Polyunsaturated Fatty Acids and Reduced Risk of Low Muscle Mass in Adults
Abstract
1. Introduction
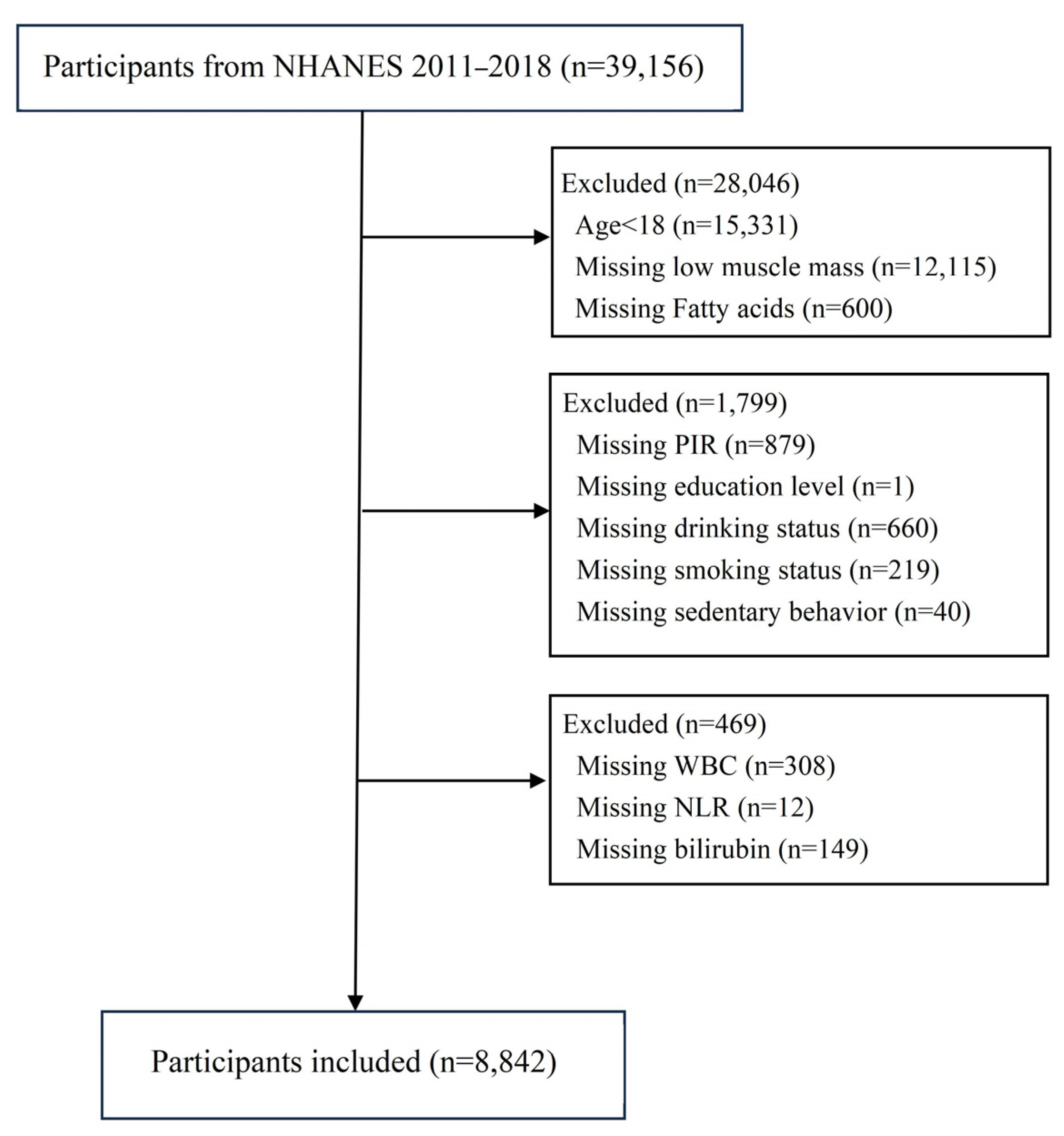
2. Materials and Methods
2.1. Data Sources
2.2. Definition of Low Muscle Mass
2.3. Fatty Acid Assessment
2.4. Definition of Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Fatty Acids and Low-Muscle-Mass Risk in the Logistic Regression Model
3.3. The Detection of Linear Relationships Between Fatty Acid Intake and Low-Muscle-Mass Risk
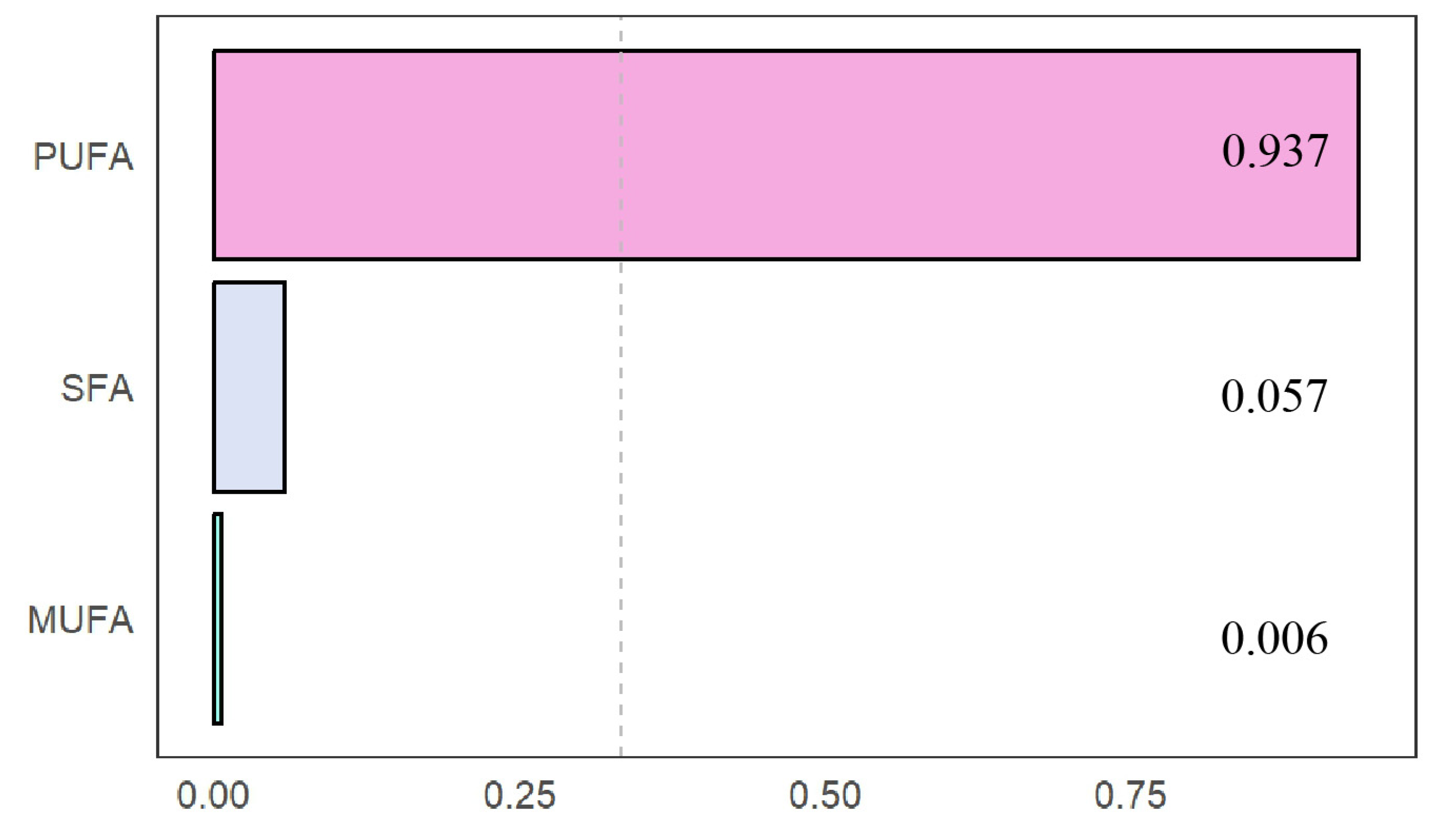
3.4. Fatty Acid Concentrations and Low-Muscle-Mass Risk in the WQS Model
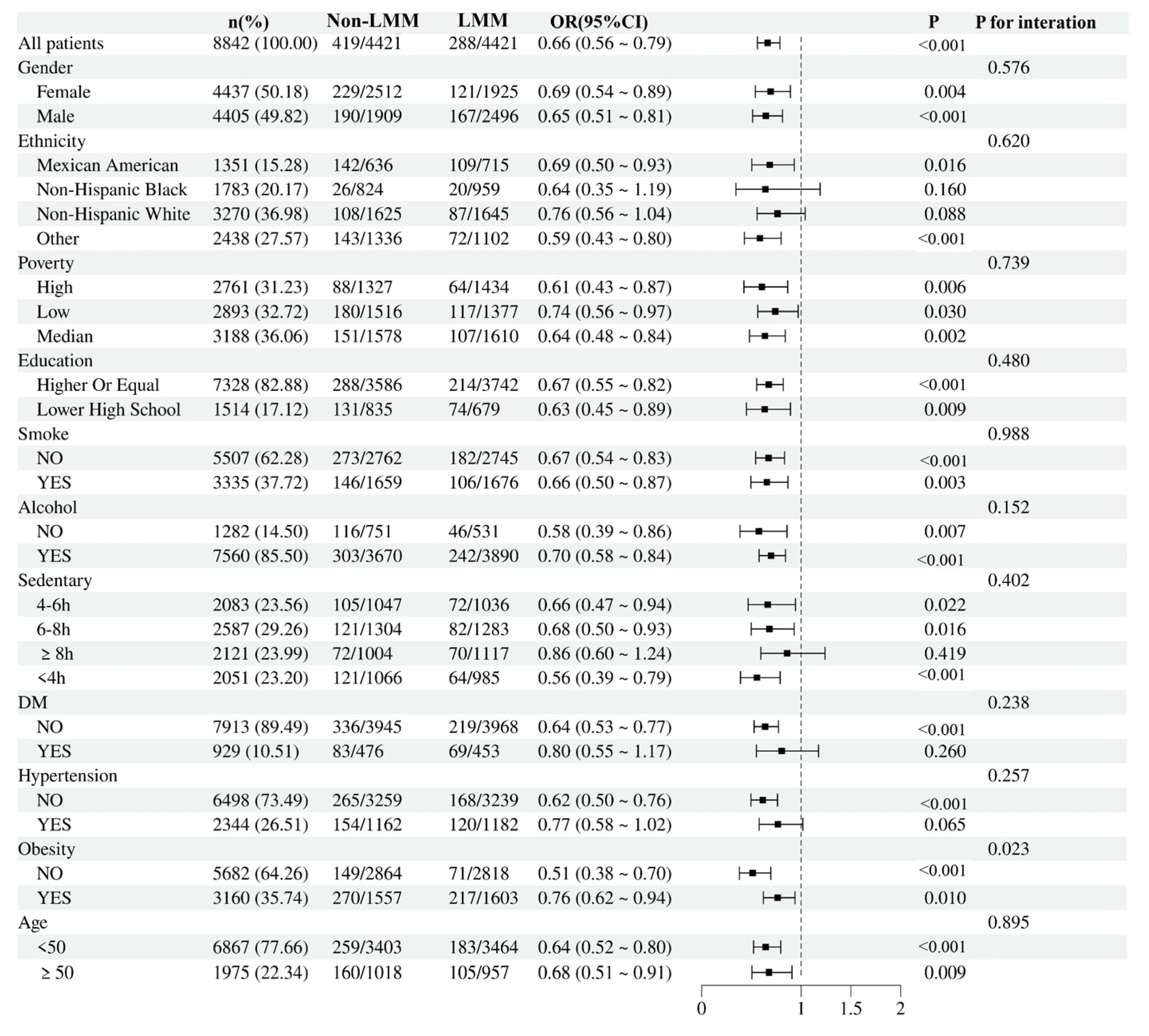
3.5. Subgroup Analysis
3.6. Mediation Effect
3.7. Associations Between Types of Polyunsaturated Fatty Acids and Low Muscle Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PUFA | polyunsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| SFA | saturated fatty acid |
| NHANES | National Health and Nutrition Examination Survey |
| RCS | restricted cubic splines |
| WQS | weighted quantile sum |
| LA | linoleic acid |
| ALA | alpha-linolenic acid |
| EPA | eicosa-pentaenoic acid |
| DHA | docosa-hexaenoic acid |
| DXA | dual-energy X-ray absorptiometry |
| MEC | mobile examination center |
| PIR | poverty income ratio |
| BMI | body mass index |
| LMM | low muscle mass |
References
- Alhmly, H.F.; Fielding, R.A. A Critical Review of Current Worldwide Definitions of Sarcopenia. Calcif. Tissue Int. 2024, 114, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Corbi, G.; Medoro, A.; Intrieri, M.; Scapagnini, G.; Davinelli, S. Relationship between monounsaturated fatty acids and sarcopenia: A systematic review and meta-analysis of observational studies. Aging Clin. Exp. Res. 2023, 35, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Gonzalez, M.C.; Prado, C.M. Sarcopenia ≠ low muscle mass. Eur. Geriatr. Med. 2023, 14, 225–228. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Montville, J.B.; Ahuja, J.K.C.; Martin, C.L.; Heendeniya, K.Y.; Omolewa-Tomobi, G.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; LaComb, R.P.; Moshfegh, A. USDA Food and Nutrient Database for Dietary Studies (FNDDS), 5.0. Procedia Food Sci. 2013, 2, 99–112. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Rawat, V.; Bortolussi, G.; Gazzin, S.; Tiribelli, C.; Muro, A.F.; Immenschuh, S. Bilirubin-Induced Oxidative Stress Leads to DNA Damage in the Cerebellum of Hyperbilirubinemic Neonatal Mice and Activates DNA Double-Strand Break Repair Pathways in Human Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1801243. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, C.; Scalise, V.; Neri, T.; Celi, A.; Susini, V.; Franzini, M.; Pedrinelli, R. Binding of Gamma-Glutamyl Transferase to TLR4 Signalling Allows Tissue Factor Activation in Monocytes. Int. J. Mol. Sci. 2022, 23, 12207. [Google Scholar] [CrossRef]
- Collins, P.F.; Stratton, R.J.; Elia, M. Nutritional support in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1385–1395. [Google Scholar] [CrossRef]
- Zhuang, C.-L.; Huang, D.-D.; Pang, W.-Y.; Zhou, C.-J.; Wang, S.-L.; Lou, N.; Ma, L.-L.; Yu, Z.; Shen, X. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: Analysis from a large-scale cohort. Medicine 2016, 95, e3164. [Google Scholar] [CrossRef] [PubMed]
- Fülster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschöpe, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Englesbe, M.J.; Lee, J.S.; He, K.; Fan, L.; Schaubel, D.E.; Sheetz, K.H.; Harbaugh, C.M.; Holcombe, S.A.; Campbell, D.A., Jr.; Sonnenday, C.J. Analytic morphomics, core muscle size, and surgical outcomes. Ann. Surg. 2012, 256, 255–261. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.K.; Ankarfeldt, M.Z.; Capra, S.; Bauer, J.; Raymond, K.; Heitmann, B.L. Lean body mass change over 6 years is associated with dietary leucine intake in an older Danish population. Br. J. Nutr. 2016, 115, 1556–1562. [Google Scholar] [CrossRef]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, W.; Würdemann, W.; Plötz, T.; Jörns, A.; Lenzen, S.; Elsner, M. Antagonism Between Saturated and Unsaturated Fatty Acids in ROS Mediated Lipotoxicity in Rat Insulin-Producing Cells. Cell. Physiol. Biochem. 2015, 36, 852–865. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Candau, R.B.; Bernardi, H. FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014, 71, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Sandri, M. Signaling pathways that control muscle mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, T.; Guo, Y.; Pei, W.; Liu, R.; Chang, M.; Wang, X. Linolenic acid ameliorates sarcopenia in C. elegans by promoting mitophagy and fighting oxidative stress. Food Funct. 2023, 14, 1498–1509. [Google Scholar] [CrossRef]
- Nisr, R.B.; Shah, D.S.; Hundal, H.S. Mono- and Polyunsaturated Fatty Acids Counter Palmitate-Induced Mitochondrial Dysfunction in Rat Skeletal Muscle Cells. Cell Physiol. Biochem. 2020, 54, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Ghzaiel, I.; Zarrouk, A.; Nury, T.; Libergoli, M.; Florio, F.; Hammouda, S.; Menetrier, F.; Avoscan, L.; Yammine, A.; Samadi, M.; et al. Antioxidant Properties and Cytoprotective Effect of Pistacia lentiscus L. Seed Oil against 7beta-Hydroxycholesterol-Induced Toxicity in C2C12 Myoblasts: Reduction in Oxidative Stress, Mitochondrial and Peroxisomal Dysfunctions and Attenuation of Cell Death. Antioxidants 2021, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Sharples, A.P.; Al-Shanti, N.; Stewart, C.E. Omega-3 fatty acid EPA improves regenerative capacity of mouse skeletal muscle cells exposed to saturated fat and inflammation. Biogerontology 2017, 18, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- González-Hedström, D.; de la Fuente-Fernández, M.; Priego, T.; Martín, A.I.; Amor, S.; López-Calderón, A.; Inarejos-García, A.M.; García-Villalón, Á.L.; Granado, M. Addition of Olive Leaf Extract to a Mixture of Algae and Extra Virgin Olive Oils Decreases Fatty Acid Oxidation and Synergically Attenuates Age-Induced Hypertension, Sarcopenia and Insulin Resistance in Rats. Antioxidants 2021, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Lalia, A.Z.; Dasari, S.; Pallauf, M.; Fitch, M.; Hellerstein, M.K.; Lanza, I.R. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 2015, 14, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Allen, J. The omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the damaging effects of tumour necrosis factor (TNF)-alpha during murine skeletal muscle cell differentiation. Lipids Health Dis. 2008, 7, 24. [Google Scholar] [CrossRef]
- Rahman, M.M.; Halade, G.V.; El Jamali, A.; Fernandes, G. Conjugated linoleic acid (CLA) prevents age-associated skeletal muscle loss. Biochem. Biophys. Res. Commun. 2009, 383, 513–518. [Google Scholar] [CrossRef]
- Patted, P.G.; Masareddy, R.S.; Patil, A.S.; Kanabargi, R.R.; Bhat, C.T. Omega-3 fatty acids: A comprehensive scientific review of their sources, functions and health benefits. Future J. Pharm. Sci. 2024, 10, 94. [Google Scholar] [CrossRef]
- Duan, H.; Song, W.; Zhao, J.; Yan, W. Polyunsaturated Fatty Acids (PUFAs): Sources, digestion, absorption, application and their potential adjunctive effects on visual fatigue. Nutrients 2023, 15, 2633. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Enrione, E.; Narayanan, V.; Li, T.; Campa, A. Association of Polyunsaturated Fatty Acids and Vitamin B6 with Inflammation in Adults from the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Curr. Dev. Nutr. 2020, 4, nzaa067_043. [Google Scholar] [CrossRef]
- Nandi, A.; Wadhwani, N.; Joshi, S. Vitamin D deficiency influences fatty acid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 57–63. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E function and requirements in relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef]

| All | Non-Sarcopenia | Sarcopenia | p Value | |
|---|---|---|---|---|
| N = 8842 | N = 8135 | N = 707 | ||
| Age, (years) | 37.0 [27.0; 48.0] | 37.0 [27.0; 48.0] | 45.0 [32.0; 53.0] | <0.001 |
| Gender, n (%) | 0.737 | |||
| Female | 4437 (50.2%) | 4087 (50.2%) | 350 (49.5%) | |
| Male | 4405 (49.8%) | 4048 (49.8%) | 357 (50.5%) | |
| Ethnicity, n (%) | <0.001 | |||
| Mexican American | 1351 (15.3%) | 1100 (13.5%) | 251 (35.5%) | |
| Non-Hispanic Black people | 1783 (20.2%) | 1737 (21.4%) | 46 (6.51%) | |
| Non-Hispanic White people | 3270 (37.0%) | 3075 (37.8%) | 195 (27.6%) | |
| Other | 2438 (27.6%) | 2223 (27.3%) | 215 (30.4%) | |
| Household economic level, n (%) | <0.001 | |||
| High | 2761 (31.2%) | 2609 (32.1%) | 152 (21.5%) | |
| Low | 2893 (32.7%) | 2596 (31.9%) | 297 (42.0%) | |
| Median | 3188 (36.1%) | 2930 (36.0%) | 258 (36.5%) | |
| Educational attainment, n (%) | <0.001 | |||
| Higher or equal | 7328 (82.9%) | 6826 (83.9%) | 502 (71.0%) | |
| Lower than high school | 1514 (17.1%) | 1309 (16.1%) | 205 (29.0%) | |
| PUFA (gm) | 17.5 [11.2; 25.8] | 17.6 [11.4; 26.1] | 15.1 [9.59; 22.1] | <0.001 |
| PUFA Quartiles (gm) | <0.001 | |||
| Q1 (≤11.2) | 2207 (25.0%) | 1974 (24.3%) | 233 (33.0%) | |
| Q2 (11.2–17.5] | 2231 (25.2%) | 2045 (25.1%) | 186 (26.3%) | |
| Q3 (17.5–25.8] | 2195 (24.8%) | 2021 (24.8%) | 174 (24.6%) | |
| Q4 (>25.8) | 2209 (25.0%) | 2095 (25.8%) | 114 (16.1%) | |
| MUFA (gm) | 26.8 [17.8; 38.4] | 27.2 [18.1; 38.7] | 23.3 [15.9; 33.9] | <0.001 |
| MUFA Quartiles (gm) | <0.001 | |||
| Q1 (≤17.8) | 2212 (25.0%) | 1989 (24.4%) | 223 (31.5%) | |
| Q2 (17.8–26.8] | 2200 (24.9%) | 1998 (24.6%) | 202 (28.6%) | |
| Q3 (26.8–38.4] | 2217 (25.1%) | 2064 (25.4%) | 153 (21.6%) | |
| Q4 (>38.4) | 2213 (25.0%) | 2084 (25.6%) | 129 (18.2%) | |
| SFA (gm) | 24.2 [15.6; 36.1] | 24.5 [15.8; 36.4] | 21.4 [13.1; 33.4] | <0.001 |
| SFA Quartiles (gm) | <0.001 | |||
| Q1 (≤15.6) | 2215 (25.1%) | 1988 (24.4%) | 227 (32.1%) | |
| Q2 (15.6–24.2] | 2204 (24.9%) | 2023 (24.9%) | 181 (25.6%) | |
| Q3 (24.2–36.1] | 2202 (24.9%) | 2048 (25.2%) | 154 (21.8%) | |
| Q4 (>36.1) | 2221 (25.1%) | 2076 (25.5%) | 145 (20.5%) | |
| Smoking status, n (%) | 0.252 | |||
| No | 5507 (62.3%) | 5052 (62.1%) | 455 (64.4%) | |
| Yes | 3335 (37.7%) | 3083 (37.9%) | 252 (35.6%) | |
| Alcohol, n (%) | <0.001 | |||
| No | 1282 (14.5%) | 1120 (13.8%) | 162 (22.9%) | |
| Yes | 7560 (85.5%) | 7015 (86.2%) | 545 (77.1%) | |
| BMI (kg/m2) | 27.6 [23.7; 32.3] | 27.1 [23.5; 31.6] | 33.5 [29.0; 39.3] | <0.001 |
| Obesity, n (%) | <0.001 | |||
| No | 5682 (64.3%) | 5462 (67.1%) | 220 (31.1%) | |
| Yes | 3160 (35.7%) | 2673 (32.9%) | 487 (68.9%) | |
| Sedentary behavior, n (%) | 0.035 | |||
| <4 h | 2051 (23.2%) | 1866 (22.9%) | 185 (26.2%) | |
| 4–6 h | 2083 (23.6%) | 1906 (23.4%) | 177 (25.0%) | |
| 6–8 h | 2587 (29.3%) | 2384 (29.3%) | 203 (28.7%) | |
| >8 h | 2121 (24.0%) | 1979 (24.3%) | 142 (20.1%) | |
| Diabetes mellitus, n (%) | <0.001 | |||
| No | 7913 (89.5%) | 7358 (90.4%) | 555 (78.5%) | |
| Yes | 929 (10.5%) | 777 (9.55%) | 152 (21.5%) | |
| Hypertension, n (%) | <0.001 | |||
| No | 6498 (73.5%) | 6065 (74.6%) | 433 (61.2%) | |
| Yes | 2344 (26.5%) | 2070 (25.4%) | 274 (38.8%) |
| Quartiles of the Fatty Acids (gm) | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| SFA | (≤15.6) | (15.6–24.2] | (24.2–36.1] | (>36.1) | |
| Crude | 1 | 0.71 [0.55–0.93] | 0.68 [0.52–0.89] | 0.59 [0.41–0.84] | 0.005 |
| Model 1 | 1 | 0.74 [0.56–0.98] | 0.71 [0.54–0.94] | 0.57 [0.39–0.83] | 0.006 |
| Model 2 | 1 | 0.71 [0.54–0.94] | 0.65 [0.47–0.89] | 0.51 [0.35–0.76] | 0.002 |
| MUFA | (≤17.8) | (17.8–26.8] | (26.8–38.4] | (>38.4) | |
| Crude | 1 | 0.92 [0.71–1.19] | 0.66 [0.50–0.86] | 0.59 [0.44–0.79] | <0.001 |
| Model 1 | 1 | 0.92 [0.71–1.20] | 0.65 [0.48–0.88] | 0.55 [0.40–0.75] | <0.001 |
| Model 2 | 1 | 0.90 [0.69–1.17] | 0.64 [0.47–0.88] | 0.52 [0.38–0.71] | <0.001 |
| PUFA | (≤11.2) | (11.2–17.5] | (17.5–25.8] | (>25.8) | |
| Crude | 1 | 0.75 [0.57–0.98] | 0.72 [0.57–0.91] | 0.48 [0.35–0.66] | <0.001 |
| Model 1 | 1 | 0.76 [0.57–1.01] | 0.70 [0.55–0.90] | 0.48 [0.34–0.67] | <0.001 |
| Model 2 | 1 | 0.78 [0.58–1.06] | 0.71 [0.54–0.93] | 0.48 [0.34–0.69] | <0.001 |
| Estimate | Standard Error | Z Value | p Value | |
|---|---|---|---|---|
| Intercept | −2.09836 | 0.07787 | −26.946 | <0.001 |
| WQS | −0.21840 | 0.04636 | −4.711 | <0.001 |
| Pathways | Mediation Proportions | 95%CI | p-Value |
|---|---|---|---|
| PUFAs→Albumin→Low muscle mass | 2.64% | 5.60 × 10−3, 0.06 | 0.03 |
| PUFAs→White blood cell→Low muscle mass | 3.75% | 0.02, 0.06 | <0.001 |
| PUFAs→Bilirubin→Low muscle mass | 1.60% | 6.42 × 10−4, 0.04 | 0.042 |
| PUFAs→Gamma-glutamyltransferase→Low muscle mass | 1.14% | 4.12 × 10−3, 0.02 | 0.004 |
| OR [95%CI]/p Value | ||
|---|---|---|
| PUFAs (gm) | Unadjusted | Adjusted Model |
| C18:3 | ||
| Per SD increment | 0.75 [0.68–0.82]/<0.001 | 0.73 [0.66–0.81]/<0.001 |
| T1 | 1 | 1 |
| T2 | 0.91 [0.69–1.19] | 0.90 [0.67–1.19] |
| T3 | 0.65 [0.48–0.88] | 0.63 [0.45–0.87] |
| p value for trend | <0.001 | <0.001 |
| C20:5 | ||
| Per SD increment | 0.97 [0.88–1.05]/0.48 | 0.98 [0.88–1.07]/0.66 |
| T1 | 1 | 1 |
| T2 | 0.97 [0.76–1.24] | 0.94 [0.73–1.21] |
| T3 | 0.76 [0.59–0.97] | 0.72 [0.55–0.95] |
| p value for trend | 0.41 | 0.33 |
| C22:5 | ||
| Per SD increment | 0.95 [0.85–1.03]/0.30 | 0.92 [0.80–1.02]/0.15 |
| T1 | 1 | 1 |
| T2 | 1.02 [0.77–1.35] | 0.89 [0.66–1.20] |
| T3 | 0.97 [0.73–1.30] | 0.81 [0.59–1.11] |
| p value for trend | 0.33 | 0.08 |
| C22:6 | ||
| Per SD increment | 0.96 [0.86–1.04]/0.39 | 0.93 [0.82–1.03]/0.20 |
| T1 | 1 | 1 |
| T2 | 0.96 [0.76–1.21] | 0.95 [0.75–1.22] |
| T3 | 0.77 [0.60–1.00] | 0.68 [0.52–0.88] |
| p value for trend | 0.24 | 0.11 |
| C18:2 | ||
| Per SD increment | 0.71 [0.64–0.78]/<0.001 | 0.71 [0.64–0.79]/<0.001 |
| T1 | 1 | 1 |
| T2 | 0.76 [0.59–0.97] | 0.77 [0.59–0.99] |
| T3 | 0.54 [0.42–0.72] | 0.52 [0.38–0.72] |
| p value for trend | <0.001 | <0.001 |
| C20:4 | ||
| Per SD increment | 0.93 [0.85–1.00]/0.06 | 0.85 [0.78–0.94]/<0.001 |
| T1 | 1 | 1 |
| T2 | 0.99 [0.78–1.27] | 0.88 [0.67–1.15] |
| T3 | 0.97 [0.76–1.23] | 0.75 [0.57–1.00] |
| p value for trend | 0.44 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Zheng, L.; Zeng, C. Polyunsaturated Fatty Acids and Reduced Risk of Low Muscle Mass in Adults. Nutrients 2025, 17, 858. https://doi.org/10.3390/nu17050858
Zou H, Zheng L, Zeng C. Polyunsaturated Fatty Acids and Reduced Risk of Low Muscle Mass in Adults. Nutrients. 2025; 17(5):858. https://doi.org/10.3390/nu17050858
Chicago/Turabian StyleZou, Haiyu, Liangrong Zheng, and Chunlai Zeng. 2025. "Polyunsaturated Fatty Acids and Reduced Risk of Low Muscle Mass in Adults" Nutrients 17, no. 5: 858. https://doi.org/10.3390/nu17050858
APA StyleZou, H., Zheng, L., & Zeng, C. (2025). Polyunsaturated Fatty Acids and Reduced Risk of Low Muscle Mass in Adults. Nutrients, 17(5), 858. https://doi.org/10.3390/nu17050858




