goldman, V.; Murphy, E.J.; Cox, R.M.; Moran, P.; Hecht, F.M. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE 2014, 9, e91027. [Google Scholar] [CrossRef]
- Czyżewska-Majchrzak, Ł.; Grzelak, T.; Kramkowska, M.; Czyżewska, K.; Witmanowski, H. The use of low-carbohydrate diet in type 2 diabetes—Benefits and risks. Ann. Agric. Environ. Med. 2014, 21, 320–326. [Google Scholar] [CrossRef]
- Guldbrand, H.; Lindström, T.; Dizdar, B.; Bunjaku, B.; Östgren, C.J.; Nystrom, F.H.; Bachrach-Lindström, M. Randomization to a low-carbohydrate diet advice improves health related quality of life compared with a low-fat diet at similar weight-loss in Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 106, 221–227. [Google Scholar] [CrossRef]
- Dyson, P. Popular diets: Are they effective for people with type 2 diabetes? Pract. Diabetes 2014, 31, 187–192. [Google Scholar] [CrossRef]
- Hernández Alcantara, G.; Jiménez Cruz, A.; Bacardí Gascón, M. Efecto de las dietas bajas en carbohidratos sobre la pérdida de peso y hemoglobina glucosilada en personas con diabetes tipo 2: Revisión sistemática. Nutr. Hosp. 2015, 32, 1960–1966. [Google Scholar] [CrossRef]
- Dyson, P. Low carbohydrate diets and type 2 diabetes: What is the latest evidence? Diabetes Ther. 2015, 6, 411–424. [Google Scholar] [CrossRef]
- van Wyk, H.J.; Davis, R.E.; Davies, J.S. A critical review of low-carbohydrate diets in people with Type 2 diabetes. Diabet. Med. 2016, 33, 148–157. [Google Scholar] [CrossRef]
- McArdle, P.D.; Mellor, D.; Rilstone, S.; Taplin, J. The role of carbohydrate in diabetes management. Pract. Diabetes 2016, 33, 237–242. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Makita, S.; Hatae, C.; Komiya, K.; Shimizu, T.; Ikeda, F.; Tamura, Y.; Ogihara, T.; Mita, T.; et al. A randomized controlled trial of 130 g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clin. Nutr. 2017, 36, 992–1000. [Google Scholar] [CrossRef]
- Liu, K.; Wang, B.; Zhou, R.; Lang, H.D.; Ran, L.; Wang, J.; Li, L.; Kang, C.; Zhu, X.H.; Zhang, Q.Y.; et al. Effect of combined use of a low-carbohydrate, high-protein diet with omega-3 polyunsaturated fatty acid supplementation on glycemic control in newly diagnosed type 2 diabetes: A randomized, double-blind, parallel-controlled trial. Am. J. Clin. Nutr. 2018, 108, 256–265. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef]
- Shafique, M.; Russell, S.; Murdoch, S.; Bell, J.D.; Guess, N. Dietary intake in people consuming a low-carbohydrate diet in the UK Biobank. J. Hum. Nutr. Diet. 2018, 31, 228–238. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Brinkworth, G.D. Nutritional adequacy of very low- and high-carbohydrate, low saturated fat diets in adults with type 2 diabetes: A secondary analysis of a 2-year randomised controlled trial. Diabetes Res. Clin. Pract. 2020, 170, 108501. [Google Scholar] [CrossRef]
- Kelly, T.; Unwin, D.; Finucane, F. Low-Carbohydrate Diets in the Management of Obesity and Type 2 Diabetes: A Review from Clinicians Using the Approach in Practice. Int. J. Environ. Res. Public Health 2020, 17, 2557. [Google Scholar] [CrossRef]
- Foley, P.J.T.; Gunson, J.T.S.; Baumann, S.L. Two Stories about Diet and Diabetes in Europe. Nurs. Sci. Q. 2020, 33, 85–90. [Google Scholar] [CrossRef]
- Chen, C.Y.; Huang, W.S.; Chen, H.C.; Chang, C.H.; Lee, L.T.; Chen, H.S.; Kang, Y.D.; Chie, W.C.; Jan, C.F.; Wang, W.D.; et al. Effect of a 90 g/day low-carbohydrate diet on glycaemic control, small, dense low-density lipoprotein and carotid intima-media thickness in type 2 diabetic patients: An 18-month randomised controlled trial. PLoS ONE 2020, 15, e0240158. [Google Scholar] [CrossRef]
- Merrill, J.D.; Soliman, D.; Kumar, N.; Lim, S.; Shariff, A.I.; Yancy, W.S., Jr. Low-Carbohydrate and Very-Low-Carbohydrate Diets in Patients With Diabetes. Diabetes Spectr. 2020, 33, 133–142. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Al-Zaid, N.S. Efficacy of Low-Carbohydrate Ketogenic Diet in the Treatment of Type 2 Diabetes. Med. Princ. Pract. 2021, 30, 223–235. [Google Scholar] [CrossRef]
- Skytte, M.J.; Samkani, A.; Astrup, A.; Frystyk, J.; Rehfeld, J.F.; Holst, J.J.; Madsbad, S.; Burling, K.; Fenger, M.; Thomsen, M.N.; et al. Effects of carbohydrate restriction on postprandial glucose metabolism, β-cell function, gut hormone secretion, and satiety in patients with Type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E7–E18. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Neudorf, H.; Mindrum, M.; Mindrum, C.; Durrer, C.; Little, J.P. A Low-Carbohydrate, High-Fat Ketogenic Diet Program Implemented by an Interdisciplinary Primary Care Team Improves Markers of Cardiometabolic Health in Adults with Type 2 Diabetes: A Retrospective Secondary Analysis. Can. J. Diabetes 2022, 46, 302–306.e4. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Shehata, M.; Panesar, A.; Summers, C.; Dale, J. The Low Carb Program for people with type 2 diabetes and pre-diabetes: A mixed methods feasibility study of signposting from general practice. BJGP Open 2022, 6, BJGPO.2021.0137. [Google Scholar] [CrossRef]
- Kumar, N.K.; Merrill, J.D.; Carlson, S.; German, J.; Yancy, W.S., Jr. Adherence to Low-Carbohydrate Diets in Patients with Diabetes: A Narrative Review. Diabetes Metab. Syndr. Obes. 2022, 15, 477–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Gram-Kampmann, E.M.; Hansen, C.D.; Hugger, M.B.; Jensen, J.M.; Brønd, J.C.; Hermann, A.P.; Krag, A.; Olsen, M.H.; Beck-Nielsen, H.; Højlund, K. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes. Metab. 2022, 24, 693–703. [Google Scholar] [CrossRef]
- Chandler, M.J.; Hildebrandt, L.A. Should patients with diabetes follow a low-carb diet? J. Am. Acad. Physician Assist. 2007, 20, 36–41. [Google Scholar] [CrossRef]
- Fields, H.; Ruddy, B.; Wallace, M.R.; Shah, A.; Millstine, D. Are Low-Carbohydrate Diets Safe and Effective? J. Osteopath. Med. 2016, 116, 788–793. [Google Scholar] [CrossRef]
- SBD Diretrizes da Sociedade Brasileira de Diabetes: 2019–2020. Sociedade Brasileira de Diabetes. São Paulo, 2020. Available online: https://profissional.diabetes.org.br/diretrizes-e-posicionamentos/ (accessed on 10 January 2024).
- Diabetes UK. Position Statement: Low-Carb Diets for People with Diabetes, 2021. Available online: https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/public/2021-05/low-carb-diets-for-people-with-diabetes-position-statement-may-2021.pdf (accessed on 10 January 2024).
- Diabetes Canada. Position Statement on Low-Carbohydrate Diets for Adults with Diabetes: A Rapid Review. Can. J. Diabetes 2020, 44, 295–299. [Google Scholar] [CrossRef]
- Diabetes Australia. Low Carbohydrate Eating for People with Diabetes Position Statement. 2018. Available online: https://www.diabetesaustralia.com.au/position-statements (accessed on 10 January 2024).
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2007, 31 (Suppl. S1), S48–S65. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Dyson, P.A.; Kelly, T.; Deakin, T.; Duncan, A.; Frost, G.; Harrison, Z.; Khatri, D.; Kunka, D.; McArdle, P.; Mellor, D.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2011, 28, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Diabetes UK. Position Statement: Low-Carb Diets for People with Diabetes; Diabetes: London, UK, 2017. [Google Scholar]
- Churuangsuk, C.; Lean, M.; Combet, E. Low and reduced carbohydrate diets: Challenges and opportunities for type 2 diabetes management and prevention. Proc. Nutr. Soc. 2020, 79, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Windt, J. Evidence that supports the prescription of low-carbohydrate high-fat diets: A narrative review. Br. J. Sports Med. 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Dening, J.; George, E.S.; Ball, K.; Mohebbi, M.; Shariful Islam, S.M. Randomised controlled trial of a web-based low carbohydrate diet intervention for adults with type 2 diabetes: The T2Diet study protocol. BMJ Open 2022, 12, e054594. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Proudfoot, J.; Creed, S.-A.; Greer, D. The Real Meal Revolution, 2nd ed.; Quivertree Publications: Cape Town, South Africa, 2013; pp. 1–298. [Google Scholar]
- Siverhus, K. Low Carbohydrate and Very Low Carbohydrate Eating Patterns in Adults with Diabetes: A Guide for Health Care Providers. Available online: https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers (accessed on 15 January 2024).
- Newson, L.; Parody, F.H. Investigating the experiences of low-carbohydrate diets for people living with Type 2 Diabetes: A thematic analysis. PLoS ONE 2022, 17, e0273422. [Google Scholar] [CrossRef]
- Webster, C.C.; Murphy, T.E.; Larmuth, K.M.; Noakes, T.D.; Smith, J.A. Diet, diabetes status, and personal experiences of individuals with Type 2 diabetes who self-selected and followed a low carbohydrate high fat diet. Diabetes Metab. Syndr. Obes. 2019, 12, 2567–2582. [Google Scholar] [CrossRef]
- Viljoen, A.; Yu, K.; Witchell, E.; Conklin, A.I. Prescribing diabetes nutrition therapy: A qualitative study of dietitians’ experiences of carbohydrate restriction in Canada. BMJ Nutr. Prev. Health 2023, 6, 83–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Class | Carbohydrate Intake | Protein Intake | Fat Intake | |
|---|---|---|---|---|
| g/day | % Total Energy 1 | % Total Energy 1 | % Total Energy 1 | |
| Very-low-carbohydrate diet [considered a ketogenic diet] | 21 to 70 2 20 to 50 4 | 4 to 14 2 0 to 20 3 ≤10 4 | - | - |
| Very-low-carbohydrate hyperproteic diet | - | 0 to 20 3 | 55 to 65 3 | 25 to 35 3 |
| Low-carbohydrate diet | 150 to 200 1 >50 to <130 4 | 30 to 39.99 2 20 to 40 3 >10 to ≤26 4 | - | - |
| Non-ketogenic low-carbohydrate diet | - | 20 to 40 3 | 20 to 30 3 | 30 to 60 3 |
| Low-carbohydrate hyperproteic diet | - | 20 to 40 3 | 30 to 60 3 | 20 to 30 3 |
| Moderate-carbohydrate diet | 200 to 325 2 130 to 230 3 | 40 to 65 2 26 to 45 3 | - | - |
| High-carbohydrate diet | >325 2 >230 4 | >65% 2 >45 4 | - | - |
| Diet | Carbohydrate Content ** | Macronutrient Composition (Total Energy Basis) | Characteristics | Foods Allowed | Foods to Avoid |
|---|---|---|---|---|---|
| Atkins | Induction phase: 20 g/day; later phases: 80–100 g/day | Carbohydrate: 5% Protein: 27% Total fat: 68% Saturated fat: 26% Alcohol: 0% | Four phases with progressively lower restrictions. The “New Atkins” diet includes a 40 g/day induction phase option for those with <40 lb (18.14 kg) overweight. | Meat, fish, and poultry Eggs Cheese Low-carbohydrate vegetables Butter and oil | Breads and pastaMost fruits and vegetables Milk Alcoholic beverages |
| Ketogenic | <50 g/day | - | Patients can check their urine for ketones or request blood tests to confirm ketotic states (elevated β-hydroxybutyrate). The diet emphasizes a period of “keto-adaptation”, whereby the body switches from glucose to fat as the main source of energy. | - | - |
| Dr Bernstein’s Diabetes Solution | 30 g/day | - | One of the original diets focused on the glycemic index, restricting foods that cause a rapid increase in blood sugar. | - | - |
| Eco-Atkins | 130 g/day | - | Vegan diet containing 31% protein, 43% fat, and 26% carbohydrate. | - | - |
| Low carbohydrate, high fat (LCHF) | <20–100 g/day | - | Focused on fat intake to promote satiety. | - | - |
| Paleo | Varies with food choices | - | Limited to foods eaten by early humans. | Meat, fish, and eggs Vegetables, fruits, and nuts | Minimize whole grains Processed foods Foods with added sugars Dairy Legumes and potatoes |
| Protein Power | 28–40 g/day | Carbohydrate: 16% Protein: 26% Total fat: 54% Saturated fat: 18% Alcohol: 4% | Focused on adequate protein intake and limited carbohydrate intake divided into 4 or 5 meals/snacks a day. | Meat, fish, and poultry Eggs Cheese Low-carbohydrate vegetables Butter, oil, and salad dressings Alcoholic beverages in moderation | Breads and pasta Fruits and vegetables Fats and oils Dairy products |
| South Beach | Phase 1: excludes most carbohydrates Phases 2 and 3: ≤140 g/day | - | Created in response to concerns about the high contents of saturated fat in the Atkins diet. Focused on restricting carbohydrates and saturated fats. Comprises three meals and three snacks a day. | - | - |
| Sugar Busters | 2–3 servings a day | - | Focused on controlling the glycemic index by minimizing the intake of refined sugars, white flour, and starches. | - | - |
| Sonoma | Varies with food choices | - | Three phases focused on controlling serving sizes. Combines Mediterranean and low-carbohydrate diets. Minimizes the intake of saturated fat, starches, and sugar. | - | - |
| Stillman | - | Carbohydrate: 3% Protein: 64% Total fat: 33% Saturated fat: 13% Alcohol: 0% | - | Lean meats and skinless poultry meat Lean fish and seafood Eggs Skim milk, cottage cheese, and other cheeses | Breads and pasta Fruits and vegetables Fats and oils Dairy products Alcoholic beverages |
| Zone | 40% | Carbohydrate: 36% Protein: 34% Total fat: 29% Saturated fat: 9% Alcohol: 1% | Focused on adequate proportions of carbohydrates, proteins (30%), and fats (30%) to aid in satiety and metabolism. The diet advocates small, frequent meals and snacks, totaling seven daily eating occasions. Protein, fat, and carbohydrates in exact proportions. | Foods with a low glycemic index Alcoholic beverages in moderation | Breads and pasta Some fruits Saturated fats |
| Classification | Carbohydrate % (Country) | Carbohydrate Content (Country) |
|---|---|---|
| Usual diet | 45–65% (BRA 2) | Individualized (BRA 2) |
| High-carbohydrate diet | >45% (AUS 6, UK 3) | >230 g (UK 3) |
| Moderate-carbohydrate diet | 26–45% (AUS 6, UK 3) | >225 g (AUS 6) |
| Low-carbohydrate diet | 26–45% (BRA 2, USA 5) <45% (CAN 4) | 130–230 g (UK 3) 50–130 g (CAN 4) <130 g (EUA 7) |
| Very-low-carbohydrate diet | 130–225 g (AUS 6) <50 g (CAN 4) | |
| Very-low-carbohydrate ketogenic diet | <26% (AU 6, UK 3, USA 1) |
| Animal Protein | Dairy | Fats | Nuts and Seeds | Vegetables |
|---|---|---|---|---|
| Eggs | Cottage cheese | Olive oil | Almonds | All leafy greens |
| Meats | Cream | Avocado | Flaxseed | Cruciferous vegetables |
| Poultry | Whole milk cream | Coconut oil | Macadamia | Aboveground vegetables |
| Game | Whole milk Greek yogurt | Macadamia oil | Walnuts | |
| Seafood | Cheeses | Pine nuts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, F.L.; Fernandes, A.C.; Kraemer, M.V.S.; Padovan, M.; Bernardo, G.L.; Uggioni, P.L.; Rafacho, A.; Proença, R.P.C. Structural Concepts, Definition, Classification, and Macronutrient and Food Composition of Carbohydrate-Restricted Diets for Individuals with Type 2 Diabetes Mellitus: A Scoping Review. Nutrients 2025, 17, 1061. https://doi.org/10.3390/nu17061061
Medeiros FL, Fernandes AC, Kraemer MVS, Padovan M, Bernardo GL, Uggioni PL, Rafacho A, Proença RPC. Structural Concepts, Definition, Classification, and Macronutrient and Food Composition of Carbohydrate-Restricted Diets for Individuals with Type 2 Diabetes Mellitus: A Scoping Review. Nutrients. 2025; 17(6):1061. https://doi.org/10.3390/nu17061061
Chicago/Turabian StyleMedeiros, Fharlley Lohann, Ana Carolina Fernandes, Mariana V. S. Kraemer, Marina Padovan, Greyce Luci Bernardo, Paula Lazzarin Uggioni, Alex Rafacho, and Rossana P. C. Proença. 2025. "Structural Concepts, Definition, Classification, and Macronutrient and Food Composition of Carbohydrate-Restricted Diets for Individuals with Type 2 Diabetes Mellitus: A Scoping Review" Nutrients 17, no. 6: 1061. https://doi.org/10.3390/nu17061061
APA StyleMedeiros, F. L., Fernandes, A. C., Kraemer, M. V. S., Padovan, M., Bernardo, G. L., Uggioni, P. L., Rafacho, A., & Proença, R. P. C. (2025). Structural Concepts, Definition, Classification, and Macronutrient and Food Composition of Carbohydrate-Restricted Diets for Individuals with Type 2 Diabetes Mellitus: A Scoping Review. Nutrients, 17(6), 1061. https://doi.org/10.3390/nu17061061





