Effect of Salt Reduction Interventions in Lowering Blood Pressure and Salt Intake in Zhejiang Province, China, 2017–2021: A Randomized Controlled Trial
Abstract
1. Background
2. Methods
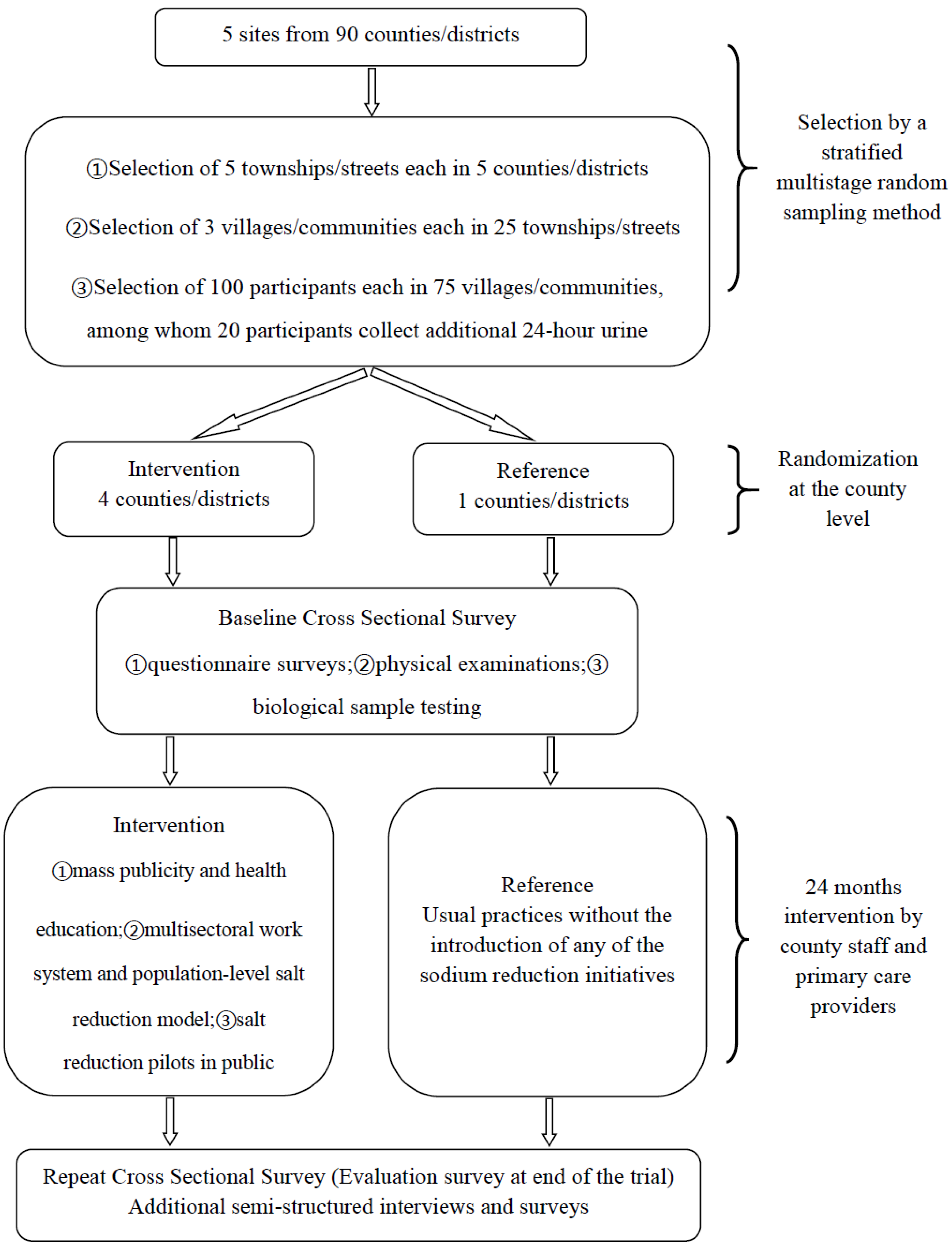
2.1. Study Setting and Overall Design
2.2. Intervention
2.3. Outcomes and Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Changes in SBP, DBP, Sodium, and Potassium Excretion
3.3. Changes in Knowledge, Attitudes, and Behaviors Associated with Sodium Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index; |
| BP | blood pressure; |
| CDC | Center for Disease Control and Prevention; |
| CI | confidence interval; |
| CVD | cardiovascular diseases; |
| DBP | diastolic blood pressure; |
| KABs | knowledge, attitudes, and behaviors; |
| OR | odds ratio; |
| RCT | randomized controlled trial; |
| SBP | systolic blood pressure; |
| SD | standard deviation; |
| SMU | second morning urine; |
| SRHPP | Salt Reduction and Hypertension Prevention Project; |
| SU | spot urine; |
| WHO | World Health Organization; |
| 24-hUKa | 24 h urinary potassium; |
| 24-hUNa | 24 h urinary sodium. |
References
- NCD Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhang, L.; Wang, X.; Hao, G.; Zhang, Z.; Shao, L.; Tian, Y.; Dong, Y.; Zheng, C.; et al. Status of Hypertension in China: Results from the China Hypertension Survey, 2012–2015. Circulation 2018, 137, 2344–2356. [Google Scholar] [CrossRef]
- Trieu, K.; Neal, B.; Hawkes, C.; Dunford, E.; Campbell, N.; Rodriguez-Fernandez, R.; Legetic, B.; McLaren, L.; Barberio, A.; Webster, J. Salt Reduction Initiatives around the World—A Systematic Review of Progress towards the Global Target. PLoS ONE 2015, 10, e0130247. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; He, F.J.; Wang, C.; MacGregor, G.A. Twenty-Four-Hour Urinary Sodium and Potassium Excretion in China: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012923. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fang, L.; Xu, J.; Chen, X.; Bai, Y.; Zhong, J. Association between 24-h urinary sodium and potassium excretion and blood pressure among Chinese adults aged 18–69 years. Sci. Rep. 2021, 11, 3474. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Guo, J.; Chen, X.; Zhang, J.; Lu, F.; Zhong, J. Calibration of 24-h urinary sodium excretion estimates from fasting morning urine in Chinese populations: A comparative analysis of 2 calibration models and a locally developed model. Am. J. Clin. Nutr. 2024, 120, 943–952. [Google Scholar] [CrossRef]
- Du, X.; Fang, L.; Xu, J.; Chen, X.; Zhang, J.; Bai, Y.; Wu, J.; Ma, J.; Yu, M.; Zhong, J. Prevalence, awareness, treatment and control of hypertension and sodium intake in Zhejiang Province, China: A cross-sectional survey in 2017. PLoS ONE 2019, 14, e0226756. [Google Scholar] [CrossRef]
- Sun, Y.; Mu, J.; Wang, D.W.; Ouyang, N.; Xing, L.; Guo, X.; Zhao, C.; Ren, G.; Ye, N.; Zhou, Y.; et al. A village doctor-led multifaceted intervention for blood pressure control in rural China: An open, cluster randomised trial. Lancet 2022, 399, 1964–1975. [Google Scholar] [CrossRef]
- Odili, A.N.; Chori, B.S.; Danladi, B.; Nwakile, P.C.; Okoye, I.C.; Abdullahi, U.; Zawaya, K.; Essien, I.; Sada, K.; Nwegbu, M.M.; et al. Urinary sodium excretion and its association with blood pressure in Nigeria: A nationwide population survey. J. Clin. Hypertens. 2020, 22, 2266–2275. [Google Scholar] [CrossRef]
- Jackson, S.L.; Cogswell, M.E.; Zhao, L.; Terry, A.L.; Wang, C.-Y.; Wright, J.; King, S.M.C.; Bowman, B.; Chen, T.-C.; Merritt, R.; et al. Association Between Urinary Sodium and Potassium Excretion and Blood Pressure Among Adults in the United States. Circulation 2018, 137, 237–246. [Google Scholar] [CrossRef]
- Chailimpamontree, W.; Kantachuvesiri, S.; Aekplakorn, W.; Lappichetpaiboon, R.; Sripaiboonkij Thokanit, N.; Vathesatogkit, P.; Kunjang, A.; Boonyagarn, N.; Sukhonthachit, P.; Chuaykarn, N.; et al. Estimated dietary sodium intake in Thailand: A nationwide population survey with 24-hour urine collections. J. Clin. Hypertens. 2021, 23, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Eyles, H.; Shields, E.; Webster, J.; Ni Mhurchu, C. Achieving the WHO sodium target: Estimation of reductions required in the sodium content of packaged foods and other sources of dietary sodium. Am. J. Clin. Nutr. 2016, 104, 470–479. [Google Scholar] [CrossRef]
- Liu, Z.; Man, Q.; Li, Y.; Yang, X.; Ding, G.; Zhang, J.; Zhao, W. Estimation of 24-hour urinary sodium and potassium excretion among Chinese adults: A cross-sectional study from the China National Nutrition Survey. Am. J. Clin. Nutr. 2024, 119, 164–173. [Google Scholar] [CrossRef]
- Xu, A.; Ma, J.; Guo, X.; Wang, L.; Wu, J.; Zhang, J.; Bai, Y.; Xu, J.; Lu, Z.; Xu, Z.; et al. Association of a Province-Wide Intervention With Salt Intake and Hypertension in Shandong Province, China, 2011–2016. JAMA Intern. Med. 2020, 180, 877–886. [Google Scholar] [CrossRef]
- Anderson, C.A.M.; Appel, L.J.; Okuda, N.; Brown, I.J.; Chan, Q.; Zhao, L.; Ueshima, H.; Kesteloot, H.; Miura, K.; Curb, J.D.; et al. Dietary Sources of Sodium in China, Japan, the United Kingdom, and the United States, Women and Men Aged 40 to 59 Years: The INTERMAP Study. J. Am. Diet. Assoc. 2010, 110, 736–745. [Google Scholar] [CrossRef]
- Anderson, C.A.M.; Cobb, L.K.; Miller, E.R.; Woodward, M.; Hottenstein, A.; Chang, A.R.; Mongraw-Chaffin, M.; White, K.; Charleston, J.; Tanaka, T.; et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: Results of the SPICE randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhao, D.; Henry, M.E.; Fang, L.; Xu, J.; Chen, X.; Zhang, J.; Bai, Y.; Wu, J.; Ma, J.; et al. Use of Salt-Restriction Spoons and Its Associations with Urinary Sodium and Potassium in the Zhejiang Province of China: Results of a Population-Based Survey. Nutrients 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.C.; Marklund, M.; Anderson, C.A.M.; Cobb, L.K.; Dalcin, A.T.; Henry, M.; Appel, L.J. Potassium-Enriched Salt Substitutes as a Means to Lower Blood Pressure: Benefits and Risks. Hypertension 2020, 75, 266–274. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Y.; Li, C.; Feng, X.; Wang, H.; Qiao, Q.; Zhang, R.; Jin, A.; Li, J.; Li, H.; et al. Effect of a Salt Substitute on Incidence of Hypertension and Hypotension Among Normotensive Adults. J. Am. Coll. Cardiol. 2024, 83, 711–722. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, C.; Yin, X.; Zhang, X.; Ji, Y.; Zheng, X.; Zhou, Q.; Wu, Y. The Guidelines for use and promotion of low sodium salt in China. J. Evid. Based Med. 2024, 17, 454–467. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef]
- Collaborators GCoD. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Loria, C.M.; Terry, A.L.; Zhao, L.; Wang, C.Y.; Chen, T.C.; Wright, J.D.; Pfeiffer, C.M.; Merritt, R.; Moy, C.S.; et al. Estimated 24-Hour Urinary Sodium and Potassium Excretion in US Adults. JAMA 2018, 319, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Lewis, C.E.; Varady, K.A.; Su, Y.R.; Madhur, M.S.; Lackland, D.T.; Reis, J.P.; Wang, T.J.; Lloyd-Jones, D.M.; Allen, N.B. Effect of Dietary Sodium on Blood Pressure: A Crossover Trial. JAMA 2023, 330, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fang, L.; Xu, J.; Chen, X.; Bai, Y.; Wu, J.; Wu, L.; Zhong, J. The association of knowledge, attitudes and behaviors related to salt with 24-h urinary sodium, potassium excretion and hypertensive status. Sci. Rep. 2022, 12, 13901. [Google Scholar] [CrossRef] [PubMed]
- Bhana, N.; Utter, J.; Eyles, H. Knowledge, Attitudes and Behaviours Related to Dietary Salt Intake in High-Income Countries: A Systematic Review. Curr. Nutr. Rep. 2018, 7, 183–197. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Cogswell, M.E.; Loria, C.M.; Chen, T.-C.; Pfeiffer, C.M.; Swanson, C.A.; Caldwell, C.G.; Perrine, A.L.; Carriquiry, K.; Liu, C.T.; et al. Urinary Excretion of Sodium, Potassium, and Chloride, but Not Iodine, Varies by Timing of Collection in a 24-Hour Calibration Study1–3. J. Nutr. 2013, 143, 1276–1282. [Google Scholar] [CrossRef]
- Dennis, B.; Stamler, J.; Buzzard, M.; Conway, R.; Elliott, P.; Moag-Stahlberg, A.; Okayama, A.; Okuda, N.; Robertson, C.; Robinson, F.; et al. INTERMAP: The dietary data—Process and quality control. J. Hum. Hypertens. 2003, 17, 609–622. [Google Scholar] [CrossRef]
- Verkaik-Kloosterman, J.; Dekkers, A.L.M.; de Borst, M.H.; Bakker, S.J.L. Estimation of the salt intake distribution of Dutch kidney transplant recipients using 24-h urinary sodium excretion: The potential of external within-person variance. Am. J. Clin. Nutr. 2019, 110, 641–651. [Google Scholar] [CrossRef]
| Characteristic | Intervention | Reference | ||||
|---|---|---|---|---|---|---|
| Preintervention | Postintervention | p Value b | Preintervention | Postintervention | p Value b | |
| Age, years | 46.5 (13.7) | 50.2 (13.8) | <0.001 * | 46.0 (13.8) | 49.8 (13.7) | <0.001 * |
| Sex | 1.00 | 1.00 | ||||
| Female | 3001 (50.0%) | 2518 (52.1%) | 765 (50.7%) | 605 (53.4%) | ||
| Male | 3002 (50.0%) | 2317 (47.9%) | 744 (49.3%) | 528 (46.6%) | ||
| Education | 1.00 | 1.00 | ||||
| Primary school or lower | 1893 (31.6%) | 1589 (33.4%) | 388 (25.7%) | 338 (28.3%) | ||
| Junior high school | 2848 (47.5%) | 2244 (47.1%) | 760 (50.4%) | 580 (48.5%) | ||
| College or higher | 1259 (21.0%) | 928 (19.5%) | 361 (23.9%) | 278 (23.2%) | ||
| Cigarette smoking | <0.001 * | 0.26 | ||||
| Never smoked | 4261 (71.0%) | 3699 (76.9%) | 1111 (73.6%) | 908 (75.8%) | ||
| Former smokers | 266 (4.4%) | 220 (4.6%) | 46 (3.0%) | 55 (4.6%) | ||
| Current smokers | 1473 (24.6%) | 893 (18.6%) | 352 (23.3%) | 235 (19.6%) | ||
| Alcohol use status | 1925 (32.1%) | 1076 (22.4%) | <0.001 * | 549 (36.4%) | 352 (29.4%) | <0.001 * |
| Physical activity | 2418 (40.3%) | 2334 (48.5%) | <0.001 * | 602 (39.9%) | 517 (43.2%) | 0.05 |
| Use of antihypertensive medications | 780 (13.0%) | 979 (20.3%) | <0.001 * | 181 (12.0%) | 239 (20.6%) | <0.001 * |
| BMI, kg/m2 | 0.043 * | <0.001 * | ||||
| Low weight | 231 (3.8%) | 187 (3.9%) | 53 (3.5%) | 26 (2.2%) | ||
| Normal | 3048 (50.1%) | 2429 (50.4%) | 726 (48.0%) | 528 (45.5%) | ||
| Overweight | 2044 (33.6%) | 1655 (34.3%) | 546 (36.1%) | 426 (36.7%) | ||
| Obese | 679 (11.2%) | 551 (11.4%) | 184 (12.2%) | 181 (15.6%) | ||
| Mean | 24.0 (3.3) | 23.9 (3.4) | 0.05 | 24.2 (3.4) | 24.5 (3.5) | <0.001 * |
| SBP, mmHg | 131.2 (19.2) | 129.8(18.0) | <0.001 * | 127.0 (18.7) | 127.8 (17.8) | 0.049 * |
| DBP, mmHg | 80.8 (10.8) | 78.9 (10.2) | <0.001 * | 80.5 (10.9) | 80.2 (10.7) | 0.47 |
| BP status | 0.69 | <0.001 * | ||||
| Normal | 1677 (27.9%) | 1282 (26.5%) | 517 (34.3%) | 339 (29.0%) | ||
| Prehypertension | 2154 (35.9%) | 1678 (34.7%) | 511 (33.9%) | 376 (32.2%) | ||
| Hypertension | 2172 (36.2%) | 1869 (38.7%) | 481 (31.9%) | 453 (38.8%) | ||
| Diabetes mellitus | 428 (7.1%) | 529 (10.9%) | <0.001 * | 137 (9.1%) | 150 (12.4%) | <0.001 * |
| 24-hUNa excretion, mg/24 h | 3701.0 (1641.8) | 3615.1 (1788.9) | 0.24 | 4227.0 (1473.7) | 4149.8 (1770.4) | 0.58 |
| 24-hUKa excretion, mg/24 h | 1441.3 (681.9) | 1676.9 (931.4) | <0.001 * | 1726.6 (671.5) | 2017.2 (783.5) | <0.001 * |
| Sodium-to-potassium ratio | 4.8 (2.2) | 3.6 (1.7) | <0.001 * | 4.6 (2.2) | 4.2 (1.9) | 0.031 * |
| Mean Change (95%CI) a | Net Difference (95%CI) b | p Value | Adjusted Net Difference (95%CI) c | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention | p Value | Reference | p Value | |||||
| Change in SBP, mmHg | 1.4 (0.9 to 1.8) | <0.001 * | −0.9 (−1.8 to −0.0) | 0.049 * | 2.3 (1.3 to 3.3) | <0.001 * | 1.3 (0.5 to 2.1) | <0.001 * |
| Change in DBP, mmHg | 1.8 (1.5 to 2.1) | <0.001 * | 0.2 (−0.4 to 0.8) | 0.47 | 1.6 (0.9 to 2.2) | <0.001 * | 1.4 (0.9 to 2.0) | <0.001 * |
| Change in 24−hUNa excretion, mg | 85.9 (−57.7 to 229.5) | 0.24 | 77.3 (−195.9 to 350.4) | 0.58 | 8.6 (−309.2 to 326.4) | 0.96 | 394.1 (133.2 to 655.0) | 0.003 * |
| Change in 24−hUKa excretion, mg | −235.5 (−304.0 to −167.1) | <0.001 * | −290.6 (−420.6 to −160.5) | <0.001 * | 55.0 (−96.7 to 206.7) | 0.48 | 240.9 (108.2 to −373.6) | <0.001 * |
| Proportion (%) | Net Difference (%) a | Adjusted OR (95%CI) b | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Reference | ||||||||
| Preintervention (d1) | Postintervention (d2) | p Value | Preintervention (d3) | Postintervention (d4) | p Value | ||||
| Knowledge | |||||||||
| Know the diagnostic criteria of hypertension | 50.3 | 65.1 | <0.001 * | 64.8 | 68.4 | 0.13 | 11.1 | 1.45 (1.33 to 1.59) | <0.001 * |
| Know the hazards of hypertension | 72.9 | 84.9 | <0.001 * | 81.1 | 87.1 | <0.001 * | 6.0 | 1.36 (1.20 to 1.53) | <0.001 * |
| Know the risk factors of hypertension | 78.2 | 88.6 | <0.001 * | 84.2 | 90.0 | <0.001 * | 4.6 | 1.27 (1.11 to 1.45) | <0.001 * |
| Know less than 6 g salt per day | 35.2 | 57.5 | <0.001 * | 53.7 | 59.4 | 0.01 * | 16.6 | 1.57 (1.43 to 1.72) | <0.001 * |
| Know that eating less salt lowers blood pressure | 63.3 | 79.8 | <0.001 * | 75.4 | 82.6 | <0.001 * | 9.3 | 1.48 (1.33 to 1.64) | <0.001 * |
| Know the hazards of high salt | 69.3 | 85.4 | <0.001 * | 78.3 | 87.7 | <0.001 * | 6.6 | 1.37 (1.22 to 1.54) | <0.001 * |
| Know what kind of people should eat a low-salt diet | 78.0 | 91.8 | <0.001 * | 84.8 | 91.7 | <0.001 * | 6.8 | 1.28 (1.12 to 1.46) | <0.001 * |
| Know how to use salt restriction spoon correctly | 8.1 | 32.3 | <0.001 * | 11.5 | 21.0 | <0.001 * | 14.7 | 1.32 (1.17 to 1.49) | <0.001 * |
| Know low-sodium salt | 29.0 | 44.6 | <0.001 * | 34.1 | 43.5 | <0.001 * | 6.1 | 1.02 (0.93 to 1.12) | 0.61 |
| Know that low-sodium salt helps control blood pressure | 19.6 | 35.9 | <0.001 * | 25.6 | 36.0 | <0.001 * | 5.9 | 1.10 (1.00 to 1.22) | 0.049 * |
| Attitude | |||||||||
| Evaluate whether low-salt diet affects taste of food | <0.001 * | 0.87 | 1.35 (1.24 to 1.47) | <0.001 * | |||||
| Great influence | 11.4 | 9.8 | 12.5 | 12.6 | −1.7 | ||||
| Has some influence, but can accept | 62.1 | 58.8 | 65.9 | 65.8 | −3.1 | ||||
| No effect | 26.5 | 31.3 | 21.6 | 21.6 | 4.8 | ||||
| Approve that low-salt diet should be promoted among the crowd | 86.4 | 93.2 | <0.001 * | 89.9 | 92.7 | 0.04 * | 3.9 | 1.14 (0.98 to 1.32) | 0.09 |
| Approve of low-salt diet | 87.8 | 91.8 | <0.001 * | 89.7 | 90.5 | 0.75 | 3.2 | 1.01 (0.88 to 1.16) | 0.90 |
| Approve of the nutrition labeling of prepackaged food | 74.9 | 77.3 | <0.001 * | 75.8 | 76.0 | 0.57 | 2.2 | 1.11 (1.00 to 1.23) | 0.06 |
| Approve that the nutrition labeling of prepackaged food will help to choose low-salt diet | 72.3 | 76.3 | <0.001 * | 74.0 | 75.4 | 0.09 | 2.7 | 1.06 (0.96 to 1.17) | 0.28 |
| Behavior | |||||||||
| Self-assessment salt level | <0.001 * | 0.26 | 1.74 (1.61 to 1.89) | <0.001 * | |||||
| Not much | 31.3 | 35.5 | 17.2 | 18.6 | 2.8 | ||||
| Moderate | 50.2 | 53.9 | 63.7 | 64.2 | 3.2 | ||||
| Excessive | 18.5 | 10.5 | 19.2 | 17.2 | −6.1 | ||||
| Received publicity or education on low-salt diet | 45.8 | 64.2 | <0.001 * | 61.7 | 72.2 | <0.001 * | 7.9 | 1.68 (1.53 to 1.83) | <0.001 * |
| Once promoted low-salt diet knowledge to people | 68.0 | 57.9 | <0.001 * | 72.3 | 63.1 | 0.33 | −0.9 | 1.17 (1.06 to 1.30) | 0.003 * |
| Pay attention to the nutrition label of prepackaged food | 20.6 | 31.0 | <0.001 * | 28.3 | 32.3 | 0.10 | 6.4 | 1.20 (1.09 to 1.33) | <0.001 * |
| Plan to reduce salt | 78.0 | 90.3 | <0.001 * | 78.2 | 87.3 | <0.001 * | 3.2 | 1.14 (1.02 to 1.28) | 0.02 * |
| Take initiative to reduce salt | 58.2 | 70.6 | <0.001 * | 58.6 | 66.2 | <0.001 * | 4.8 | 1.12 (1.02 to 1.22) | 0.02 * |
| Using or used salt restriction spoon | 11.1 | 39.0 | <0.001 * | 15.3 | 24.0 | <0.001 * | 19.1 | 1.37 (1.22 to 1.53) | <0.001 * |
| Using salt restriction spoon correctly | 5.1 | 26.0 | <0.001 * | 7.4 | 16.5 | <0.001 * | 11.8 | 1.36 (1.19 to 1.56) | <0.001 * |
| Using or used low-sodium salt | 15.0 | 21.8 | <0.001 * | 18.5 | 21.0 | 0.19 | 4.3 | 1.04 (0.93 to 1.17) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zhu, Y.; Guo, J.; Chen, X.; Zhang, J.; Lu, F.; Xu, C.; Liang, M.; Wang, M.; Zhong, J.; et al. Effect of Salt Reduction Interventions in Lowering Blood Pressure and Salt Intake in Zhejiang Province, China, 2017–2021: A Randomized Controlled Trial. Nutrients 2025, 17, 893. https://doi.org/10.3390/nu17050893
Du X, Zhu Y, Guo J, Chen X, Zhang J, Lu F, Xu C, Liang M, Wang M, Zhong J, et al. Effect of Salt Reduction Interventions in Lowering Blood Pressure and Salt Intake in Zhejiang Province, China, 2017–2021: A Randomized Controlled Trial. Nutrients. 2025; 17(5):893. https://doi.org/10.3390/nu17050893
Chicago/Turabian StyleDu, Xiaofu, Ying Zhu, Jing Guo, Xiangyu Chen, Jie Zhang, Feng Lu, Chunxiao Xu, Mingbin Liang, Meng Wang, Jieming Zhong, and et al. 2025. "Effect of Salt Reduction Interventions in Lowering Blood Pressure and Salt Intake in Zhejiang Province, China, 2017–2021: A Randomized Controlled Trial" Nutrients 17, no. 5: 893. https://doi.org/10.3390/nu17050893
APA StyleDu, X., Zhu, Y., Guo, J., Chen, X., Zhang, J., Lu, F., Xu, C., Liang, M., Wang, M., Zhong, J., & Li, Y. (2025). Effect of Salt Reduction Interventions in Lowering Blood Pressure and Salt Intake in Zhejiang Province, China, 2017–2021: A Randomized Controlled Trial. Nutrients, 17(5), 893. https://doi.org/10.3390/nu17050893






