Effect of Combined Vitamin C and Thiamine Therapy on Myocardial and Inflammatory Markers in Cardiac Surgery: A Randomized Controlled Clinical Trial
Highlights
- Combined vitamin C and thiamine (B1) therapy significantly reduced myocardial injury biomarkers (CK-MB, Troponin-I, LDH) compared to vitamin C alone.
- Interleukin-6 (IL-6) levels were significantly lower in the immediate period postoperatively, suggesting the occurrence of early inflammatory modulation after cardiopulmonary bypass (CPB).
- A novel four-time perioperative administration protocol (induction, post-CPB, 12 h, 24 h) optimized the protective effects of antioxidant therapy in cardiac surgery.
- These findings suggest that vitamin C and thiamine supplementation could be an effective strategy to reduce oxidative stress and myocardial injury in patients undergoing cardiac surgery.
- This novel perioperative dosing strategy could be incorporated into perioperative cardiac care protocols to enhance cardioprotective outcomes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Population
2.3.1. Inclusion Criteria
- Scheduled for elective, non-emergent open cardiac surgery;
- Aged 18 years or older;
- Left ventricular ejection fraction (LVEF) > 35%;
- No known coagulopathy before surgery.
2.3.2. Exclusion Criteria
- Administration of vitamin C or thiamine within 7 days before surgery;
- Known allergies to vitamin C or thiamine;
- Indications for thiamine administration (such as chronic alcohol use);
- Participation in multiple simultaneous research studies;
- Autoimmune diseases or ongoing immunosuppressive therapy;
- History of renal calculi and risk of iron overload disorders (e.g., thalassemia and hemochromatosis);
- Preoperative creatinine clearance < 40 mL/min or serum creatinine > 1.8 mg/dL;
- Known bleeding disorders or current anticoagulant therapy;
- Active infection, malignancy, or tumor;
- History of atrial fibrillation;
- Pregnancy;
- Mechanical ventilation or vasopressor/inotropic use on the day of surgery.
2.4. Sample Size
2.5. Randomization and Blinding
2.6. Intervention and Control Groups
- Intervention group—vitamin C combined with vitamin B1 group (Group BC)—received 1000 mg vitamin C and 100 mg thiamine dissolved in 50 mL normal saline, administered intravenously over 30 min.
- Control group—vitamin C alone group (Group C)—received 1000 mg vitamin C dissolved in 50 mL normal saline with an equivalent fluid volume administered intravenously over 30 min.
2.7. Informed Consent and Ethical Approval
2.8. Anesthesia and Surgical Protocols
2.9. Intraoperative Monitoring
2.10. Outcomes
2.10.1. Primary Outcome
2.10.2. Secondary Outcomes
- Hemodynamic parameters: To evaluate systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) from pre-induction through the 24 h postoperative period between the group with combined vitamin C and B1 and the group with vitamin C alone.
- Left ventricular function: To assess the percentage change in LVEF from the preoperative baseline to 24 h postoperatively, we compared the combined vitamin C and B1 group with the group with vitamin C alone.
2.11. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Inflammatory Biomarkers (Table 2)
- CRP: Levels were similar between the groups at all time points, including 24 h postoperatively.
- IL-6: Group BC showed significantly lower levels immediately after surgery (median 63.1 vs. 114.4 pg/mL, p = 0.003), but no difference was observed at 24 h (p = 0.394) (Figure 2).
- WBC count was slightly lower in Group BC at 24 h postoperatively, but the difference was not statistically significant (mean 10,351.2 vs. 11,938.4 per microliter, p = 0.055).
| Preoperative | Immediate Postoperative | 24 h Postoperative | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Laboratory Results | Group C | Group BC | p-Value | Group C | Group BC | p-Value | Group C | Group BC | p-Value |
| CK-MB (IU/L), median (IQR) | 1 (1, 1.2) | 1 (1, 1.2) | 0.928 | 22.1 (13.2, 27.6) | 14.8 (9.3, 18.6) | 0.008 | 12.8 (9.1,18.4) | 7.9 (4.5, 15.6) | 0.048 |
| Troponin-I (ng/mL), median (IQR) | 5.9 (3.8, 12.8) | 4.4 (2.7, 12.3) | 0.342 | 1606.8 (983.7, 2568.9) | 618 (388.6, 1312.9) | 0.005 | 3603.7 (2010.9, 6108) | 1589.5 (683.5, 2865.9) | 0.005 |
| LDH (U/L), median (IQR) | 184 (161, 202.5) | 169 (151, 200) | 0.39 | 332 (295.5, 361.5) | 257 (230, 294) | <0.001 | 405 (335, 471) | 330 (273, 384) | 0.005 |
| WBC (per microliter), median (IQR) | 7360 (5960, 9310) | 6580 (5970, 7750) | 0.234 | 14,958.7 (6481.9) | 13,256.7 (4841.4) | 0.237 | 11,938.4 (3872.7) | 10,351.2 (2520.4) | 0.055 |
| CRP (mg/L), median (IQR) | 1 (1, 3.1) | 1 (1, 2.2) | 0.833 | 1.2 (1, 2.4) | 1 (1, 2.1) | 0.45 | 125.3 (36.7) | 127.8 (47.6) | 0.814 |
| IL-6 (pg/mL), median (IQR) | 3.7 (2.7, 6) | 3.6 (1.8, 4.4) | 0.245 | 114.4 (78.7, 177.4) | 63.1 (47.2, 104) | 0.003 | 99 (79.9, 125.6) | 102.5 (85.1, 175.8) | 0.394 |
| Lactate (mmol/L), median (IQR) | 1.1 (0.8, 1.4) | 1 (0.9, 1.3) | 0.989 | 2.8 (2, 3.9) | 2.9 (2, 4) | 0.078 | 2 (1.2, 2.5) | 1.7 (1.4, 2) | 0.431 |
| Creatinine (mg/dL), mean (SD) | 1 (0.2) | 1 (0.3) | 0.492 | 1 (0.2) | 0.9 (0.2) | 0.637 | 0.8 (0.7, 1.1) | 0.9 (0.7, 1.1) | 0.909 |
| LVEF (%), mean (SD) | 63.4 (10.9) | 62.1 (11.3) | 0.631 | 65.5 (13.3) | 62.5 (11.9) | 0.342 | 65.5 (11.2) | 64.5 (9.8) | 0.691 |
3.3. Cardiac Biomarkers (Table 2)
- CK-MB: Group BC demonstrated significantly lower levels both immediately (median 14.8 vs. 22.1 IU/L, p = 0.008) and at 24 h postoperatively (median 7.9 vs. 12.8 IU/L, p = 0.048).
- Troponin-I: The levels were significantly reduced in Group BC immediately (median 618 vs. 1606.8 ng/mL, p = 0.005) and at 24 h after surgery (median 1589.5 vs. 3603.7 ng/mL, p = 0.005).
- LDH: Group BC showed significantly lower levels of LDH both immediately (median 257 vs. 332 U/L, p < 0.001) and 24 h after surgery (median 330 vs. 405 U/L, p = 0.005).
3.4. Lactate Levels (Table 2)
3.5. Hemodynamic Parameters (Figure 2)
3.6. Left Ventricular Ejection Fraction (LVEF)
3.7. Intraoperative Outcomes (Table 3)
- CPB time: Group BC had a significantly shorter mean CPB duration (107.8 vs. 128.6 min, p = 0.009).
- Arrhythmias: The incidence of arrhythmias during CPB was significantly lower in Group BC (0 vs. 16.1%, p = 0.022).
| Intraoperative Variables | Group C (n = 31) | Group BC (n = 33) | p-Value |
|---|---|---|---|
| Heart disease, n (%) | 0.56 | ||
| - Coronary artery disease | 13 (41.9) | 19 (57.6) | |
| - Mitral stenosis | 2 (6.5) | 1 (3) | |
| - Mitral regurgitation | 8 (25.8) | 7 (21.2) | |
| - Aortic stenosis | 6 (19.4) | 6 (18.2) | |
| - Aortic regurgitation | 2 (6.5) | 0 (0) | |
| Type of valvular surgery | 0.854 | ||
| - Valvular repair | 6 (33.3) | 6 (42.9) | |
| - Valvular replacement | 12 (66.7) | 8 (57.1) | |
| Cardiopulmonary bypass time (min), mean (SD) | 128.6 (32.2) | 107.8 (29.9) | 0.009 |
| Aortic cross clamp time (min), mean (SD) | 87.1 (31) | 74.3 (26.4) | 0.08 |
| Arrhythmia during CPB | 5 (16.1) | 0 (0) | 0.022 |
| Anti-arrhythmic agent requirement | 1 (3.2) | 0 (0) | 0.484 |
| Electrical defibrillation | 3 (9.7) | 0 (0) | 0.108 |
| Intraoperative inotropes, n (%) | 31 (100) | 32 (97) | 1 |
| - Epinephrine, n (%) | 11 (35.5) | 10 (31.2) | 0.929 |
| - maximum dose (mcg/kg/min), median (IQR) | 0.2 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.178 |
| duration (h), mean (SD) | 1.4 (0.7) | 1.5 (0.9) | 0.73 |
| - Norepinephrine, n (%) | 17 (54.8) | 18 (56.2) | 1 |
| - maximum dose (mcg/kg/min), median (IQR) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.385 |
| duration (h), mean (SD) | 1.7 (1) | 1.9 (1.2) | 0.612 |
| - Dobutamine, n (%) | 24 (77.4) | 23 (71.9) | 0.829 |
| - maximum dose (mcg/kg/min), median (IQR) | 5 (5, 7.2) | 5 (5, 6.5) | 0.527 |
| Duration (h), mean (SD) | 1.5 (0.8) | 1.3 (0.8) | 0.211 |
| Vasoactive-inotropic score | |||
| - After separate CPB, median (IQR) | 8 (5, 17.5) | 10 (5, 15) | 0.962 |
| - At the end of surgery, median (IQR) | 5 (0.8, 7) | 5 (0, 6) | 0.645 |
| Intraoperative blood components | |||
| - Packed red cell, n (%) | 25 (80.6) | 26 (78.8) | 1 |
| Packed red cell (units), median (IQR) | 1 (1, 2) | 1 (1, 2) | 0.725 |
| - Fresh frozen plasma, n (%) | 30 (96.8) | 32 (97) | 1 |
| Fresh frozen plasma (units), median (IQR) | 3 (3, 3) | 3 (3, 4.2) | 0.526 |
| - Platelet, n (%) | 22 (71) | 24 (72.7) | 1 |
| Platelet (units), median (IQR) | 6 (6, 6) | 6 (6, 6) | 0.555 |
3.8. Postoperative Outcomes (Table 4)
| Postoperative Clinical Outcomes | Group C (n = 31) | Group BC (n = 33) | p-Value |
|---|---|---|---|
| ICU stays (days), median (IQR) | 3 (3, 4) | 3 (3, 4) | 0.701 |
| Hospital stays (days), median (IQR) | 8 (8, 10.5) | 8 (7, 10) | 0.407 |
| Time to first extubation (h), median (IQR) | 4.5 (1.1, 14.1) | 4 (0, 13.8) | 0.515 |
| Duration of mechanical ventilation (h), median (IQR) | 4.5 (1.1, 14.1) | 4 (0, 13.8) | 0.515 |
| Non-invasive ventilation, n (%) | 31 (100) | 30 (90.9) | 0.239 |
| Postoperative complications, n (%) | 27 (87.1) | 32 (97) | 0.19 |
| Myocardial infarction | 0 | 0 | 0.515 |
| Cardiac arrest with ROSC | 0 | 0 | 0.515 |
| Cardiac arrhythmia | 21 (77.8) | 14 (43.8) | 0.017 |
| Neurological complications | 2 (7.4) | 2 (6.2) | 1 |
| Pulmonary complications | 22 (81.5) | 29 (90.6) | 0.45 |
| Renal complications | 4 (14.8) | 2 (6.2) | 0.398 |
| Postoperative inotropic agents | 21 (67.7) | 25 (75.8) | 0.664 |
| - Epinephrine, n (%) | 5 (23.8) | 6(24) | 1 |
| - maximum dose (mcg/kg/min), median (IQR) | 0 (0, 0) | 0.1 (0, 0.2) | 0.714 |
| duration (h), mean (SD) | 2.6 (2.9) | 7.1 (9.9) | 0.35 |
| - Norepinephrine, n (%) | 15 (71.4) | 15 (60) | 0.617 |
| maximum dose (mcg/kg/min), median (IQR) | 0 (0, 0.1) | 0 (0, 0.1) | 0.676 |
| Duration (h), mean (SD) | 7.5 (7.5) | 10.2 (8.6) | 0.374 |
| - Dobutamine, n (%) | 14 (66.7) | 12 (48) | 0.33 |
| - maximum dose (mcg/kg/min), mean (SD) | 5.4 (2.4) | 5.3 (3) | 0.918 |
| duration (h), mean (SD) | 14.8 (8.5) | 7.7 (8.8) | 0.049 |
| - Nitroglycerine, n (%) | 4 (19) | 3 (12) | 0.686 |
| maximum dose (mg/h), mean (SD) | 7.5 (2.9) | 10.3 (5.7) | 0.421 |
| duration (h), mean (SD) | 13.8 (8) | 12.8 (11.5) | 0.9 |
| Vasoactive-inotropic score, median (IQR) | |||
| - At the end of surgery | 1.4 (0, 5) | 1.7 (0, 6) | 0.592 |
| - 6 h, median (IQR) | 0 (0, 5) | 0 (0, 3.3) | 0.787 |
| - 12 h, median (IQR) | 0 (0, 3) | 0 (0, 3.3) | 0.891 |
| - 24 h, median (IQR) | 0 (0, 0) | 0 (0, 0) | 0.942 |
| Blood transfusion within 24 h, n (%) | 30 (96.8) | 26 (78.8) | 0.054 |
4. Discussion
4.1. Cardiac Biomarkers
4.2. Inflammatory Response
4.3. Lactate
4.4. Postoperative Outcomes
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCOS | Low cardiac output syndrome |
| CPB | Cardiopulmonary bypass |
| CABG | Coronary bypass graft |
| CK-MB | Creatine kinase-MB |
| LDH | Lactate dehydrogenase |
| ICU | Intensive care unit |
| IL-6 | Interleukin -6 |
| PCA | Patient controlled analgesia |
| CRP | C-reactive protein |
| WBC | White blood cells |
| HR | Heart rate |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| MAP | Mean arterial pressure |
| LVEF | Left ventricular ejection fraction |
| TTE | Transthoracic echocardiogram |
| ACEI | Angiotensin-converting enzyme inhibitor |
| ARB | Angiotensin receptor blocker |
| ASA-PS | American Society of Anesthesiologist-Physical Status classification |
| BMI | Body mass index |
| BW | Body weight |
| CVD | Cerebrovascular disease |
| CVP | Central venous pressure |
| ECG | Electrocardiogram |
| IQR | Interquartile range |
| LVH | Left ventricular hypertrophy |
| NYHA | New York Heart Association |
| SD | Standard deviation |
| TIA | Transient ischemic attack |
| ROSC | Return of spontaneous circulation |
| POAF | Postoperative atrial fibrillation |
References
- Schoonen, A.; van Klei, W.A.; van Wolfswinkel, L.; van Loon, K. Definitions of low cardiac output syndrome after cardiac surgery and their effect on the incidence of intraoperative LCOS: A literature review and cohort study. Front. Cardiovasc. Med. 2022, 9, 926957. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Loubon, C.; Fernández-Molina, M.; Carrascal-Hinojal, Y.; Fulquet-Carreras, E. Cardiac surgery-associated acute kidney injury. Ann. Card. Anaesth. 2016, 19, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Durante, A.; Limite, L.R.; Cianflone, D. Postoperative arrhythmias after cardiac surgery: Incidence, risk factors, and therapeutic management. Cardiol. Res. Pract. 2014, 2014, 615987. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granda, M.J.; Barrio, J.M.; Cuerpo, G.; Valerio, M.; Muñoz, P.; Hortal, J.; Pinto, A.G.; Bouza, E. Infectious complications following major heart surgery from the day of the surgery to hospital discharge. BMC Infect. Dis. 2024, 24, 73. [Google Scholar] [CrossRef]
- Nambiar, P.M.; Bhan, A.; Mehta, Y. Prolonged Stay in ICU after Cardiac Surgery: Challenges—A Review. J. Card. Crit. Care TSS 2024, 8, 70–79. [Google Scholar] [CrossRef]
- Kraft, F.; Schmidt, C.; Van Aken, H.; Zarbock, A. Inflammatory response and extracorporeal circulation. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 113–123. [Google Scholar] [CrossRef]
- Ferreira, L.O.; Vasconcelos, V.W.; Lima, J.S.; Vieira Neto, J.R.; da Costa, G.E.; Esteves, J.C.; de Sousa, S.C.; Moura, J.A.; Santos, F.R.S.; Leitão Filho, J.M.; et al. Biochemical Changes in Cardiopulmonary Bypass in Cardiac Surgery: New Insights. J. Pers. Med. 2023, 13, 1506. [Google Scholar] [CrossRef]
- Falter, F.; Salter, R.; Fernandes, J.; Burt, C.; Drummond, K.; Ramalingam, G.; Nashef, S. Predictive role of cardiopulmonary bypass exposure indexed to body surface area on postoperative organ dysfunction: A retrospective cohort study. Interdiscip. CardioVascular Thorac. Surg. 2024, 39, ivae171. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Vílchez, M.J.; Ibáñez, S.; Huertas, J.R.; Palacio, M.A.; Muñoz-Hoyos, A. Oxidative stress is evident in erythrocytes as well as plasma in patients undergoing heart surgery involving cardiopulmonary bypass. Free Radic. Res. 2003, 37, 11–17. [Google Scholar] [CrossRef]
- Landis, R.C.; Brown, J.R.; Fitzgerald, D.; Likosky, D.S.; Shore-Lesserson, L.; Baker, R.A.; Hammon, J.W. Attenuating the Systemic Inflammatory Response to Adult Cardiopulmonary Bypass: A Critical Review of the Evidence Base. J. Extra-Corpor. Technol. 2014, 46, 197–211. [Google Scholar] [CrossRef]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients—Review and Pragmatic Approach. Nutrients 2018, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra-de Man, A.M.E.; Oudemans-van Straaten, H.M.; Elbers, P.W.G. Vitamin C and thiamine in critical illness. BJA Educ. 2019, 19, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Alshafey, M.K.; Elrakhawy, H.M.; Rezk, M.E.; Moustafa, H.M. Role of ascorbic acid in reduction of the incidence of the atrial fibrillation in patients under B-blocker and undergoing coronary artery bypass graft operation in early post-operative period. J. Egypt. Soc. Cardio-Thorac. Surg. 2017, 25, 198–203. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Quang, D.D. Unraveling the antioxidant potential of thiamine: Thermochemical and kinetics studies in aqueous phase using DFT. Vietnam J. Chem. 2019, 57, 485–490. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar] [CrossRef]
- Yamada, Y.; Kusakari, Y.; Akaoka, M.; Watanabe, M.; Tanihata, J.; Nishioka, N.; Bochimoto, H.; Akaike, T.; Tachibana, T.; Minamisawa, S. Thiamine treatment preserves cardiac function against ischemia injury via maintaining mitochondrial size and ATP levels. J. Appl. Physiol. 2021, 130, 26–35. [Google Scholar] [CrossRef]
- Smith, T.J.; Johnson, C.R.; Koshy, R.; Hess, S.Y.; Qureshi, U.A.; Mynak, M.L.; Fischer, P.R. Thiamine deficiency disorders: A clinical perspective. Ann. N. Y. Acad. Sci. 2021, 1498, 9–28. [Google Scholar] [CrossRef]
- Donnino, M.W.; Cocchi, M.N.; Smithline, H.; Carney, E.; Chou, P.P.; Salciccioli, J. Coronary artery bypass graft surgery depletes plasma thiamine levels. Nutrition 2010, 26, 133–136. [Google Scholar] [CrossRef]
- Andersen, L.W.; Liu, X.; Peng, T.J.; Giberson, T.A.; Khabbaz, K.R.; Donnino, M.W. Pyruvate dehydrogenase activity and quantity decreases after coronary artery bypass grafting: A prospective observational study. Shock 2015, 43, 250–254. [Google Scholar] [CrossRef]
- Page, G.L.; Laight, D.; Cummings, M. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 2011, 65, 684–690. [Google Scholar] [CrossRef]
- Manzanares, W.; Hardy, G. Thiamine supplementation in the critically ill. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Alizadeh, M.; Chehri, G.; Jafari-Vayghyan, H.; Foroumandi, E.; Maleki, V.; Ebrahimi, B.; Sadeghpour, A.; Alizadehasl, A.; Tabaee, A.S. The effect of vitamin C supplementation on cardiac enzymes after coronary artery bypass graft: A double-blind Randomized Control Trial. Curr. Nutr. Food Sci. 2020, 16, 833–838. [Google Scholar] [CrossRef]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Chase, M.; Cocchi, M.N.; Sulmonte, C.; Balkema, J.; MacDonald, M.; Montissol, S.; Senthilnathan, V.; et al. Thiamine as an adjunctive therapy in cardiac surgery: A randomized, double-blind, placebo-controlled, phase II trial. Crit. Care 2016, 20, 92. [Google Scholar] [CrossRef]
- Jahangirifard, A.; Salajegheh, S.; Arab, S.; Mirtajani, S.B.; Radmand, G.; Farzanegan, B. Thiamine can decrease lactate and creatinine level after coronary artery Bypass surgery in patients with mild systolic dysfunction. J. Cell. Mol. Anesth. 2019, 3, 136–142. [Google Scholar] [CrossRef]
- Emadi, N.; Nemati, M.H.; Ghorbani, M.; Allahyari, E. The Effect of High-Dose Vitamin C on Biochemical Markers of Myocardial Injury in Coronary Artery Bypass Surgery. Braz. J. Cardiovasc. Surg. 2019, 34, 517–524. [Google Scholar] [CrossRef]
- Shokri-Mashhadi, N.; Aliyari, A.; Hajhashemy, Z.; Saadat, S.; Rouhani, M.H. Is it time to reconsider the administration of thiamine alone or in combination with vitamin C in critically ill patients? A meta-analysis of clinical trial studies. J. Intensive Care 2022, 10, 8. [Google Scholar] [CrossRef]
- Rodrigo, R.; Korantzopoulos, P.; Cereceda, M.; Asenjo, R.; Zamorano, J.; Villalabeitia, E.; Baeza, C.; Aguayo, R.; Castillo, R.; Carrasco, R.; et al. A randomized controlled trial to prevent post-operative atrial fibrillation by antioxidant reinforcement. J. Am. Coll. Cardiol. 2013, 62, 1457–1465. [Google Scholar] [CrossRef]
- Tai, Y.H.; Wu, H.L.; Chu, Y.H.; Huang, C.H.; Ho, S.T.; Lin, T.C.; Lu, C.C. Vitamin C supplementation attenuates oxidative stress and improves erythrocyte deformability in cardiac surgery with cardiopulmonary bypass. Chin. J. Physiol. 2022, 65, 241–249. [Google Scholar] [CrossRef]
- Rozemeijer, S.; Hemilä, H.; van Baaren, M.; de Man, A.M. Vitamin C may reduce troponin and CKMB levels after PCI and CABG: A meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 475. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Alizadehasl, A.; Kyavar, M.; Sadeghi, T.; Moludi, J.; Gholizadeh, F.; Totonchi, Z.; Ghadrdoost, B. Impact of vitamin C supplementation on post-cardiac surgery ICU and hospital length of stay. Anesthesiol. Pain Med. 2015, 5, e25337. [Google Scholar] [CrossRef]
- Polymeropoulos, E.; Bagos, P.; Papadimitriou, M.; Rizos, I.; Patsouris, E.; Τoumpoulis, I. Vitamin C for the Prevention of Postoperative Atrial Fibrillation after Cardiac Surgery: A Meta-Analysis. Adv. Pharm. Bull. 2016, 6, 243–250. [Google Scholar] [CrossRef]
- Eskandari, P.; Pouladi, S.; Anvaripour, A.R. The effects of Vitamin C on Inflammatory Markers and Atrial Fibrillation in Patients Undergoing Coronary Artery Bypass Surgery in Bushehr Heart Center: A Randomized Controlled Clinical Trial. Iran. South Med. J. 2020, 23, 528–540. [Google Scholar] [CrossRef]
- Stef, A.; Bodolea, C.; Bocsan, I.C.; Cainap, S.S.; Achim, A.; Serban, A.; Solomonean, A.G.; Tintiuc, N.; Buzoianu, A.D. The Value of Biomarkers in Major Cardiovascular Surgery Necessitating Cardiopulmonary Bypass. Rev. Cardiovasc. Med. 2024, 25, 355. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Daulasim, A.; Souibri, M.; Nguyen, L.S. Incidence and prognosis associated with troponin elevation after cardiac surgery: A prospective cohort study. BMJ Open 2022, 12, e057375. [Google Scholar] [CrossRef]
- Gulhar, R.; Ashraf, M.A.; Jialal, I. Physiology, Acute Phase Reactants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519570/ (accessed on 5 February 2025).
- Watt, D.G.; Horgan, P.G.; McMillan, D.C. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: A systematic review. Surgery 2015, 157, 362–380. [Google Scholar] [CrossRef]
- Sloop, G.D.; De Mast, Q.; Pop, G.; Weidman, J.J.; St Cyr, J.A. The Role of Blood Viscosity in Infectious Diseases. Cureus 2020, 12, e7090. [Google Scholar] [CrossRef]
- Hsu, P.S.; Chen, J.L.; Sung, S.Y.; Tsai, Y.T.; Lin, C.Y.; Wu, Y.F.; Tsai, C.S. Inflammatory Biomarkers and Blood Physical Property Transformations Following On-Pump Coronary Artery Bypass Graft Surgery. J. Pers. Med. 2023, 13, 1434. [Google Scholar] [CrossRef]
- Andersen, L.W.; Holmberg, M.J.; Doherty, M.; Khabbaz, K.; Lerner, A.; Berg, K.M.; Donnino, M.W. Postoperative Lactate Levels and Hospital Length of Stay After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1454–1460. [Google Scholar] [CrossRef]
- Badreldin, A.M.; Doerr, F.; Elsobky, S.; Brehm, B.R.; Abul-dahab, M.; Lehmann, T.; Bayer, O.; Wahlers, T.; Hekmat, K. Mortality prediction after cardiac surgery: Blood lactate is indispensible. Thorac. Cardiovasc. Surg. 2013, 61, 708–717. [Google Scholar] [CrossRef]
- Fink, M.P. Bench-to-bedside review: Cytopathic hypoxia. Crit. Care 2002, 6, 491–499. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Moroz, G.; Ismoilov, S.; Shmyrev, V.; Efremov, S.; Abubakirov, M.; Batalov, V.; Landoni, G.; Lembo, R.; Bogachev-Prokophiev, A.; et al. Sustained High-dose Thiamine Supplementation in High-risk Cardiac Patients Undergoing Cardiopulmonary Bypass: A Pilot Feasibility Study (The APPLY trial). J. Cardiothorac. Vasc. Anesth. 2020, 34, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Gandhi, H.; Shah, K.; Pandya, H.; Patel, R.; Keshwani, S.; Yadav, N. Hydrocortisone, Vitamin C and thiamine for the treatment of sepsis and septic shock following cardiac surgery. Indian J. Anaesth. 2018, 62, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Charalambous, M.; Anastasiou, T.; Aggeli, K.; Soteriades, E.S. Preoperative and postoperative administration of vitamin C in cardiac surgery patients-settings, dosages, duration, and clinical outcomes: A narrative review. Ann. Med. Surg. 2024, 86, 3591–3607. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yuan, L.; Wang, H.; Li, C.; Cai, J.; Hu, Y.; Ma, C. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int. J. Surg. 2017, 37, 58–64. [Google Scholar] [CrossRef]
- Shi, R.; Li, Z.H.; Chen, D.; Wu, Q.C.; Zhou, X.L.; Tie, H.T. Sole and combined vitamin C supplementation can prevent postoperative atrial fibrillation after cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Clin. Cardiol. 2018, 41, 871–878. [Google Scholar] [CrossRef]
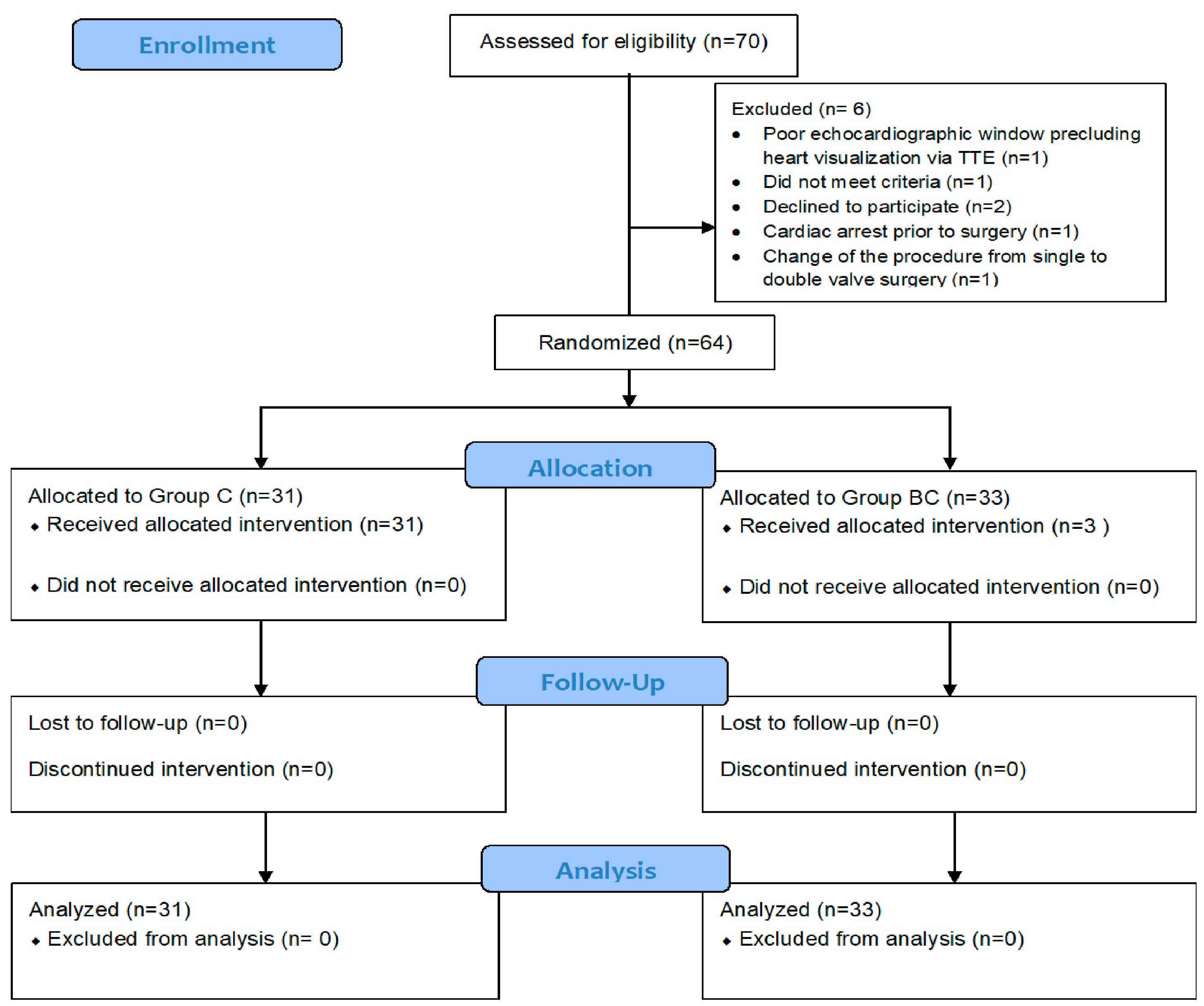

| Patient Characteristics | Group C (n = 31) | Group BC (n = 33) | p-Value |
|---|---|---|---|
| Age (years), median (IQR) | 59 (53, 65.5) | 60 (54, 65) | 0.814 |
| Sex | 1 | ||
| Male, n (%) | 20 (64.5) | 21 (63.6) | |
| Female, n (%) | 11 (35.5) | 12 (36.4) | |
| BW (kg), mean (SD) | 64.4 (13) | 66.6 (12.1) | 0.483 |
| BMI (kg/m2), median (IQR) | 24.6 (22.8, 26.6) | 25.4 (22.9, 27.2) | 0.727 |
| Comorbidity, n (%) | 23 (74.2) | 27 (81.8) | 0.664 |
| Ischemic heart disease | 7 (30.4) | 9 (33.3) | 1 |
| Heart failure | 0 (0) | 1 (3.7) | 1 |
| Hypertension | 16 (69.6) | 14 (51.9) | 0.325 |
| Dyslipidemia | 18 (78.3) | 17 (63) | 0.386 |
| Diabetes | 10 (43.5) | 12 (44.4) | 1 |
| Insulin use | 0 (0) | 1 (3.7) | 1 |
| Chronic lung disease | 2 (8.7) | 1 (3.7) | 0.588 |
| CVD (previous stroke or TIA) | 2 (8.7) | 0 (0) | 0.207 |
| Smoking | 4 (17.4) | 5 (18.5) | 1 |
| Current medications, n (%) | 29 (93.5) | 31 (93.9) | 1 |
| Beta-blocker | 18 (62.1) | 20 (64.5) | 1 |
| Calcium channel blocker | 9 (31) | 11 (35.5) | 0.927 |
| ACEI | 13 (44.8) | 5 (16.1) | 0.032 |
| ARB | 5 (17.2) | 5 (16.1) | 1 |
| Diuretics | 16 (55.2) | 8 (25.8) | 0.04 |
| Digoxin | 1 (3.4) | 0 (0) | 0.483 |
| Nitrate | 11 (37.9) | 15 (48.4) | 0.578 |
| Anticoagulant | 1 (3.4) | 0 (0) | 0.483 |
| Aspirin | 18 (62.1) | 22 (71) | 0.648 |
| Statin | 26 (89.7) | 20 (64.5) | 0.046 |
| ASA-PS class, median (IQR) | 3 (3, 3) | 3 (3, 3) | 0.533 |
| EuroScore-II (%), median (IQR) | 0.9 (0.8, 1.3) | 0.9 (0.8, 1.2) | 0.444 |
| NYHA class, median (IQR) | 2 (2, 2) | 2 (2, 2) | 0.844 |
| LVH by ECG, n (%) | 22 (71) | 13 (39.4) | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saetang, M.; Wasinwong, W.; Oofuvong, M.; Tanasansutthiporn, J.; Rattanapittayaporn, L.; Petsakul, S.; Duangpakdee, P.; Rodneam, P.; Boonthum, P.; Khunakanan, S.; et al. Effect of Combined Vitamin C and Thiamine Therapy on Myocardial and Inflammatory Markers in Cardiac Surgery: A Randomized Controlled Clinical Trial. Nutrients 2025, 17, 1006. https://doi.org/10.3390/nu17061006
Saetang M, Wasinwong W, Oofuvong M, Tanasansutthiporn J, Rattanapittayaporn L, Petsakul S, Duangpakdee P, Rodneam P, Boonthum P, Khunakanan S, et al. Effect of Combined Vitamin C and Thiamine Therapy on Myocardial and Inflammatory Markers in Cardiac Surgery: A Randomized Controlled Clinical Trial. Nutrients. 2025; 17(6):1006. https://doi.org/10.3390/nu17061006
Chicago/Turabian StyleSaetang, Mantana, Wirat Wasinwong, Maliwan Oofuvong, Jutarat Tanasansutthiporn, Laortip Rattanapittayaporn, Sutthasinee Petsakul, Pongsanae Duangpakdee, Puripong Rodneam, Parin Boonthum, Supphamongkhon Khunakanan, and et al. 2025. "Effect of Combined Vitamin C and Thiamine Therapy on Myocardial and Inflammatory Markers in Cardiac Surgery: A Randomized Controlled Clinical Trial" Nutrients 17, no. 6: 1006. https://doi.org/10.3390/nu17061006
APA StyleSaetang, M., Wasinwong, W., Oofuvong, M., Tanasansutthiporn, J., Rattanapittayaporn, L., Petsakul, S., Duangpakdee, P., Rodneam, P., Boonthum, P., Khunakanan, S., Churuangsuk, C., Sriwimol, W., Chantarokon, A., Nuanjun, K., & Yongsata, D. (2025). Effect of Combined Vitamin C and Thiamine Therapy on Myocardial and Inflammatory Markers in Cardiac Surgery: A Randomized Controlled Clinical Trial. Nutrients, 17(6), 1006. https://doi.org/10.3390/nu17061006







