Dehydration and Malnutrition—Similar Yet Different: Data from a Prospective Observational Study in Older Hospitalized Patients
Highlights
- Dehydration and malnutrition are distinct conditions that do not frequently co-occur.
- Geriatric patients with malnutrition are not at higher risk for dehydration, and vice versa.
- A higher BMI and increased creatinine levels were predictive for dehydration.
Abstract
1. Introduction
2. Subjects and Methods
2.1. Respondents
2.2. Comprehensive Geriatric Assessment and Further Trial Measurements
2.3. Definition of Dehydration
2.4. Assessment and Definition of Malnutrition
2.5. Ethical Approval and Informed Consent
2.6. Statistical Methods
3. Results
3.1. Characteristics of Study Participants
3.2. Prevalence of Malnutrition and Dehydration
3.3. Comparison of Normohydrated and Dehydrated Patients
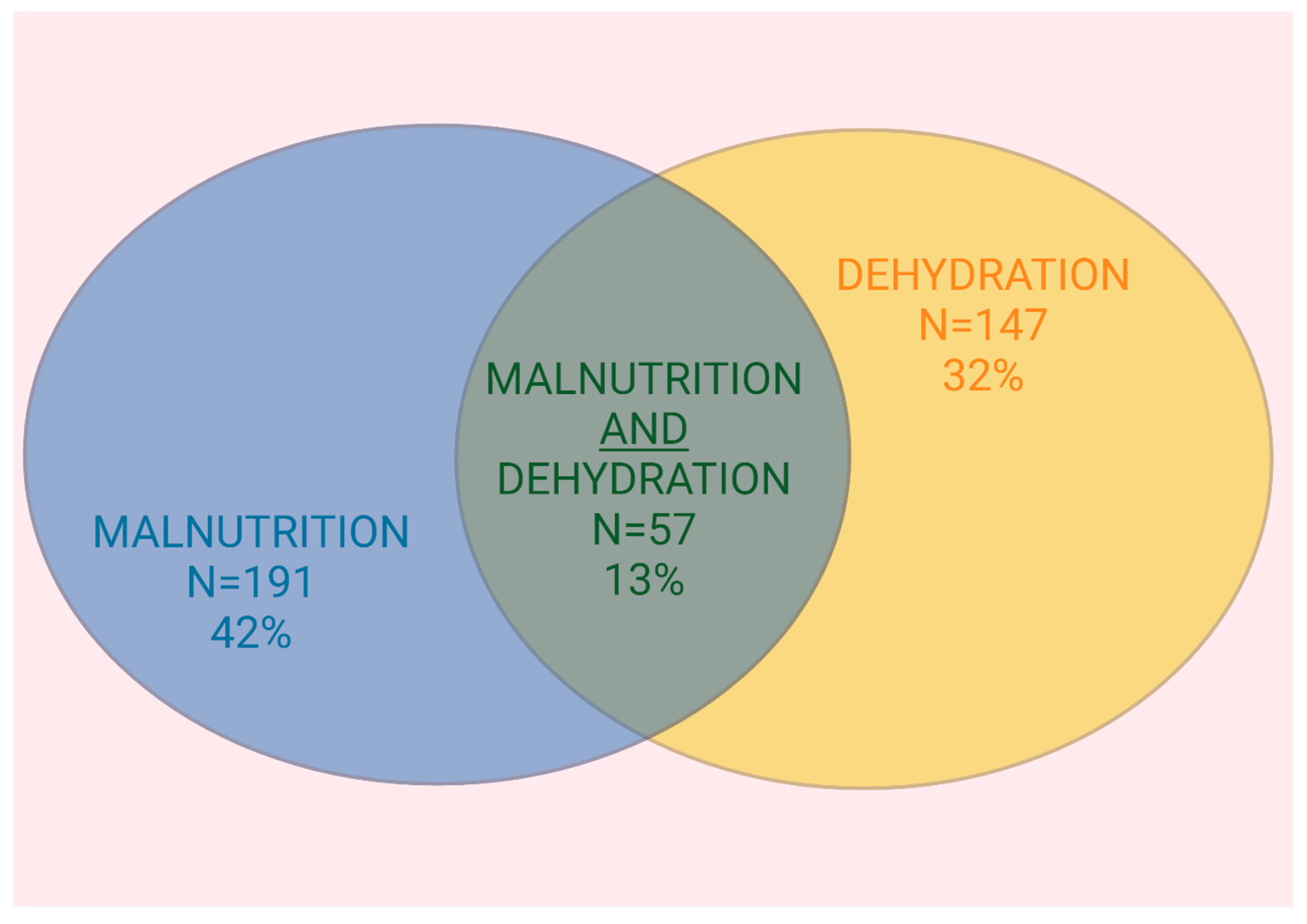
3.4. Overlap Between Malnutrition and Dehydration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef] [PubMed]
- de Souto Barreto, P.; Cesari, M.; Morley, J.E.; Roberts, S.; Landi, F.; Cederholm, T.; Rolland, Y.; Vellas, B.; Fielding, R. Appetite Loss and Anorexia of Aging in Clinical Care: An ICFSR Task Force Report. J. Frailty Aging 2022, 11, 129–134. [Google Scholar] [CrossRef]
- Phillips, P.A.; Bretherton, M.; Johnston, C.I.; Gray, L. Reduced osmotic thirst in healthy elderly men. Am. J. Physiol. 1991, 261, R166–R171. [Google Scholar] [CrossRef] [PubMed]
- Bech, C.B.; Svendsen, J.A.; Knudsen, A.W.; Munk, T.; Beck, A.M. The association between malnutrition and dehydration in older adults admitted to a geriatric unit: An observational study. Clin. Nutr. ESPEN 2023, 57, 598–605. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Marzetti, E. Anorexia of Aging: Metabolic Changes and Biomarker Discovery. Clin. Interv. Aging 2022, 17, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Sieske, L.; Janssen, G.; Babel, N.; Westhoff, T.H.; Wirth, R.; Pourhassan, M. Inflammation, Appetite and Food Intake in Older Hospitalized Patients. Nutrients 2019, 11, 1986. [Google Scholar] [CrossRef]
- Minaglia, C.; Giannotti, C.; Boccardi, V.; Mecocci, P.; Serafini, G.; Odetti, P.; Monacelli, F. Cachexia and advanced dementia. J. Cachexia Sarcopenia Muscle 2019, 10, 263–277. [Google Scholar] [CrossRef]
- Pourhassan, M.; Sieske, L.; Janssen, G.; Babel, N.; Westhoff, T.H.; Wirth, R. The impact of acute changes of inflammation on appetite and food intake among older hospitalised patients. Br. J. Nutr. 2020, 124, 1069–1075. [Google Scholar] [CrossRef]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef]
- Lambert, K.; Carey, S. Dehydration in geriatrics: Consequences and practical guidelines. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 36–41. [Google Scholar] [CrossRef]
- Li, S.; Xiao, X.; Zhang, X. Hydration Status in Older Adults: Current Knowledge and Future Challenges. Nutrients 2023, 15, 2609. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Zamarripa, F.; Shade, R.; Phillips, P.A.; McKinley, M.; Fox, P.T.; Blair-West, J.; Denton, D.A.; Egan, G.F. Effect of aging on regional cerebral blood flow responses associated with osmotic thirst and its satiation by water drinking: A PET study. Proc. Natl. Acad. Sci. USA 2008, 105, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.A.; Rolls, B.J.; Ledingham, J.G.; Forsling, M.L.; Morton, J.J.; Crowe, M.J.; Wollner, L. Reduced thirst after water deprivation in healthy elderly men. N. Engl. J. Med. 1984, 311, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Mehta, R.L.; Burdmann, E.A.; Cerdá, J.; Feehally, J.; Finkelstein, F.; García-García, G.; Godin, M.; Jha, V.; Lameire, N.H.; Levin, N.W.; et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: A multinational cross-sectional study. Lancet 2016, 387, 2017–2025. [Google Scholar] [CrossRef]
- Inouye, S.K.; Viscoli, C.M.; Horwitz, R.I.; Hurst, L.D.; Tinetti, M.E. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann. Intern. Med. 1993, 119, 474–481. [Google Scholar] [CrossRef]
- Marcomini, I.; Pisoni, L.; Mellino, A.; Labaran, R.; Milani, L. Evaluation of Delirium Among Elders in the Emergency Department: A Cross-Sectional Study. Dimens. Crit. Care. Nurs. 2024, 43, 130–135. [Google Scholar] [CrossRef]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Ali, A.; Bunn, D.K.; Jennings, A.; John, W.G.; Kerry, S.; Lindner, G.; Pfortmueller, C.A.; Sjöstrand, F.; et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: Adding value to pathology laboratory reports. BMJ Open 2015, 5, e008846. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Cmaj 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Heidenblut, S.; Zank, S. Entwicklung eines neuen Depressionsscreenings für den Einsatz in der Geriatrie. Z. Gerontol. Geriatr. 2010, 43, 170–176. [Google Scholar] [CrossRef]
- Dorwart, W.V.; Chalmers, L. Comparison of methods for calculating serum osmolality form chemical concentrations, and the prognostic value of such calculations. Clin. Chem. 1975, 21, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report from the Global Clinical Nutrition Community. JPEN J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Skipper, A.; Coltman, A.; Tomesko, J.; Charney, P.; Porcari, J.; Piemonte, T.A.; Handu, D.; Cheng, F.W. Adult Malnutrition (Undernutrition) Screening: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 669–708. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Watson, P.; Neal, K.R.; Ljungqvist, O.; Maughan, R.J.; Sahota, O.; Lobo, D.N. Hydration and outcome in older patients admitted to hospital (The HOOP prospective cohort study). Age Ageing 2015, 44, 943–947. [Google Scholar] [CrossRef]
- Lueg, G.; Wirth, R.; Kwiatkowski, J.; Rösler, A.; Jäger, M.; Gehrke, I.; Volkert, D.; Pourhassan, M. Low Self-Perception of Malnutrition in Older Hospitalized Patients. Clin. Interv. Aging 2020, 15, 2219–2226. [Google Scholar] [CrossRef]
- Charlton, K.E.; Batterham, M.J.; Bowden, S.; Ghosh, A.; Caldwell, K.; Barone, L.; Mason, M.; Potter, J.; Meyer, B.; Milosavljevic, M. A high prevalence of malnutrition in acute geriatric patients predicts adverse clinical outcomes and mortality within 12 months. e-SPEN J. 2013, 8, e120–e125. [Google Scholar] [CrossRef][Green Version]
- Kaiser, M.J.; Bauer, J.M.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef]
- Heybeli, C.; Uzun, O.; Smith, L.; Veronese, N.; Rahmati, M.; Hajek, A.; Soysal, P. Associations between malnutrition and dehydration among older adults: A cross-sectional observational study. Nutr. Clin. Pract. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Yonemitsu, T.; Miyai, N.; Yonemitsu, A. Factors associated with hypertonic dehydration in older Japanese outpatients. J. Gen. Fam. Med. 2025. [Google Scholar] [CrossRef]
- Martin-Taboada, M.; Vila-Bedmar, R.; Medina-Gómez, G. From Obesity to Chronic Kidney Disease: How Can Adipose Tissue Affect Renal Function? Nephron 2021, 145, 609–613. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef]
- Bak, A.; Tsiami, A. Review on mechanisms, importance of homeostasis and fluid imbalances in the elderly. Curr. Res. Nutr. Food Sci. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Theodoridis, X.; Poulia, K.A.; Chourdakis, M. What’s new about hydration in dementia? Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Clavé, P.; Tomsen, N. Prevalence of dehydration in older hospitalized patients with oropharyngeal dysphagia. Geroscience 2024. [Google Scholar] [CrossRef]
- Makhnevich, A.; Perrin, A.; Porreca, K.; Lee, J.Y.; Sison, C.; Gromova, V.; Accardi, K.; David, I.; Burch, L.; Chua, V.; et al. Oropharyngeal Dysphagia in Hospitalized Older Adults with Dementia: A Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2024, 25, 105267. [Google Scholar] [CrossRef]
- Takei, Y. Comparative physiology of body fluid regulation in vertebrates with special reference to thirst regulation. Jpn. J. Physiol. 2000, 50, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Ellershaw, J.E.; Sutcliffe, J.M.; Saunders, C.M. Dehydration and the dying patient. J. Pain Symptom Manag. 1995, 10, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.F.; Bartal, N.; Opstad, J. The Sensation of Thirst in Dying Patients Receiving IV Hydration. J. Palliat. Care 1995, 11, 17–21. [Google Scholar] [CrossRef]
- Marino, F.E. Evolution of the thirst mechanism in Homo: The need and limitations of thirst and hydration. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 298, 111745. [Google Scholar] [CrossRef] [PubMed]
- Serwah, N.; Marino, F.E. The combined effects of hydration and exercise heat stress on choice reaction time. J. Sci. Med. Sport 2006, 9, 157–164. [Google Scholar] [CrossRef]
- Beck, A.M.; Seemer, J.; Knudsen, A.W.; Munk, T. Narrative Review of Low-Intake Dehydration in Older Adults. Nutrients 2021, 13, 3142. [Google Scholar] [CrossRef]
| All (n = 454) | |
|---|---|
| Gender | |
| Female, n (%) | 315 (69) |
| Male, n (%) | 139 (31) |
| Age (y), mean ± SD | 82.21 ± 6.82 |
| Nutritional status | |
| Height (m), mean ± SD | 1.65 ± 0.09 |
| Body weight (kg), mean ± SD | 72.70 ± 17.83 |
| BMI (kg/m2), mean ± SD | 26.50 ± 6.02 |
| Calf circumference (cm), mean ± SD | 33.63 ± 4.82 |
| Previous weight loss, (kg), mean ± SD | 3.95 ± 6.32 |
| Duration of previous weight loss (month), mean ± SD | |
| <3 months | 131 (30) |
| <6 months | 30 (7) |
| GLIM criteria for the diagnosis of malnutrition | |
| Malnourished, n (%) | 191 (42) |
| Not malnourished, n (%) | 263 (58) |
| MNA-SF, median (IQR) | 8 (6–9) |
| Normal nutritional status, n (%) | 20 (5) |
| Risk of malnutrition, n (%) | 230 (50) |
| Malnourished, n (%) | 204 (45) |
| Geriatric assessment | |
| Barthel Index, median (IQR) | 50 (40–60) |
| MoCa, median (IQR) | 18 (15–21) |
| DiA-S, median (IQR) | 3 (1–5) |
| Clinical Frailty Scale, median (IQR) | 5 (5–6) |
| Frailty, n (%) | 357 (79) |
| No frailty, n (%) | 97 (21) |
| Osmolality (mmol/L), mean ± SD | 291.54 ± 10.40 |
| Hydration status | |
| Normohydrated, n (%) | 307 (68) |
| Dehydrated, n (%) | 147 (32) |
| Creatinine (mg/dL), mean ± SD | 1.11 ± 0.54 |
| Normohydrated (n = 307, 68%) | Dehydrated (n = 147, 32%) | p Value | |
|---|---|---|---|
| Gender | |||
| Female, n (%) | 225 (71) | 90 (29) | 0.012 |
| Male, n (%) | 82 (59) | 57 (41) | |
| Age (y), mean ± SD | 81.91 ± 6.90 | 82.93 ± 6.65 | 0.147 |
| Height (m), mean ± SD | 1.65 ± 0.08 | 1.66 ± 0.09 | 0.191 |
| Body weight (kg), mean ± SD | 70.93 ± 16.93 | 76.30 ± 19.04 | 0.004 |
| BMI (kg/m2), mean ± SD | 26.0 ± 5.68 | 27.61 ± 6.51 | 0.010 |
| Calf circumference (cm), mean ± SD | 33.50 ± 4.72 | 33.92 ± 4.91 | 0.397 |
| Previous weight loss, (kg), mean ± SD | 3.87 ± 6.30 | 4.03 ± 6.52 | 0.772 |
| Duration of previous weight loss (month), mean ± SD | |||
| <3 months | 92 (30) | 39 (27) | 0.533 |
| <6 months | 17 (5) | 13 (9) | |
| GLIM criteria for the diagnosis of malnutrition | |||
| Malnourished, n (%) | 134 (44) | 57 (39) | 0.361 |
| Not malnourished, n (%) | 173 (56) | 90 (61) | |
| Geriatric assessment | |||
| MNA-SF, median (IQR) | 8 (6–9) | 8 (6–9) | 0.991 |
| Normal nutritional status, n (%) | 12 (4) | 8 (6) | 0.756 |
| Risk of malnutrition, n (%) | 156 (51) | 74 (50) | |
| Malnourished, n (%) | 139 (45) | 65 (44) | |
| Barthel Index, median (IQR) | 50 (40–60) | 50 (40–60) | 0.357 |
| MoCa, median (IQR) | 18 (15–21) | 18 (15–21) | 0.371 |
| DiA-S, median (IQR) | 3 (1–5) | 3 (1–5) | 0.270 |
| Clinical Frailty Scale, median (IQR) | 5 (5–6) | 5 (5–6) | 0.093 |
| Frailty, n (%) | 240 (78) | 117 (80) | 0.807 |
| No frailty, n (%) | 67 (22) | 30 (20) | |
| Osmolality (mOsm/kg), mean ± SD | 286.44 ± 8.05 | 302.01 ± 6.00 | <0.001 |
| Creatinine (mg/dL), mean ± SD | 0.92 ± 0.41 | 1.41 ± 0.64 | <0.001 |
| Dehydration (Yes/No) | ||||||
|---|---|---|---|---|---|---|
| 95% CI for Exp(B) | ||||||
| B | SE | Exp(B) | Lower | Upper | p Value | |
| Malnutrition (yes/no) | 0.040 | 0.330 | 1.041 | 0.545 | 1.989 | 0.903 |
| Age (year) | 0.008 | 0.019 | 1.008 | 0.971 | 1.047 | 0.661 |
| Gender (female/male) | −0.063 | 0.273 | 0.939 | 0.550 | 1.603 | 0.816 |
| BMI (kg) | 0.072 | 0.031 | 1.075 | 1.011 | 1.142 | 0.020 |
| Previous weight loss (kg) | 0.024 | 0.024 | 1.024 | 0.976 | 1.073 | 0.331 |
| Calf circumference (cm) | −0.052 | 0.037 | 0.949 | 0.883 | 1.020 | 0.158 |
| MoCA score | −0.018 | 0.026 | 0.982 | 0.933 | 1.034 | 0.489 |
| DiA-S score | 0.009 | 0.051 | 1.009 | 0.914 | 1.114 | 0.857 |
| CSF (points) | 0.078 | 0.102 | 1.081 | 0.886 | 1.319 | 0.444 |
| Barthel Index | 0.001 | 0.008 | 1.001 | 0.985 | 1.017 | 0.892 |
| Creatinine (mg/dL) | 2.185 | 0.336 | 8.889 | 4.601 | 17.174 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuendorff, N.R.; Wirth, R.; Stoev, K.; Schnepper, M.; Levermann, I.; Wang, B.; Giehl, C.; Trampisch, U.S.; Funk, L.; Pourhassan, M. Dehydration and Malnutrition—Similar Yet Different: Data from a Prospective Observational Study in Older Hospitalized Patients. Nutrients 2025, 17, 1004. https://doi.org/10.3390/nu17061004
Neuendorff NR, Wirth R, Stoev K, Schnepper M, Levermann I, Wang B, Giehl C, Trampisch US, Funk L, Pourhassan M. Dehydration and Malnutrition—Similar Yet Different: Data from a Prospective Observational Study in Older Hospitalized Patients. Nutrients. 2025; 17(6):1004. https://doi.org/10.3390/nu17061004
Chicago/Turabian StyleNeuendorff, Nina Rosa, Rainer Wirth, Kiril Stoev, Maria Schnepper, Isabel Levermann, Baigang Wang, Chantal Giehl, Ulrike Sonja Trampisch, Lukas Funk, and Maryam Pourhassan. 2025. "Dehydration and Malnutrition—Similar Yet Different: Data from a Prospective Observational Study in Older Hospitalized Patients" Nutrients 17, no. 6: 1004. https://doi.org/10.3390/nu17061004
APA StyleNeuendorff, N. R., Wirth, R., Stoev, K., Schnepper, M., Levermann, I., Wang, B., Giehl, C., Trampisch, U. S., Funk, L., & Pourhassan, M. (2025). Dehydration and Malnutrition—Similar Yet Different: Data from a Prospective Observational Study in Older Hospitalized Patients. Nutrients, 17(6), 1004. https://doi.org/10.3390/nu17061004







