Urinary Iodine Concentration and Thyroid Hormone Metabolism in Pregnant Women and Neurodevelopment in Their Children: A Longitudinal Canadian Birth Cohort
Abstract
:1. Introduction
1.1. Iodine and Thyroid Hormone Metabolism
1.2. Maternal TH Metabolism and Iodine During Pregnancy
1.3. Assessment of Iodine Concentration
1.4. Iodine Deficiency and Iodine Excess and Maternal TH Metabolism
1.5. Maternal Iodine and Neurodevelopmental Outcomes
1.6. Aims and Hypotheses
2. Materials and Methods
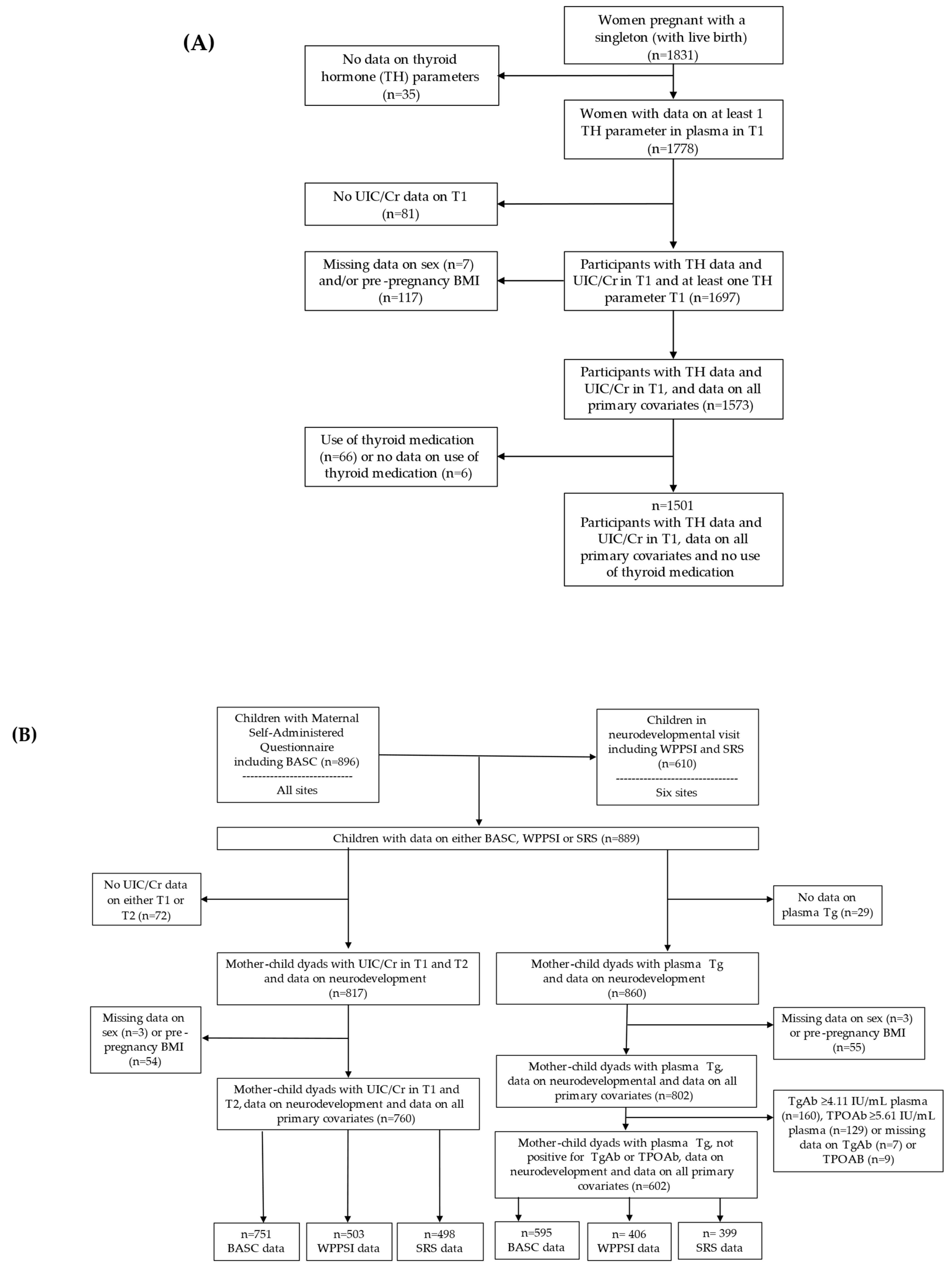
2.1. Cohort Profile
2.2. Iodine and Creatinine Concentrations During Pregnancy
2.3. Thyroid Parameters During Pregnancy
2.4. Neurodevelopmental Outcomes
2.4.1. Cognitive Function
2.4.2. Behavioural and Social Functioning
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Study Sample Characteristics
3.2. Urinary Iodine and Urinary Creatinine Concentration
3.3. Thyroid Parameters Concentrations During Pregnancy
3.4. Neurodevelopmental Outcomes in Offspring at 3–4 Years of Age
3.5. Associations Between Maternal UIC/Cr and Thyroid Parameters
3.6. Sensitivity Analyses
3.6.1. UIC/Cr and Thyroid Parameters Excluding Thyroid-Ab-pos
3.6.2. UIC/Cr Quartiles and Thyroid Parameters
3.6.3. UIC/Cr and Thyroid Parameters Excluding Low and High UCr
3.7. Associations Between Maternal UIC/Cr_Avg and Offspring Neurodevelopment
4. Discussion
4.1. Main Findings
4.2. Assessment of Urinary Iodine Concentrations in the MIREC Cohort
4.3. High UIC/Cr and Thyroid Parameters in T1
4.4. Low UIC/Cr and Thyroid Parameters in T1
4.5. Iodine Concentrations and Tg During Pregnancy and Neurodevelopmental Outcome
4.6. Deiodinase Activity and Sodium/Iodine Symporter (NIS)
4.7. Other Factors Influencing Thyroid Metabolism
4.8. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | autism spectrum disorder |
| BASC-2 | Behavior Assessment System for Children, Second Edition |
| β-hCG | human chorionic gonadotropin beta |
| BMI | body mass index |
| CES-D 10 | Center for Epidemiological Studies Depression Scale |
| D2 | deiodinase type 2 |
| D3 | deiodinase type 3 |
| EAR | estimated average requirement |
| hCG | human chorionic gonadotropin |
| HOME score | Home Observation for Measurement of the Environment score |
| HPT axis | hypothalamic-pituitary-thyroid axis |
| I− | iodide |
| ICP-MS | inductively coupled plasma mass spectrometry |
| INSPQ | Institut National de Santé Publique du Québec |
| IQ | intelligence quotient |
| IUCPQ | Institut Universitaire de Cardiologie et de Pneumologie de Québec |
| LOD | limit of detection |
| FSIQ | full-scale IQ |
| fT3 | free T3 |
| fT4 | free T4 |
| MSAQ | maternal self-administered questionnaire |
| MIREC study | Maternal–Infant Research on Environmental Chemicals study |
| MIREC-CD+ study | MIREC-Child Development Plus study |
| Na+, K+-ATPase | sodium potassium-activated adenosine 5′-triphosphatase pump |
| NIS | sodium/iodine symporter |
| PFAS | per- and polyfluoroalkyl substances |
| PIQ | performance IQ |
| rT3 | reverse T3 |
| SRS-2 | Social Responsiveness Scale, Second Edition |
| T1 | trimester 1 |
| T2 | trimester 2 |
| T3 | triiodothyronine |
| T4 | tetra-iodothyronine thyroxine |
| tT4 | total T4 |
| TBG | thyroxine-binding globulin |
| Tg | thyroglobulin |
| TgAb | Tg antibodies |
| TH | Thyroid hormone |
| Thyroid-Ab-pos | positive thyroid antibodies |
| TPO | thyroid peroxidase |
| TPOAb | TPO antibodies |
| TSH | thyroid-stimulating hormone |
| UCr | urinary creatine concentration |
| UIC | urinary iodine concentration |
| UIC/Cr | urinary iodine concentration divided by urinary creatinine concentration |
| UIC/Cr_Avg | average UIC/Cr of the two samples collected in the first and second trimester |
| UL | upper intake level |
| VIQ | verbal IQ |
| WHO | World Health Organization |
| WPPSI-III | Wechsler Preschool and Primary Scale of Intelligence, Third Edition |
References
- Ramhøj, L.; Axelstad, M.; Baert, Y.; Cañas-Portilla, A.I.; Chalmel, F.; Dahmen, L.; De La Vieja, A.; Evrard, B.; Haigis, A.; Hamers, T. New Approach Methods to Improve Human Health Risk Assessment of Thyroid Hormone System Disruption—A PARC Project. Front. Toxicol. 2023, 5, 1189303. [Google Scholar] [CrossRef]
- Coscia, F.; Taler-Verčič, A.; Chang, V.T.; Sinn, L.; O’Reilly, F.J.; Izoré, T.; Renko, M.; Berger, I.; Rappsilber, J.; Turk, D. The Structure of Human Thyroglobulin. Nature 2020, 578, 627–630. [Google Scholar] [CrossRef]
- Kansagra, S.M.; McCudden, C.R.; Willis, M.S. The Challenges and Complexities of Thyroid Hormone Replacement. Lab. Med. 2010, 41, 338–348. [Google Scholar] [CrossRef]
- Greenspan, F.S.; Gardner, D.G. Basic and Clinical Endocrinology, 7th ed.; McGraw-Hill Companies: New York, NY, USA, 2004; pp. 215–294. [Google Scholar]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess Iodine Intake: Sources, Assessment, and Effects on Thyroid Function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Trimarchi, F.; Vermiglio, F. Thyroid Physiology in Pregnancy. Endocr. Pract. 2014, 20, 589–596. [Google Scholar] [CrossRef]
- Petca, A.; Dimcea, D.A.; Dumitrașcu, M.C.; Șandru, F.; Mehedințu, C.; Petca, R. Management of Hyperthyroidism during Pregnancy: A Systematic Literature Review. J. Clin. Med. 2023, 12, 1811. [Google Scholar] [CrossRef]
- Ohara, N.; Tsujino, T.; Maruo, T. The Role of Thyroid Hormone in Trophoblast Function, Early Pregnancy Maintenance, and Fetal Neurodevelopment. J. Obstet. Gynaecol. Can. 2004, 26, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Teng, Y.; Ru, X.; Liu, Z.; Han, Y.; Tao, F.; Huang, K. Sex-Specific Effect of Maternal Thyroid Hormone Trajectories on Preschoolers’ Behavioral Development: A Birth Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e2037–e2046. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Russell, G.; Baragwanath, G.; Matthews, J.; Vaidya, B.; Thompson-Coon, J.O. Maternal Thyroid Hormone Insufficiency during Pregnancy and Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. Clin. Endocrinol. 2018, 88, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Methods to Assess Iron and Iodine Status. Br. J. Nutr. 2008, 99, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, S.; Zhang, X.; Xie, X.; Wang, D.; Mao, J.; Teng, X.; Shan, Z.; Teng, W. The Urine Iodine to Creatinine as an Optimal Index of Iodine during Pregnancy in an Iodine Adequate Area in China. J. Clin. Endocrinol. Metab. 2016, 101, 1290–1298. [Google Scholar] [CrossRef]
- Bilek, R.; Dvořáková, M.; Grimmichova, T.; Jiskra, J. Iodine, Thyroglobulin and Thyroid Gland. Physiol. Res. 2020, 69, S225. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.; Bülow, I.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Laurberg, P. Serum Tg—A Sensitive Marker of Thyroid Abnormalities and Iodine Deficiency in Epidemiological Studies. J. Clin. Endocrinol. Metab. 2001, 86, 3599–3603. [Google Scholar] [PubMed]
- Krzeczkowski, J.E.; Hall, M.; McGuckin, T.; Lanphear, B.; Bertinato, J.; Ayotte, P.; Chevrier, J.; Goodman, C.; Green, R.; Till, C. Iodine Status in a Large Canadian Pregnancy Cohort. Am. J. Obstet. Gynecol. MFM 2023, 5, 100784. [Google Scholar] [CrossRef] [PubMed]
- Mégier, C.; Dumery, G.; Luton, D. Iodine and Thyroid Maternal and Fetal Metabolism during Pregnancy. Metabolites 2023, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- Candido, A.C.; Vieira, A.A.; de Souza Ferreira, E.; Moreira, T.R.; do Carmo Castro Franceschini, S.; Cotta, R.M.M. Prevalence of Excessive Iodine Intake in Pregnancy and its Health Consequences: Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2023, 201, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Levie, D.; Derakhshan, A.; Shu, H.; Broeren, M.A.; De Poortere, R.A.; Peeters, R.P.; Bornehag, C.; Demeneix, B.; Korevaar, T.I. The Association of Maternal Iodine Status in Early Pregnancy with Thyroid Function in the Swedish Environmental Longitudinal, Mother and Child, Asthma and Allergy Study. Thyroid 2019, 29, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of Inadequate Iodine Status in UK Pregnant Women on Cognitive Outcomes in their Children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Reyes, R.; Glinoer, D.; Van Oyen, H.; Vandevijvere, S. High Prevalence of Thyroid Disorders in Pregnant Women in a Mildly Iodine-Deficient Country: A Population-Based Study. J. Clin. Endocrinol. Metab. 2013, 98, 3694–3701. [Google Scholar] [CrossRef]
- Derakhshan, A.; Korevaar, T.I.; Taylor, P.N.; Levie, D.; Guxens, M.; Jaddoe, V.W.; Nelson, S.M.; Tiemeier, H.; Peeters, R.P. The Association of Maternal Thyroid Autoimmunity during Pregnancy with Child IQ. J. Clin. Endocrinol. Metab. 2018, 103, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Ohashi, T.; Yamaguchi, S.; Yamaguchi, M.; Shishiba, Y.; Irie, M. Iodine Status of Pregnant and Postpartum Japanese Women: Effect of Iodine Intake on Maternal and Neonatal Thyroid Function in an Iodine-Sufficient Area. J. Clin. Endocrinol. Metab. 2011, 96, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Orito, Y.; Oku, H.; Kubota, S.; Amino, N.; Shimogaki, K.; Hata, M.; Manki, K.; Tanaka, Y.; Sugino, S.; Ueta, M. Thyroid Function in Early Pregnancy in Japanese Healthy Women: Relation to Urinary Iodine Excretion, Emesis, and Fetal and Child Development. J. Clin. Endocrinol. Metab. 2009, 94, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Levie, D.; Bath, S.C.; Guxens, M.; Korevaar, T.I.; Dineva, M.; Fano, E.; Ibarluzea, J.M.; Llop, S.; Murcia, M.; Rayman, M.P. Maternal Iodine Status during Pregnancy is Not Consistently Associated with Attention-Deficit Hyperactivity Disorder or Autistic Traits in Children. J. Nutr. 2020, 150, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.M.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; Dyer, M.; van Wijngaarden, E.; Yeates, A.J. Associations between Maternal Urinary Iodine Assessment, Dietary Iodine Intakes and Neurodevelopmental Outcomes in the Child: A Systematic Review. Thyroid. Res. 2021, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T.A.; Guxens, M.; Rebagliato, M.L.; Dineva, M.; Bath, S.C.; Hunziker, S.; Sunyer, J.; Delgado-Saborit, J.M.; Irizar Loibide, A.; Lertxundi, N. Association of Maternal Thyroglobulin with Gestational Thyroid Function and Offspring IQ and Brain Morphology. J. Clin. Endocrinol. Metab. 2024, dgae679. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global Iodine Nutrition: Where do we Stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Qiao, C.; L’Abbé, M.R. Iodine Status of Canadian Children, Adolescents, and Women of Childbearing Age. J. Nutr. 2021, 151, 3710–3717. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Fraser, W.D.; Fisher, M.; Davis, K.; Liang, C.L.; Lupien, N.; Bastien, S.; Velez, M.P.; von Dadelszen, P.; Hemmings, D.G. Cohort Profile: The Maternal-infant Research on Environmental Chemicals Research Platform. Paediatr. Perinat. Epidemiol. 2013, 27, 415–425. [Google Scholar] [CrossRef]
- Fisher, M.; Muckle, G.; Lanphear, B.; Arbuckle, T.E.; Braun, J.M.; Zidek, A.; Vélez, M.P.; Lupien, N.; Bastien, S.; Ashley-Martin, J. Cohort Profile Update: The Canadian Maternal–Infant Research on Environmental Chemicals Child Development Study (MIREC-CD PLUS). Paediatr. Perinat. Epidemiol. 2023, 37, 719–732. [Google Scholar] [CrossRef]
- Goodman, C.V.; Hall, M.; Green, R.; Chevrier, J.; Ayotte, P.; Martinez-Mier, E.A.; McGuckin, T.; Krzeczkowski, J.; Flora, D.; Hornung, R. Iodine Status Modifies the Association between Fluoride Exposure in Pregnancy and Preschool Boys’ Intelligence. Nutrients 2022, 14, 2920. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Till, C.; Green, R.; Grundy, J.G.; Hornung, R.; Neufeld, R.; Martinez-Mier, E.A.; Ayotte, P.; Muckle, G.; Lanphear, B. Community Water Fluoridation and Urinary Fluoride Concentrations in a National Sample of Pregnant Women in Canada. Environ. Health Perspect. 2018, 126, 107001. [Google Scholar] [CrossRef]
- Bath, S.C.; Pop, V.J.; Furmidge-Owen, V.L.; Broeren, M.A.; Rayman, M.P. Thyroglobulin as a Functional Biomarker of Iodine Status in a Cohort Study of Pregnant Women in the United Kingdom. Thyroid 2017, 27, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Konrade, I.; Kalere, I.; Strele, I.; Makrecka-Kuka, M.; Jekabsone, A.; Tetere, E.; Veisa, V.; Gavars, D.; Rezeberga, D.; Pīrāgs, V. Iodine Deficiency during Pregnancy: A National Cross-Sectional Survey in Latvia. Public Health Nutr. 2015, 18, 2990–2997. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, C.; Zhang, X.; Shan, Z.; Teng, W. Reference Intervals of the Ratio of Urine Iodine to Creatinine in Pregnant Women in an Iodine-Replete Area of China. Biol. Trace Elem. Res. 2021, 199, 62–69. [Google Scholar] [CrossRef]
- Hall, M.; Hornung, R.; Chevrier, J.; Ayotte, P.; Lanphear, B.; Till, C. Fluoride Exposure and Thyroid Hormone Levels in Pregnancy: The MIREC Cohort. Environ. Int. 2024, 184, 108442. [Google Scholar] [CrossRef]
- Abbott Laboratories. Architect Anti-Tg (2K46) User Manual; Abbott Laboratories: Chicago, IL, USA, 2015. [Google Scholar]
- Abbott Laboratories. Architect Anti-TPO (2K47) User Manual; Abbott Laboratories: Chicago, IL, USA, 2015. [Google Scholar]
- Wechsler, D. Wechsler Pre-School and Primary Scale of Intelligence III Administration and Scoring Manual; Psychological Corporation: San Antonio, TX, USA, 2002. [Google Scholar]
- Wechsler, D. WPPSI-III: Wechsler Preschool and Primary Scale of Intelligence–Third Edition: Canadian Manual; Harcourt Assessment: Toronto, ON, Canada, 2004. [Google Scholar]
- Reynolds, C.R.; Kamphaus, R.W. Behavior Assessment System for Children, (BASC-2); American Guidance Service: Circle Pines, MN, USA, 2004. [Google Scholar]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale, 2nd ed.; Western Psychology Services: Torrance, CA, USA, 2012. [Google Scholar]
- Abbott Laboratories. Architect Thyroglobulin (5P20) User Manual; Abbott Laboratories: Chicago, IL, USA, 2018. [Google Scholar]
- Walsh, J.P.; Bremner, A.P.; Feddema, P.; Leedman, P.J.; Brown, S.J.; O’Leary, P. Thyrotropin and Thyroid Antibodies as Predictors of Hypothyroidism: A 13-Year, Longitudinal Study of a Community-Based Cohort using Current Immunoassay Techniques. J. Clin. Endocrinol. Metab. 2010, 95, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary Creatinine Concentrations in the US Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Lanphear, B.; Chevrier, J.; Hornung, R.; Green, R.; Goodman, C.; Ayotte, P.; Martinez-Mier, E.A.; Zoeller, R.T.; Till, C. Fluoride Exposure and Hypothyroidism in a Canadian Pregnancy Cohort. Sci. Total Environ. 2023, 869, 161149. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Furmidge-Owen, V.L.; Redman, C.W.; Rayman, M.P. Gestational Changes in Iodine Status in a Cohort Study of Pregnant Women from the United Kingdom: Season as an Effect Modifier. Am. J. Clin. Nutr. 2015, 101, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Mullan, K.; McMullan, P.; Kayes, L.; McCance, D.; Hunter, A.; Woodside, J.V. Thyroglobulin Levels among Iodine Deficient Pregnant Women Living in Northern Ireland. Eur. J. Clin. Nutr. 2022, 76, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Levie, D.; Korevaar, T.I.; Bath, S.C.; Murcia, M.; Dineva, M.; Llop, S.; Espada, M.; Van Herwaarden, A.E.; De Rijke, Y.B.; Ibarluzea, J.M. Association of Maternal Iodine Status with Child IQ: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab. 2019, 104, 5957–5967. [Google Scholar] [CrossRef] [PubMed]
- Balucan, F.S.; Morshed, S.A.; Davies, T.F. Thyroid Autoantibodies in Pregnancy: Their Role, Regulation and Clinical Relevance. J. Thyroid. Res. 2013, 2013, 182472. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Boelaert, K. Iodine Deficiency and Thyroid Disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Increased Sensitivity of the Thyroid in Iodine-Depleted Rats to the Goitrogenic Effects of Thyrotropin. J. Clin. Investig. 1968, 47, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Dineva, M.; Fishpool, H.; Rayman, M.P.; Mendis, J.; Bath, S.C. Systematic Review and Meta-Analysis of the Effects of Iodine Supplementation on Thyroid Function and Child Neurodevelopment in Mildly-to-Moderately Iodine-Deficient Pregnant Women. Am. J. Clin. Nutr. 2020, 112, 389–412. [Google Scholar] [CrossRef]
- Ghassabian, A.; Steenweg-de Graaff, J.; Peeters, R.P.; Ross, H.A.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; White, T.; Tiemeier, H. Maternal Urinary Iodine Concentration in Pregnancy and Children’s Cognition: Results from a Population-Based Birth Cohort in an Iodine-Sufficient Area. BMJ Open 2014, 4, e005520. [Google Scholar] [CrossRef]
- Zhou, S.J.; Skeaff, S.A.; Ryan, P.; Doyle, L.W.; Anderson, P.J.; Kornman, L.; Mcphee, A.J.; Yelland, L.N.; Makrides, M. The Effect of Iodine Supplementation in Pregnancy on Early Childhood Neurodevelopment and Clinical Outcomes: Results of an Aborted Randomised Placebo-Controlled Trial. Trials 2015, 16, 563. [Google Scholar] [CrossRef]
- Gowachirapant, S.; Jaiswal, N.; Melse-Boonstra, A.; Galetti, V.; Stinca, S.; Mackenzie, I.; Thomas, S.; Thomas, T.; Winichagoon, P.; Srinivasan, K. Effect of Iodine Supplementation in Pregnant Women on Child Neurodevelopment: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Richard, K.; McKinnon, B.; Mortimer, R.H. Effect of Iodide on Human Choriogonadotropin, Sodium-Iodide Symporter Expression, and Iodide Uptake in BeWo Choriocarcinoma Cells. J. Clin. Endocrinol. Metab. 2007, 92, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Grossklaus, R.; Liesenkötter, K.; Doubek, K.; Völzke, H.; Gaertner, R. Iodine Deficiency, Maternal Hypothyroxinemia and Endocrine Disrupters Affecting Fetal Brain Development: A Scoping Review. Nutrients 2023, 15, 2249. [Google Scholar] [CrossRef] [PubMed]
- Soechitram, S.D.; Berghuis, S.A.; Visser, T.J.; Sauer, P.J. Polychlorinated Biphenyl Exposure and Deiodinase Activity in Young Infants. Sci. Total Environ. 2017, 574, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.T. Fluoride Exposure Induces Inhibition of Sodium/Iodide Symporter (NIS) Contributing to Impaired Iodine Absorption and Iodine Deficiency: Molecular Mechanisms of Inhibition and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 1086. [Google Scholar] [CrossRef]
- Mervish, N.A.; Pajak, A.; Teitelbaum, S.L.; Pinney, S.M.; Windham, G.C.; Kushi, L.H.; Biro, F.M.; Valentin-Blasini, L.; Blount, B.C.; Wolff, M.S. Thyroid Antagonists (Perchlorate, Thiocyanate, and Nitrate) and Childhood Growth in a Longitudinal Study of US Girls. Environ. Health Perspect. 2016, 124, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.E.; Wang, J.; Murr, A.S.; Bailey, J.R.; Buckalew, A.R. High-Throughput Screening of ToxCast PFAS Chemical Library for Potential Inhibitors of the Human Sodium Iodide Symporter. Chem. Res. Toxicol. 2023, 36, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.M.; Rauch, S.A.; Marie, N.S.; Mattman, A.; Lanphear, B.P.; Venners, S.A. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in US Adults: Variation According to TPOAb and Iodine Status (NHANES 2007–2008). Environ. Health Perspect. 2016, 124, 935–942. [Google Scholar] [CrossRef]
- Andersen, S.L.; Sørensen, L.K.; Krejbjerg, A.; Møller, M.; Laurberg, P. Challenges in the Evaluation of Urinary Iodine Status in Pregnancy: The Importance of Iodine Supplement Intake and Time of Sampling. Eur. Thyroid. J. 2014, 3, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Knudsen, N.; Pedersen, I.B.; Rasmussen, L.B.; Carlé, A.; Vejbjerg, P. The Danish Investigation on Iodine Intake and Thyroid Disease, DanThyr: Status and Perspectives. Eur. J. Endocrinol. 2006, 155, 219–228. [Google Scholar] [CrossRef]
- World Health Organization. Biological Monitoring of Chemical Exposure in the Workplace: Guidelines; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Gołkowski, F.; Buziak-Bereza, M.; Trofimiuk, M.; Bałdys-Waligórska, A.; Szybiński, Z.; Huszno, B. Increased Prevalence of Hyperthyroidism as an Early and Transient Side-Effect of Implementing Iodine Prophylaxis. Public Health Nutr. 2007, 10, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Delange, F.; De Benoist, B.; Alnwick, D. Risks of Iodine-Induced Hyperthyroidism After Correction of Iodine Deficiency by Iodized Salt. Thyroid 1999, 9, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.Y.; Inoue, K.; Rhee, C.M.; Leung, A.M. Risks of Iodine Excess. Endocr. Rev. 2024, 45, 858–879. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Mao, J.; Wang, W.; Xie, X.; Peng, S.; Wang, Z.; Han, C.; Zhang, X.; Wang, D. Gestation-specific Changes in Maternal Thyroglobulin during Pregnancy and Lactation in an Iodine-sufficient Region in China: A Longitudinal Study. Clin. Endocrinol. 2017, 86, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, M.; Yang, L.; Wu, J.; Hu, Y.; Han, J.; Gu, Y.; Li, X.; Wang, H.; Ma, L. Evaluation of Median Urinary Iodine Concentration Cut-Off for Defining Iodine Deficiency in Pregnant Women After a Long Term USI in China. Nutr. Metab. 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]

| Thyroid Hormone Data and UIC/Cr T1 a (n = 1501) | Neurodevelopmental Data and UIC/Cr_Avg b (n = 760) | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age at pregnancy (years; mean ± SD) | 32.3 ± 5.0 | 32.8 ± 4.7 |
| Race (n; %) | ||
| White | 1287 (86%) | 690 (91%) |
| Other | 214 (14%) | 70 (9%) |
| Level of education of mother at pregnancy (n; %) | ||
| College diploma or less | 551 (37%) | 241 (32%) |
| University undergraduate or graduate degree | 950 (63%) | 519 (68%) |
| Pre-pregnancy BMI (kg/m2; mean; SD) | 24.8 ± 5.4 | 25.0 ± 5.6 |
| Parity (n; %) | ||
| 0 | 661 (44%) | 339 (45%) |
| 1 | 611 (41%) | 310 (41%) |
| ≥2 | 229 (15%) | 111 (15%) |
| CESD-10 mother (n = 731; mean; range) | ∙ | 5.6 (0–23) |
| Alcohol use during pregnancy c (n; %) | 250 (18%) | 134 (18%) |
| Second-hand smoke during pregnancy d (n; %) | 83 (5.5%) | 34 (4.5%) |
| Use of thyroid medication e (n; %) | ∙ | 32 (4.2%) |
| Maternal thyroid auto antibodies in T1 | ||
| TgAb-positive f (≥4.11 IU/mL plasma; n; %) | 279 (19%) | 144 (19%) |
| TPOAb-positive g (≥5.61 IU/mL plasma; n; %) | 192 (13%) | 120 (17%) |
| Use of prenatal vitamins h | 1314 (88%) | 660 (87%) |
| Child characteristics | ||
| Child male sex (n; %) | 800 (53%) | 364 (48%) |
| Gestational age in weeks at birth (mean ± SD) | ∙ | 39.5 ± 1.6 |
| Number of months exclusively breastfeeding (n = 754; mean ± SD) | ∙ | 5.0 ± 3.2 |
| HOME total score (n = 489; mean ± SD) | ∙ | 47.5 ± 4.3 |
| Child age at WPPSI and SRS in years (n = 505; mean ± SD) | ∙ | 3.4 ± 0.3 |
| Thyroid Parameter Data and UIC/Cr T1 a (n = 1501) | Neurodevelopmental Data and UIC/Cr T1 and T2 b (n = 760) | |
|---|---|---|
| UIC/Cr Average T1 and T2 (μg/g; median; IQR) | ∙ | 310.5 (216.2–446.6) c |
| <150 μg/g | ∙ | 77 (10%) |
| 150–500 μg/g | ∙ | 549 (72%) |
| ≥500 μg/g | ∙ | 134 (18%) |
| UIC/Cr T1 (μg/g; median; IQR) | 266.5 (162.8–423.7) c,d | 275.0 (169.6–456.8) c,d |
| <150 μg/g | 328 (22%) | 142 (19%) |
| 150–500 μg/g | 925 (62%) | 479 (63%) |
| ≥500 μg/g | 248 (17%) | 139 (18%) |
| UIC/Cr T2 (μg/g; median; IQR) | ∙ | 317.6 (200.7–463.5) c,e |
| <150 μg/g | ∙ | 102 (13%) |
| 150–500 μg/g | ∙ | 497 (65%) |
| ≥500 μg/g | ∙ | 161 (21%) |
| Tg in plasma (ng/mL; median; IQR) | 13.9 (8.7–21.5) f | 14.2 (8.4–21.7) g |
| Urinary creatinine T1 (mg/dL; mean ± SD) | 76.9 ± 59.7 | 75.5 ± 57.7 |
| Urinary creatinine T2 (mg/dL; mean ± SD) | ∙ | 69.9 ± 52.2 |
| Unadjusted urinary iodine T1 (μg/L; median; IQR) | 151.9 (75.9–278.5) c,d | 158.2 (77.2–303.8) c,d |
| <150 μg/L (n; %; “Insufficient”) | 717 (48%) | 350 (46%) |
| 150–249 μg/L (n; %; “Adequate”) | 341 (23%) | 169 (22%) |
| 250–499 μg/L (n; %; “Above requirements”) | 326 (22%) | 172 (23%) |
| ≥500 μg/L (n; %; “Excessive”) | 117 (8%) | 69 (9%) |
| Unadjusted urinary iodine T2 (μg/L; median; IQR) | ∙ | 164.6 (88.6–303.8) c,e |
| <150 μg/L (n; %; “Insufficient”) | ∙ | 336 (44%) |
| 150–249 μg/L (n; %; “Adequate”) | ∙ | 173 (23%) |
| 250–499 μg/L (n; %; “Above requirements”) | ∙ | 184 (24%) |
| ≥500 μg/L (n; %; “Excessive”) | ∙ | 67 (9%) |
| UIC/Cr Trimester 1 (μg/g) | TSH (μIU/mL) a (n = 1426) | tT4 (ng/mL) (n = 1499) | fT4 (pg/mL) (n = 1478) | Tg (ng/mL) b (n = 1485) | TgAb (IU/mL) c (n = 1481) | TPOAb (IU/mL) d (n = 1474) | TgAb ≥ 4.11 IU/mL; n (% of Participants in Category) (n = 1481) | TPOAb ≥ 5.61 IU/mL; n (% of Participants in Category) (n = 1474) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <150 | 308 | 1.26 ± 1.07 e | 328 | 107.1 ± 19.2 | 322 | 13.4 ± 2.4 | 324 | 20.2 ± 18.5 e | 322 | 12.3 ± 57.1 | 320 | 25.0 ± 98.5 | 61 (19%) | 47 (15%) |
| 150–500 | 885 | 1.37 ± 0.98 e | 924 | 104.8 ± 20.8 | 911 | 13.5 ± 2.7 | 915 | 17.0 ± 15.7 e | 913 | 10.9 ± 53.9 | 908 | 21.2 ± 83.8 f | 184 (20%) f | 125 (14%) f |
| ≥500 | 233 | 1.38 ± 0.82 | 247 | 106.2 ± 20.5 | 245 | 13.3 ± 2.5 | 246 | 16.3 ± 11.9 | 246 | 7.7 ± 39.9 | 246 | 19.3 ± 105.7 f | 34 (14%) f | 20 (8%) f |
| Neurodevelopmental Outcomes | n | Boys | Girls |
|---|---|---|---|
| WPPSI Full Scale IQ (mean ± SD) | 503 | 104.5 ± 14.2 | 109.2 ± 12.1 |
| WPPSI Verbal IQ (mean ± SD) | 500 | 107.1 ± 13.8 | 111.9 ± 12.1 |
| WPPSI Performance IQ (mean ± SD) | 498 | 101.4 ± 15.1 | 104.4 ± 14.0 |
| SRS-2 Total T-score (mean ± SD) | 498 | 46.6 ± 6.7 | 44.3 ± 5.6 |
| BASC-2 T-score Composite score Externalizing Problems (mean ± SD) | 751 | 50.7 ± 8.2 | 48.0 ± 7.7 |
| BASC-2 T-score Composite score Internalizing Problems (mean ± SD) | 742 | 52.0 ± 8.4 | 52.2 ± 8.7 |
| Thyroid Parameter | UIC/Cr T1 Categories | Primary Model a | Secondary Model b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | B | 95% CI | p | n | B | 95% CI | p | ||
| Log TSH (µIU/mL) | <150 versus 150–500 µg/g | 1426 | −0.11 | −0.23, −0.00 | 0.05 | 1305 | −0.10 | −0.22, 0.02 | 0.11 |
| ≥500 versus 150–500 µg/g | −0.02 | −0.14, 0.10 | 0.75 | −0.01 | −0.14, 0.12 | 0.91 | |||
| Log fT4 (pg/mL) | <150 versus 150–500 µg/g | 1478 | −0.00 | −0.03, 0.02 | 0.67 | 1354 | −0.01 | −0.03, 0.02 | 0.48 |
| ≥500 versus 150–500 µg/g | −0.02 | −0.05, 0.01 | 0.12 | −0.02 | −0.04, 0.01 | 0.20 | |||
| tT4 (ng/mL) | <150 versus 150–500 µg/g | 1499 | 1.74 | −0.81, 4.28 | 0.18 | 1372 | 2.35 | −0.34, 5.04 | 0.09 |
| ≥500 versus 150–500 µg/g | 1.28 | −1.54, 4.11 | 0.37 | 1.77 | −1.13, 4.68 | 0.23 | |||
| Log Tg (ng/mL) | <150 versus 150–500 µg/g | 1485 | 0.19 | 0.05, 0.33 | 0.01 | 1358 | 0.18 | 0.03, 0.33 | 0.02 |
| ≥500 versus 150–500 µg/g | 0.03 | −0.13, 0.18 | 0.74 | 0.03 | −0.13, 0.19 | 0.72 | |||
| Logistic Regression | |||||||||
| TgAb ≥ 4.11 IU/mL (versus <4.11) | <150 versus 150–500 µg/g | 1481 | −0.06 | −0.39, 0.27 | 0.72 | 1354 | −0.06 | −0.41, 0.29 | 0.75 |
| ≥500 versus 150–500 µg/g | −0.46 | −0.86, −0.06 | 0.03 | −0.44 | −0.86, −0.03 | 0.04 | |||
| TPOAb ≥ 5.61 IU/mL (versus <5.61) | <150 versus 150–500 µg/g | 1474 | 0.11 | −0.25, 0.48 | 0.54 | 1348 | 0.05 | −0.35, 0.44 | 0.82 |
| ≥500 versus 150–500 µg/g | −0.60 | −1.10, −0.10 | 0.02 | −0.62 | −1.13, −0.11 | 0.02 | |||
| Primary Model a | Secondary Model b | Interaction Term Sex c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | B | 95% CI | p | n | B | 95% CI | p | p | ||
| WPPSI-Full Scale IQ | UIC/Cr Average T1 and T2 (μg/g × 0.01) | 503 | −0.03 | −0.36, 0.30 | 0.86 | 455 | 0.01 | −0.32, 0.33 | 0.97 | 0.27 |
| <150 versus 150–500 µg/g | −0.37 | −3.94, 3.21 | 0.84 | −1.45 | −5.05, 2.14 | 0.43 | ||||
| ≥500 versus 150–500 µg/g | −0.56 | −3.60, 2.47 | 0.72 | −0.85 | −3.93, 2.24 | 0.59 | ||||
| Log Tg d | 406 | 0.17 | −1.72, 2.05 | 0.86 | 361 | 0.56 | −1.35, 2.46 | 0.57 | 0.88 | |
| WPPSI-Verbal IQ | UIC/Cr Average T1 and T2 (μg/g × 0.01) | 500 | −0.05 | −0.37, 0.27 | 0.74 | 452 | −0.03 | −0.35, 0.29 | 0.85 | 0.32 |
| <150 versus 150–500 µg/g | 0.23 | −3.29, 3.75 | 0.90 | −0.63 | −4.21, 2.94 | 0.73 | ||||
| ≥500 versus 150–500 µg/g | 0.10 | −2.86, 3.07 | 0.95 | −0.38 | −3.41, 2.66 | 0.81 | ||||
| Log Tg d | 404 | −0.19 | −1.99, 1.61 | 0.84 | 359 | 0.19 | −1.68, 2.06 | 0.84 | 0.89 | |
| WPPSI-Performance IQ | UIC/Cr Average T1 and T2 (μg/g × 0.01) | 498 | 0.02 | −0.34, 0.38 | 0.90 | 451 | 0.06 | −0.30, 0.43 | 0.73 | 0.31 |
| <150 versus 150–500 µg/g | −0.75 | −4.69, 3.18 | 0.71 | −1.69 | −5.72, 2.34 | 0.41 | ||||
| ≥500 versus 150–500 µg/g | −1.10 | −4.46, 2.26 | 0.52 | −0.99 | −4.47, 2.48 | 0.57 | ||||
| Log Tg d | 401 | 0.38 | −1.69, 2.44 | 0.72 | 357 | 0.86 | −1.27, 2.98 | 0.43 | 0.89 | |
| SRS-Total T-Score | UIC/Cr Average T1 and T2 (μg/g × 0.01) | 498 | 451 | |||||||
| <150 versus 150–500 µg/g | −0.53 | −2.23, 1.18 | 0.54 | −0.13 | −1.85, 1.59 | 0.88 | ||||
| ≥500 versus 150–500 µg/g | −1.26 | −2.70, 0.18 | 0.09 | −1.06 | −2.53, 0.41 | 0.16 | ||||
| Log Tg d | 399 | −0.12 | −1.02, 0.78 | 0.80 | 356 | −0.14 | −1.07, 0.78 | 0.77 | 0.04 | |
| boys as reference | 399 | −1.07 | −2.33, 0.19 | 0.10 | 356 | −1.20 | −2.48, 0.09 | 0.07 | ||
| girls as reference | 399 | 0.83 | −0.43, 2.10 | 0.20 | 356 | 0.96 | −0.35, 2.27 | 0.15 | ||
| BASC-T-score Composite score Externalizing Problems | UIC/Cr Average T1 and T2 (μg/g × 0.01) | 751 | 455 | |||||||
| <150 versus 150–500 µg/g | 0.57 | −1.35, 2.50 | 0.56 | 0.62 | −1.62, 2.86 | 0.59 | ||||
| ≥500 versus 150–500 µg/g | −0.97 | −2.51, 0.58 | 0.22 | −1.07 | −2.98, 0.85 | 0.27 | ||||
| Log Tg d | 595 | −0.13 | −1.06, 0.79 | 0.77 | 361 | 0.12 | −1.09, 1.33 | 0.85 | 0.74 | |
| BASC-T-score Composite score Internalizing Problems | UIC/Cr Average T1 and T2 (μg/g × 0.01) | 744 | 453 | |||||||
| <150 versus 150–500 µg/g | 0.55 | −1.52, 2.62 | 0.60 | 1.45 | −0.83, 3.74 | 0.21 | ||||
| ≥500 versus 150–500 µg/g | 0.87 | −0.79, 2.53 | 0.30 | 0.94 | −1.02, 2.91 | 0.35 | ||||
| Log Tg d | 588 | −0.83 | −1.81, 0.15 | 0.10 | 359 | −0.02 | −1.24, 1.21 | 0.98 | 0.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berghuis, S.A.; Hall, M.; Krzeczkowski, J.E.; Goodman, C.V.; Chevrier, J.; Ayotte, P.; Lanphear, B.; Till, C. Urinary Iodine Concentration and Thyroid Hormone Metabolism in Pregnant Women and Neurodevelopment in Their Children: A Longitudinal Canadian Birth Cohort. Nutrients 2025, 17, 830. https://doi.org/10.3390/nu17050830
Berghuis SA, Hall M, Krzeczkowski JE, Goodman CV, Chevrier J, Ayotte P, Lanphear B, Till C. Urinary Iodine Concentration and Thyroid Hormone Metabolism in Pregnant Women and Neurodevelopment in Their Children: A Longitudinal Canadian Birth Cohort. Nutrients. 2025; 17(5):830. https://doi.org/10.3390/nu17050830
Chicago/Turabian StyleBerghuis, Sietske A., Meaghan Hall, John E. Krzeczkowski, Carly V. Goodman, Jonathan Chevrier, Pierre Ayotte, Bruce Lanphear, and Christine Till. 2025. "Urinary Iodine Concentration and Thyroid Hormone Metabolism in Pregnant Women and Neurodevelopment in Their Children: A Longitudinal Canadian Birth Cohort" Nutrients 17, no. 5: 830. https://doi.org/10.3390/nu17050830
APA StyleBerghuis, S. A., Hall, M., Krzeczkowski, J. E., Goodman, C. V., Chevrier, J., Ayotte, P., Lanphear, B., & Till, C. (2025). Urinary Iodine Concentration and Thyroid Hormone Metabolism in Pregnant Women and Neurodevelopment in Their Children: A Longitudinal Canadian Birth Cohort. Nutrients, 17(5), 830. https://doi.org/10.3390/nu17050830







