Choline During Pregnancy and Child Neurodevelopment: A Systematic Review of Randomized Controlled Trials and Observational Studies
Abstract
1. Introduction
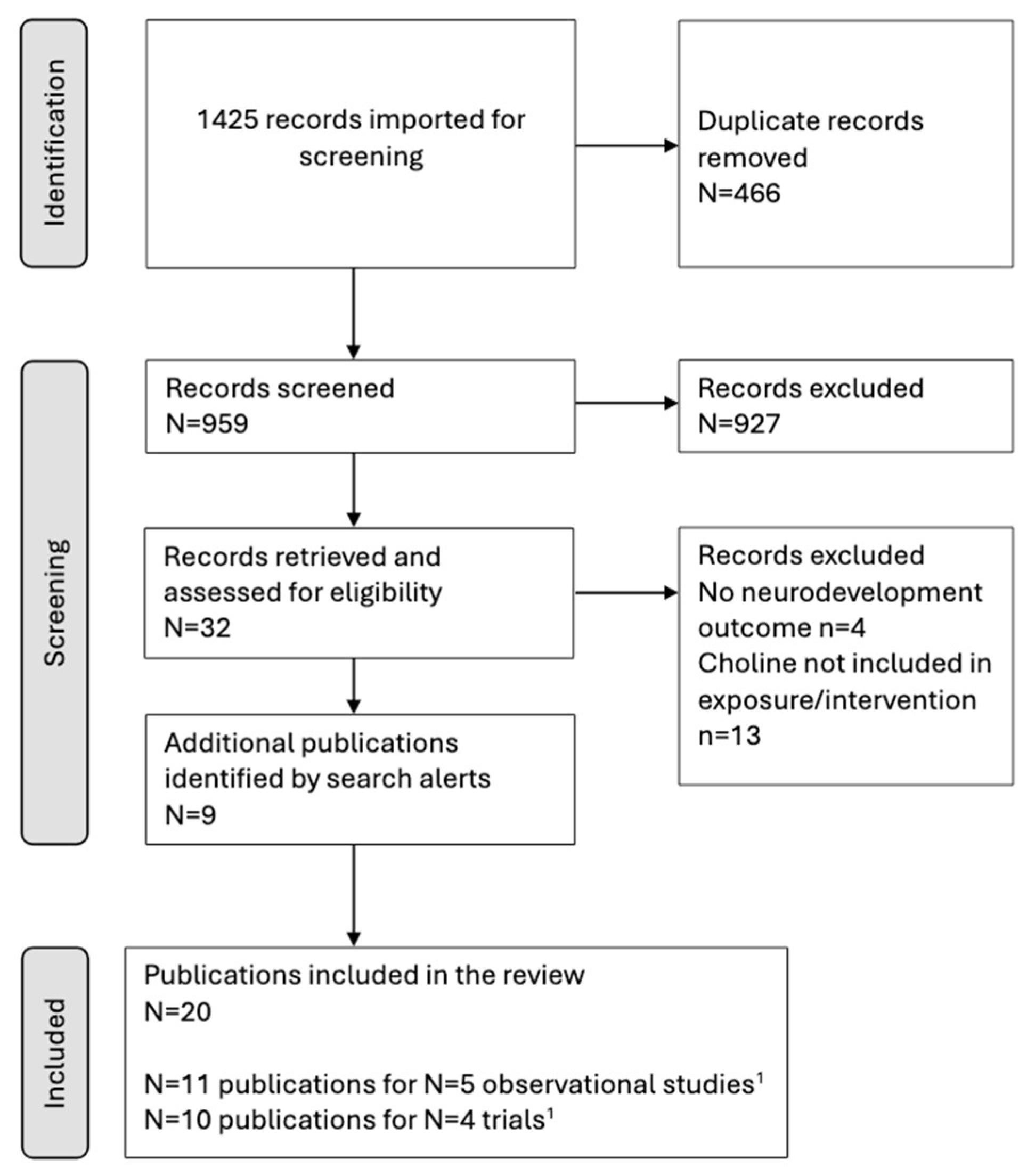
2. Materials and Methods
3. Results
3.1. Participants
3.1.1. Clinical Trials
3.1.2. Observational Studies
3.2. Exposure or Intervention
3.2.1. Clinical Trials
3.2.2. Observational Studies
3.3. Neurodevelopmental Outcomes
3.3.1. Clinical Trials
3.3.2. Observational Studies
| Author/Setting | Participants | Interventions | Outcome Measure and Follow-Up | Results |
|---|---|---|---|---|
| Cheatham et al. 2012 [25] Chapel Hill NC, U.S. | N:140 Healthy | Period:18 wk. of gestation to 90 d postpartum Treatment: 750 mg/d choline as phosphatidylcholine or placebo (corn oil) | Age: 10 and 12 mo. Outcome: MCDI-SF; MSEL; Short-term Visuospatial Memory Task; Long-term Episodic Memory Task | No effect of treatment on any outcome |
| Ross et al. 2013 [26] Denver CO, U.S. | N:100 Healthy | Period: Second trimester to 70–90 d postpartum Treatment: 900 mg/d choline as phosphatidylcholine or placebo (corn oil). Infants 100 mg/d phosphatidylcholine or placebo in an oral suspension. | Age: 5 and 13 wk. Primary Outcome: normal or abnormal P50 inhibition ratio according to electrophysiological recordings of cerebral inhibition Outcome: latencies and amplitudes of the individual P50 responses according to electrophysiological recordings of cerebral inhibition Age: 6 mo. Outcome: MSEL | Infants whose mothers received choline versus placebo were more likely to have normal inhibition at 5 wks (beneficial effect). No difference at 13 wks. No effect on MSEL |
| Ross et al. 2016 [37] | N:49 | Age: 40 mo. Outcome: CBCL-parent report | Fewer attention problems were reported and less social withdrawal in choline versus the placebo group. Aggression, emotionality, anxiety/depression, sleep, somatic, internalizing, externalizing, and total scores did not differ. | |
| Hunter et al. 2021 [42] | N:15 Black American mothers | Infants whose mothers received choline versus placebo were more likely to have normal P50 inhibition (beneficial effect, unclear whether 5 week and/or 13-wk assessment). Males of mothers who received choline had lower CBCL withdrawn and attention problem scores, but no other differences and no differences among females. | ||
| Caudill et al. 2018 [27] Ithaca NY, U.S. | N:29 Healthy | Period: third wk. of gestation until delivery Treatment: 480 or 930 mg/d choline chloride | Age: 4, 7, 10, and 13 mo. Primary outcome: mean reaction time for eye shifts according to a Visual Attention Task Outcome: Number of predictive eye movements (made in anticipation of a stimulus presenting) according to a Visual-Attention Task. | Reaction time averaged across the four ages was significantly faster for infants born to mothers in the 930 vs. 480 mg/d choline group. The number of anticipatory eye movements did not differ between groups. |
| Nevins et al. 2018 [40] | N:20 | Age: 7 y Outcome: Tower of London task-total score, correct moves, and planning time | The total score did not differ between choline groups, nor did planning time. Children born to mothers in the 930 vs. 480 mg/d choline group had more correct moves at the first attempt. | |
| Bahnfleth et al. 2019 [41] | N:20 | Age: 7 y Outcome: Color Location Memory Task | Children whose mothers were in the 930 vs. 480 mg/d choline group passed more levels of increasing difficulty. | |
| Bahnfleth et al. 2022 [38] | N:20 | Age: 7 y Outcome: Sustained Attention Task | A total of 930 versus 480 mg/d choline group had a better overall score as well as more correct responses. | |
| Jacobson et al. 2018 [28] Cape Town, South Africa | N: 69 heavy drinkers | Period: by 23 wk. of gestation until delivery Treatment: 2000 mg/d of choline bitartrate or placebo (not defined) | Age: 6.5 and 12 mo. Outcomes: eyeblink conditioning, Fagan Test of Infant Intelligence (visual recognition memory) | At 6.5 mo., the choline group was more likely to be classified as meeting the criterion for eyeblink conditioning. At 12 but not 6.5 mo. the choline group had faster recognition memory processing speed. No difference in recognition processing speed |
| Warton et al. 2021 [39] | N: 67 | Age: 1–7 wk. Outcomes: Regional brain volumes were measured using structural magnetic resonance imaging. Subcortical regions were manually segmented. All volumes were adjusted for age and total intracranial volume. | Six of the twelve regions in the brain were larger in the choline than in the placebo group. Larger right putamen and corpus callosum were related to higher Fagan scores at 12 mo. An NS trend toward partial mediation of the choline effect on recognition memory. |
| Author | Participants | Exposure | Follow-Up | Confounder Adjustments | Results |
|---|---|---|---|---|---|
| Signore et al. 2008 [29] AB, U.S. Infant Growth Project cohort | N:400 Healthy women receiving care from the public health department | Period: 16–18, 24–26, 30–32, and 36–38 wk pregnancy and cord. Measure: Serum total and free choline. | Age: 5 y Outcomes: WPPSI-R | Maternal PPVT-R raw score, HSQ score, poverty status, maternal race, education level, smoking, alcohol intake, gestational age at delivery, and infant sex. | No association between maternal or cord serum total and free choline at any time point for any neurodevelopmental outcome. |
| Villamor et al. 2012 [30] MA, U.S. Project Viva cohort | N:2128 Healthy women receiving care at a large practice | Period: first and second trimester Measure: Choline intake measured by FFQ | Age: 3 y Outcomes: PPVT-III, WRAVMA | Maternal ethnicity, age, parity, smoking, body mass index, PPVT-III, education, energy intake, fish and iron intake; paternal education; household income, infant sex, and primary language. | No association between choline intake and neurodevelopmental outcomes. |
| Boeke et al. 2013 [12] | N:895 | Age: 7 y Outcomes: WRAML-2, KBIT-2 | Top quartile choline intake in the second trimester was associated with modestly better WRAML (child visual memory) at 7 y. No other associations. | ||
| Wu et al. 2012 [11] Vancouver, Canada | N:154 Healthy | Period: 16, and 36 wk. of pregnancy. Measure: Plasma choline | Age: 18 mo. Outcome: Bayley-III | Maternal IQ, ethnicity, age phosphatidylethanolamine docosahexaenoic acid, infant sex, and breastfeeding duration. | A positive association was found between better infant cognitive test scores and higher maternal plasma choline at 16 wks. |
| Freedman et al. 2019 [31] Colorado, U.S. | N: 201 Healthy | Period: 16 wk. of pregnancy. Measure: Serum choline | Age: 1 mo. Outcome: cerebral auditory evoked potential as P50 amplitude Age: 3 mo. Outcome: IBQ-R | The primary analyses explored whether choline lessens any detrimental effects of maternal infection in pregnancy. Maternal age, education obesity, depression, and infant sex. | A significant interaction between choline status and infection where choline levels were associated with lower P50 amplitudes (beneficial effect) among infants whose mothers had experienced an infection. Similarly, choline was associated with increased IBQ-R Regulation, but not Surgency or Negativity for infants whose mothers were infected. |
| Freedman et al. 2020 [32] | N:89 Women who experienced a viral respiratory infection or no infections during pregnancy | Age: 3 mo. Outcome: IBQ-R | The primary analyses explored whether choline status ≥7.5 mM lessens any detrimental effects of maternal viral respiratory infection in pregnancy. Maternal age, education, obesity, anxiety, depression, and infant sex. | A significant interaction between choline and viral respiratory infection, where children whose mothers had choline status 37.5 mM had higher regulation scores, particularly within regulation the subscale of attention if their mother had experienced infection. The effect of other regulation subscales, nor surgency or negativity were reported. | |
| Hoffman et al. 2020 [33] | N: 201 | Period: 16 wk. of pregnancy. Measure: plasma choline and metabolite betaine | Age: 1 mo. Outcome: cerebral auditory evoked potential as P50 amplitude Age: 3 mo. Outcome: IBQ-R | The primary analyses explored whether choline lessens any detrimental effects of prenatal marijuana use. Maternal health, sociodemographic, prenatal and delivery characteristics, and infant sex. | A significant interaction between choline status and marijuana use where choline levels were associated with lower P50 amplitudes (beneficial effect) among infants whose mothers had used marijuana during pregnancy. Similarly, choline was associated with increased IBQ-R Regulation, but not surgency or negativity for infants whose mothers used marijuana. |
| Hunter et al. 2021 [42] | N: 149 N:23 Black Americans N:126 White Americans | The primary analyses explored whether choline influences the risk of predisposition to mental health for black Americans. | Higher choline at 16 weeks but not 28 weeks, was inversely associated with lower P50 (beneficial effect) in infants with Black American mothers. Similarly, higher choline was associated with better IBQ-R regulation scores among infants of Black American women. Choline >7 mM at 16 weeks was associated with better regulation scores in all women, particularly if they had experienced infection in pregnancy. | ||
| Hunter et al. 2022 [34] | N: 82 | Period: 16 and 28 wk. of pregnancy. Measure: plasma choline and metabolite betaine | Age: 18, 30, 40, 48 mo. Outcome: CBCL (average scores across all time points) | Maternal age, prenatal cannabis use, prenatal infections, lifetime depressive disorder, gestational age at birth, and infant sex. | Maternal choline concentrations >7.07 mM were associated with lower attention problem scores, fewer sleep problems (beneficial effect), and lower withdrawn scores among males only. Choline was associated with attention scores, particularly among children whose mothers had used cannabis or had an infection during pregnancy. |
| Hunter et al. 2021 [35] | N: 48 | Age: 4 y Outcome: WPPSI-IV | Maternal age, pre-pregnancy body mass index, depression, gestational age at birth, birth weight, child age at follow-up assessment, sex, and biological father in the household. | The Processing Speed Score was positively associated with choline concentration >7.07 mM, but no other WPPSI scores were. | |
| Irvine et al. 2023 [36] Alberta, Canada APrON study cohort | N: 309 Metropolitan pregnant women >16 years | Period: 2nd or 3rd trimester Measure: Choline intake measured by 24 hr FFQ | Age: 3–4 y Outcomes: WPPSI-IV, NEPSY-II, MABC-2, Spatial Span, Boy-Girl Stroop, DCCS | Maternal pre-pregnancy body mass index, age, education, income, ethnicity, parity, delivery mode, infant birth weight, sex, and age at assessment, and prenatal serum omega-3, iron, vitamin B12, magnesium, copper, zinc, and selenium status. The interaction with maternal folate status was also explored. | No associations were found for choline intake with all but the DCCS where high choline intake (223 mg/day) and high folate status were associated with lower odds of children receiving a passing score on the DCCS. |
3.4. Quality and Risk of Bias
3.4.1. Clinical Trials
3.4.2. Observational Studies
3.5. Efficacy
3.5.1. Clinical Trials
3.5.2. Observational Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Adequate Intake |
| Bayley | Bayley Scales of Infant Development |
| CBCL | Child Behavior Checklist |
| DCCS | Dimensional Change Card Sort |
| Fagan | Fagan Test of Infant Intelligence |
| FFQ | Food Frequency Questionnaire |
| K-ABC | Kaufman Assessment Battery |
| KBIT | Kaufman Brief Intelligence Test |
| MABC | Movement Assessment Battery for Children |
| MCDI | MacArthur-Bates Communicative Development Inventories |
| MCDI-SF | Mac- Arthur-Bates Short Form Vocabulary Checklist |
| MSEL | Mullen Scales of Early Learning |
| NEPSY | A Developmental Neuropsychological Assessment |
| NOS | Newcastle-Ottawa Scale |
| PPVT | The Peabody Picture Vocabulary Test |
| R | Revised |
| U.S. | United States (of America) |
| WPPSI | Wechsler Preschool and Primary Scale of Intelligence |
| WRAML | Wide Range Assessment of Memory and Learning |
| WRAVMA | Wide Range Assessment of Visual Motor Abilities |
References
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Australian Health Survey: Nutrition First Results—Foods and Nutrients; Australian Bureau of Statistics: Canberra, Australia, 2014.
- Probst, Y.; Sulistyoningrum, D.C.; Netting, M.J.; Gould, J.F.; Wood, S.; Makrides, M.; Best, K.P.; Green, T.J. Estimated Choline Intakes and Dietary Sources of Choline in Pregnant Australian Women. Nutrients 2022, 14, 3819. [Google Scholar] [CrossRef] [PubMed]
- Probst, Y.; Guan, V.; Neale, E. Development of a Choline Database to Estimate Australian Population Intakes. Nutrients 2019, 11, 913. [Google Scholar] [CrossRef]
- Vennemann, F.B.; Ioannidou, S.; Valsta, L.M.; Dumas, C.; Ocké, M.C.; Mensink, G.B.M.; Lindtner, O.; Virtanen, S.M.; Tlustos, C.; D’addezio, L.; et al. Dietary intake and food sources of choline in European populations. Br. J. Nutr. 2015, 114, 2046–2055. [Google Scholar] [CrossRef]
- Derbyshire, E.; Obeid, R. Choline, Neurological Development and Brain Function: A Systematic Review Focusing on the First 1000 Days. Nutrients 2020, 12, 1731. [Google Scholar] [CrossRef]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., III; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L., 3rd. Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar]
- Wu, B.T.; Dyer, R.A.; King, D.J.; Richardson, K.J.; Innis, S.M. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 2012, 7, e43448. [Google Scholar] [CrossRef]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Irvine, N.; England-Mason, G.; Field, C.J.; Dewey, D.; Aghajafari, F. Prenatal Folate and Choline Levels and Brain and Cognitive Development in Children: A Critical Narrative Review. Nutrients 2022, 14, 364. [Google Scholar] [CrossRef]
- Obeid, R.; Derbyshire, E.; Schön, C. Association between Maternal Choline, Fetal Brain Development, and Child Neurocognition: Systematic Review and Meta-Analysis of Human Studies. Adv. Nutr. 2022, 13, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.; Maes, M. The Role of Choline in Neurodevelopmental Disorders-A Narrative Review Focusing on ASC, ADHD and Dyslexia. Nutrients 2023, 15, 2876. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Dewani, D.; Reddy, L.S.; Patel, A. Choline Supplementation in Pregnancy: Current Evidence and Implications. Cureus 2023, 15, e48538. [Google Scholar] [CrossRef]
- Spoelstra, S.K.; Eijsink, J.J.H.; Hoenders, H.J.R.; Knegtering, H. Maternal choline supplementation during pregnancy to promote mental health in offspring. Early Interv. Psychiatry 2023, 17, 643–651. [Google Scholar] [CrossRef]
- Serwatka, C.A.; Griebel-Thompson, A.K.; Eiden, R.D.; Kong, K.L. Nutrient Supplementation during the Prenatal Period in Substance-Using Mothers: A Narrative Review of the Effects on Offspring Development. Nutrients 2023, 15, 2990. [Google Scholar] [CrossRef]
- Zhong, W.; Hu, L.; Zhao, Y.; Li, Z.; Zhuo, Y.; Jiang, X.; Li, J.; Zhao, X.; Che, L.; Feng, B.; et al. Effects of Dietary Choline Levels During Pregnancy on Reproductive Performance, Plasma Metabolome and Gut Microbiota of Sows. Front. Vet. Sci. 2021, 8, 771228. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010, 63, e1–e73. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 2 June 2024).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.M.P.F.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Cifasd, T.; Coles, C.D.; Kable, J.A.; Keen, C.L.; Jones, K.L.; Wertelecki, W.; Granovska, I.V.; Pashtepa, A.O.; Chambers, C.D. Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern. Child. Health J. 2015, 19, 2605–2614. [Google Scholar]
- Kable, J.A.; Keen, C.; Uriu-Adams, J.; Jones, K.; Yevtushok, L.; Kulikovsky, Y.; Wertelecki, W.; Pedersen, T.; Chambers, C. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol. 2015, 49, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, C.L.; Goldman, B.D.; Fischer, L.M.; da Costa, K.-A.; Reznick, J.S.; Zeisel, S.H. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2012, 96, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.G.; Hunter, S.K.; McCarthy, L.; Beuler, J.; Hutchison, A.K.; Wagner, B.D.; Leonard, S.; Stevens, K.E.; Freedman, R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiatry 2013, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Signore, C.; Ueland, P.M.; Troendle, J.; Mills, J.L. Choline concentrations in human maternal and cord blood and intelligence at 5 y of age. Am. J. Clin. Nutr. 2008, 87, 896–902. [Google Scholar] [CrossRef]
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Perinat. Epidemiol. 2012, 26, 328–335. [Google Scholar] [CrossRef]
- Freedman, R.; Hunter, S.K.; Law, A.J.; Wagner, B.D.; D’Alessandro, A.; Christians, U.; Noonan, K.; Wyrwa, A.; Hoffman, M.C. Higher Gestational Choline Levels in Maternal Infection Are Protective for Infant Brain Development. J. Pediatr. 2019, 208, 198–206.e2. [Google Scholar] [CrossRef]
- Freedman, R.; Hunter, S.K.; Law, A.J.; D’Alessandro, A.; Noonan, K.; Wyrwa, A.; Hoffman, M.C. Maternal choline and respiratory coronavirus effects on fetal brain development. J. Psychiatr. Res. 2020, 128, 1–4. [Google Scholar] [CrossRef]
- Hoffman, M.C.; Hunter, S.K.; D’Alessandro, A.; Noonan, K.; Wyrwa, A.; Freedman, R. Interaction of maternal choline levels and prenatal Marijuana’s effects on the offspring. Psychol. Med. 2020, 50, 1716–1726. [Google Scholar] [CrossRef]
- Hunter, S.K.; Hoffman, M.C.; D’Alessandro, A.; Wyrwa, A.; Noonan, K.; Zeisel, S.H.; Law, A.J.; Freedman, R. Prenatal choline, cannabis, and infection, and their association with offspring development of attention and social problems through 4 years of age. Psychol. Med. 2022, 52, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Hoffman, M.C.; D’Alessandro, A.; Walker, V.K.; Balser, M.; Noonan, K.; Law, A.J.; Freedman, R. Maternal prenatal choline and inflammation effects on 4-year-olds’ performance on the Wechsler Preschool and Primary Scale of Intelligence-IV. J. Psychiatr. Res. 2021, 141, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Irvine, N.; England-Mason, G.; Field, C.J.; Letourneau, N.; Bell, R.C.; Giesbrecht, G.F.; Kinniburgh, D.W.; MacDonald, A.M.; Martin, J.W.; Dewey, D.; et al. Associations between maternal folate status and choline intake during pregnancy and neurodevelopment at 3-4 years of age in the Alberta Pregnancy Outcomes and Nutrition (APrON) study. J. Dev. Orig. Health Dis. 2023, 14, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.G.; Hunter, S.K.; Hoffman, M.C.; McCarthy, L.; Chambers, B.M.; Law, A.J.; Leonard, S.; Zerbe, G.O.; Freedman, R. Perinatal Phosphatidylcholine Supplementation and Early Childhood Behavior Problems: Evidence for CHRNA7 Moderation. Am. J. Psychiatry 2016, 173, 509–516. [Google Scholar] [CrossRef]
- Bahnfleth, C.L.; Strupp, B.J.; Caudill, M.A.; Canfield, R.L. Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J. 2022, 36, e22054. [Google Scholar] [CrossRef]
- Warton, F.L.; Molteno, C.D.; Warton, C.M.R.; Wintermark, P.; Lindinger, N.M.; Dodge, N.C.; Zöllei, L.; van der Kouwe, A.J.; Carter, R.C.; Jacobson, J.L.; et al. Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol. Clin. Exp. Res. 2021, 45, 1762–1774. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.; Canfield, R.L. Maternal choline supplementation during pregnancy improves executive functioning in children at age 7 y (E10–06). Curr. Dev. Nutr. 2018, 2, 2172. [Google Scholar]
- Bahnfleth, C.; Canfield, R.; Nevins, J.; Caudill, M.; Strupp, B. Prenatal choline supplementation improves child color- location memory task performance at 7 y of age (FS05-01-19). Curr. Dev. Nutr. 2019, 3, nzz052.FS05-01-19. [Google Scholar] [CrossRef]
- Hunter, S.K.; Hoffman, M.C.; McCarthy, L.; D’alessandro, A.; Wyrwa, A.; Noonan, K.; Christians, U.; Nakimuli-Mpungu, E.; Zeisel, S.H.; Law, A.J.; et al. Black American Maternal Prenatal Choline, Offspring Gestational Age at Birth, and Developmental Predisposition to Mental Illness. Schizophr. Bull. 2021, 47, 896–905. [Google Scholar] [CrossRef]
- Thomas, J.D.; Tran, T.D. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus 2012, 22, 619–630. [Google Scholar] [CrossRef]
| Author | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Cheatham et al., 2012 [25] | + | + | + | ? | + | − | ? |
| Ross et al., 2013 [26] | − | ? | ? | ? | − | ? | ? |
| Caudill et al., 2018 [27] | + | + | + | ? | − | − | ? |
| Jacobson et al., 2018 [28] | + | + | + | ? | + | − | ? |
| Author | Year | Selection | Comparability | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort Studies | Q1 | Q2 | Q3 | Q4 | Q1 | Q1 | Q2 | Q3 | ||
| Signore et al. [29] | 2008 | * | * | * | * | ** | * | * | - | 8 |
| Villamor et al. [30] | 2021 | * | * | - | * | ** | - | * | * | 7 |
| Boeke et al. [12] | 2013 | * | * | - | * | ** | - | * | - | 6 |
| Wu et al. [11] | 2012 | - | * | * | * | * | - | * | - | 5 |
| Hoffman et al. [33] | 2020 | * | * | * | * | * | * | * | * | 8 |
| Hunter et al. [42] | 2021 | * | * | * | * | * | * | * | - | 7 |
| Hunter et al. [35] | 2021 | * | * | * | * | ** | * | * | - | 7 |
| Hunter et al. [34] | 2022 | * | * | * | * | * | * | * | - | 8 |
| Irvine et al. [36] | 2023 | * | * | - | * | * | * | * | - | 6 |
| Selection | Comparability | Exposure | ||||||||
| Case Controls | Q1 | Q2 | Q3 | Q4 | Q1 | Q1 | Q2 | Q3 | ||
| Freedman et al. [31] | 2019 | * | * | * | * | * | * | * | - | 8 |
| Freedman et al. [32] | 2020 | * | * | * | * | * | * | * | - | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, J.F.; Hines, S.; Best, K.P.; Grzeskowiak, L.E.; Jansen, O.; Green, T.J. Choline During Pregnancy and Child Neurodevelopment: A Systematic Review of Randomized Controlled Trials and Observational Studies. Nutrients 2025, 17, 886. https://doi.org/10.3390/nu17050886
Gould JF, Hines S, Best KP, Grzeskowiak LE, Jansen O, Green TJ. Choline During Pregnancy and Child Neurodevelopment: A Systematic Review of Randomized Controlled Trials and Observational Studies. Nutrients. 2025; 17(5):886. https://doi.org/10.3390/nu17050886
Chicago/Turabian StyleGould, Jacqueline F., Sonia Hines, Karen P. Best, Luke E. Grzeskowiak, Olivia Jansen, and Tim J. Green. 2025. "Choline During Pregnancy and Child Neurodevelopment: A Systematic Review of Randomized Controlled Trials and Observational Studies" Nutrients 17, no. 5: 886. https://doi.org/10.3390/nu17050886
APA StyleGould, J. F., Hines, S., Best, K. P., Grzeskowiak, L. E., Jansen, O., & Green, T. J. (2025). Choline During Pregnancy and Child Neurodevelopment: A Systematic Review of Randomized Controlled Trials and Observational Studies. Nutrients, 17(5), 886. https://doi.org/10.3390/nu17050886







