Breastfeeding Shapes the Gut Microbiota and Its Structure Is Associated with Weight Gain Trajectories in Mexican Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Measurements
2.2. Maternal Diet
2.3. Breast Milk Composition
2.4. Microbiota Analysis
2.5. Bioinformatics and Statistical Analysis
3. Results
3.1. Maternal Diet and Breast Milk Composition
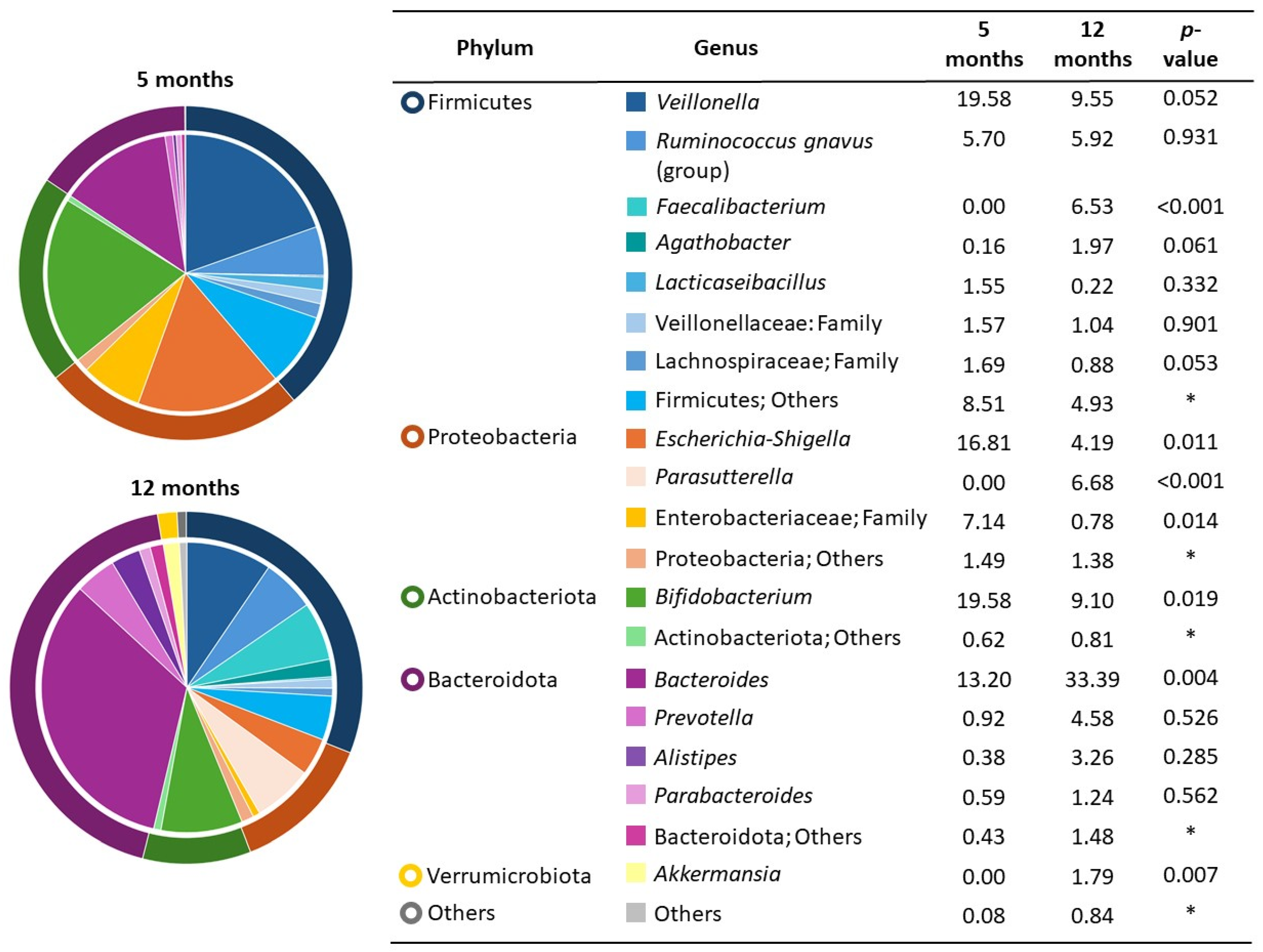
3.2. Infant Fecal Microbiota and Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, M.; Lamb, K.E.; Grimes, C.; Laws, R.; Bolton, K.; Ong, K.K.; Campbell, K. Rapid Weight Gain during Infancy and Subsequent Adiposity: A Systematic Review and Meta-Analysis of Evidence. Obes. Rev. 2018, 19, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Leth-Møller, M.; Kampmann, U.; Hede, S.; Ovesen, P.G.; Hulman, A.; Knorr, S. Breastfeeding and Infant Growth in Relation to Childhood Overweight—A Longitudinal Cohort Study. Am. J. Clin. Nutr. 2025; in press. [Google Scholar] [CrossRef]
- Zheng, M.; Hesketh, K.D.; Vuillermin, P.; Dodd, J.; Wen, L.M.; Baur, L.A.; Taylor, R.; Byrne, R.; Mihrshahi, S.; Sly, P.D.; et al. Determinants of Rapid Infant Weight Gain: A Pooled Analysis of Seven Cohorts. Pediatr. Obes. 2022, 17, e12928. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ramírez, A.D.; Murillo-Zamora, E.; Trujillo-Hernández, B.; Delgado-Enciso, I.; Sánchez-Ramírez, C.A. Birth Weight, Slowness in Eating and Feeding Practices as Independent Determinants of Rapid Weight Gain. Acta Paediatr. 2024, 113, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Shinn, L.M.; Tangney, C.C.; Busche, C.; Sharp, C.M.; Mullen, M.C. Demographic Correlates of Infant Feeding Practices and Growth Performance in the First Year of Life. Int. J. Pediatr. 2018, 2018, 6569204. [Google Scholar] [CrossRef]
- Li, Y.; Ren, L.; Wang, Y.; Li, J.; Zhou, Q.; Peng, C.; Li, Y.; Cheng, R.; He, F.; Shen, X. The Effect of Breast Milk Microbiota on the Composition of Infant Gut Microbiota: A Cohort Study. Nutrients 2022, 14, 5397. [Google Scholar] [CrossRef]
- Larsen, J.K.; Bode, L. Obesogenic Programming Effects during Lactation: A Narrative Review and Conceptual Model Focusing on Underlying Mechanisms and Promising Future Research Avenues. Nutrients 2021, 13, 299. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut Microbiota and BMI throughout Childhood: The Role of Firmicutes, Bacteroidetes, and Short-Chain Fatty Acid Producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Baek, G.H.; Yoo, K.M.; Kim, S.Y.; Lee, D.H.; Chung, H.; Jung, S.C.; Park, S.K.; Kim, J.S. Collagen Peptide Exerts an Anti-Obesity Effect by Influencing the Firmicutes/Bacteroidetes Ratio in the Gut. Nutrients 2023, 15, 2610. [Google Scholar] [CrossRef]
- Fricker, A.D.; Sejane, K.; Desai, M.; Snyder, M.W.; Duran, L.; Mackelprang, R.; Bode, L.; Ross, M.G.; Flores, G.E. A Pilot Study Exploring the Relationship Between Milk Composition and Microbial Capacity in Breastfed Infants. Nutrients 2025, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Silva-Zolezzi, I.; Hidalgo-Miranda, A.; Estrada-Gil, J.; Fernandez-Lopez, J.C.; Uribe-Figueroa, L.; Contreras, A.; Balam-Ortiz, E.; del Bosque-Plata, L.; Velazquez-Fernandez, D.; Lara, C.; et al. Analysis of Genomic Diversity in Mexican Mestizo Populations to Develop Genomic Medicine in Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 8611–8616. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Gaona-Pineda, E.B.; Cuevas-Nasu, L.; Morales-Ruan, C.; Valenzuela-Bravo, D.G.; Humaran, I.M.G.; Ávila-Arcos, M.A. Prevalencias de Sobrepeso y Obesidad en Población Escolar y Adolescente de México. Ensanut Continua 2020–2022. Salud Publica Mex 2023, 65, s218–s224. [Google Scholar] [CrossRef] [PubMed]
- González-Castell, L.D.; Unar-Munguía, M.; Bonvecchio-Arenas, A.; Rivera-Pasquel, M.; Lozada-Tequeanes, A.L.; Ramírez-Silva, C.I.; Álvarez-Peña, I.J.; Cobo-Armijo, F.; Rivera-Dommarco, J. Lactancia Materna. Salud Publica Mex. 2024, 66, 500–510. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; ISBN 924154693X. [Google Scholar]
- Ramirez-Silva, I.; Rivera, J.A.; Trejo-Valdivia, B.; Stein, A.D.; Martorell, R.; Romieu, I.; Barraza-Villarreal, A.; Avila-Jiménez, L.; Ramakrishnan, U. Relative Weight Gain Through Age 4 Years is Associated with Increased Adiposity, and Higher Blood Pressure and Insulinemia at 4–5 Years of Age in Mexican Children. J. Nutr. 2018, 148, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Adair, L.S.; Fall, C.H.; Osmond, C.; Stein, A.D.; Martorell, R.; Ramirez-Zea, M.; Sachdev, H.S.; Dahly, D.L.; Bas, I.; Norris, S.A.; et al. Associations of Linear Growth and Relative Weight Gain during Early Life with Adult Health and Human Capital in Countries of Low and Middle Income: Findings from Five Birth Cohort Studies. Lancet 2013, 382, 525–534. [Google Scholar] [CrossRef]
- Ong, K.K.L.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between Postnatal Catch-up Growth and Obesity in Childhood: Prospective Cohort Study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef]
- Quizán Plata, T.; Ortega, M.I. Diseño y Validación de una Herramienta para Identificar Riesgo Dietario en Mujeres Adultas de Bajo Ingreso. Nutr. Clin. 2000, 4, 128–135. [Google Scholar]
- Ortega, M.; Quizan, T.; Morales, G.; Preciado, M. Cálculo de Ingestión Dietaría y Coeficientes de Adecuación a Partir de Registro de 24h y Frecuencia de Consumo de Alimentos Estimación de Consumo de Alimentos; Centro de Investigación en Alimentación y Desarrollo A.C.; Secretaría de Educación Pública/Conaculta: Hermosillo, Mexico, 1999. [Google Scholar]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 4 December 2021).
- Arguelles-López, A.; Trujillo-Rivera, O.A.; Calderón-de-la-Barca, A.M. Dietary Patterns of Sonoran Breastfeeding Women are Associated to Exclusive or Partial Breastfeeding Regimes. Bol. Med. Hosp. Infant. Mex. 2024, 81, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lucas, A.; Gibbs, J.A.; Lyster, R.L.; Baum, J.D. Creamatocrit: Simple Clinical Technique for Estimating Fat Concentration and Energy Value of Human Milk. Br. Med. J. 1978, 1, 1018–1020. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Chen, R.Y.; Mostafa, I.; Hibberd, M.C.; Das, S.; Mahfuz, M.; Naila, N.N.; Islam, M.M.; Huq, S.; Alam, M.A.; Zaman, M.U.; et al. A Microbiota-Directed Food Intervention for Undernourished Children. N. Engl. J. Med. 2021, 384, 1517–1528. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic Balancing of Intestinal Short-Chain Fatty Acids: The Crucial Role of Bacterial Metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Mejía-León, M.E.; Petrosino, J.; Ajami, N.J.; Dominguez-Bello, M.; Calderon de la Barca, A.M. Fecal Microbiota Imbalance in Mexican Children with Type 1 Diabetes. Sci. Rep. 2014, 4, 3814. [Google Scholar] [CrossRef]
- Alderete, T.L.; Jones, R.B.; Shaffer, J.P.; Holzhausen, E.A.; Patterson, W.B.; Kazemian, E.; Chatzi, L.; Knight, R.; Plows, J.F.; Berger, P.K.; et al. Early Life Gut Microbiota Is Associated with Rapid Infant Growth in Hispanics from Southern California. Gut Microbes 2021, 13, 1961203. [Google Scholar] [CrossRef]
- Aguayo-Patrón, S.V.; Trujillo-Rivera, O.A.; Cornejo-Granados, F.; Ochoa-Leyva, A.; Calderón de la Barca, A.M. HLA-Haplotypes Influence Microbiota Structure in Northwestern Mexican Schoolchildren Predisposed for Celiac Disease or Type 1 Diabetes. Microorganisms 2023, 11, 1412. [Google Scholar] [CrossRef]
- Odiase, E.; Frank, D.N.; Young, B.E.; Robertson, C.E.; Kofonow, J.M.; Davis, K.N.; Berman, L.M.; Krebs, N.F.; Tang, M. The Gut Microbiota Differ in Exclusively Breastfed and Formula-Fed United States Infants and Are Associated with Growth Status. J. Nutr. 2023, 153, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- McKeen, S.; Roy, N.C.; Mullaney, J.A.; Eriksen, H.; Lovell, A.; Kussman, M.; Young, W.; Fraser, K.; Wall, C.R.; McNabb, W.C. Adaptation of the Infant Gut Microbiome during the Complementary Feeding Transition. PLoS ONE 2022, 17, e0270213. [Google Scholar] [CrossRef]
- Marin-Gómez, W.; Grande, M.J.; Pérez-Pulido, R.; Galvez, A.; Lucas, R. Changes in the Bacterial Diversity of Human Milk during Late Lactation Period (Weeks 21 to 48). Foods 2020, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Sagheddu, V.; Patrone, V.; Miragoli, F.; Puglisi, E.; Morelli, L. Infant Early Gut Colonization by Lachnospiraceae: High Frequency of Ruminococcus Gnavus. Front. Pediatr. 2016, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, B.; Li, J.; Dong, X.; Lin, M.; Zhang, M.; Zhao, J.; Dai, Y.; Chen, L. Association between Sn-2 Fatty Acid Profiles of Breast Milk and Development of the Infant Intestinal Microbiome. Food Funct. 2018, 9, 1028–1037. [Google Scholar] [CrossRef]
- Daniel, A.I.; Shama, S.; Ismail, S.; Bourdon, C.; Kiss, A.; Mwangome, M.; Bandsma, R.H.J.; O’Connor, D.L. Maternal BMI Is Positively Associated with Human Milk Fat: A Systematic Review and Meta-Regression Analysis. Am. J. Clin. Nutr. 2021, 113, 1009–1022. [Google Scholar] [CrossRef]
- Olga, L.; Vervoort, J.; van Diepen, J.A.; Gross, G.; Petry, C.J.; Prentice, P.M.; Chichlowski, M.; van Tol, E.A.; Hughes, I.A.; Dunger, D.B.; et al. Associations between Breast Milk Intake Volume, Macronutrient Intake, and Infant Growth in a Longitudinal Birth Cohort: The Cambridge Baby Growth and Breastfeeding Study (CBGS-BF). Br. J. Nutr. 2022, 130, 56–64. [Google Scholar] [CrossRef]
- Gulick, C.N.; Peddie, M.C.; Cameron, C.; Bradbury, K.; Rehrer, N.J. Physical Activity, Dietary Protein and Insulin-like Growth Factor 1: Cross-Sectional Analysis Utilising UK Biobank. Growth Horm. IGF Res. 2020, 55, 101353. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, J.C.; Smethers, A.D.; Eosso, J.R.; Papas, M.A.; Stallings, V.A.; Mennella, J.A. Impact of Early Rapid Weight Gain on Odds for Overweight at One Year Differs between Breastfed and Formula-Fed Infants. Pediatr. Obes. 2020, 15, e12688. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; Calatayud Arroyo, M.; García-Mantrana, I.; Parra-Llorca, A.; Escuriet, R.; Martínez-Costa, C.; Collado, M.C. Perinatal Environment Shapes Microbiota Colonization and Infant Growth: Impact on Host Response and Intestinal Function. Microbiome 2020, 8, 167. [Google Scholar] [CrossRef]
- Perng, W.; Ringham, B.M.; Glueck, D.H.; Sauder, K.A.; Starling, A.P.; Belfort, M.B.; Dabelea, D. An Observational Cohort Study of Weit- and Length-Derived Anthropometric Indicators with Body Composition at Birth and 5 Mo: The Healthy Start Study. Amer. J. Clin. Nutr. 2017, 106, 559–567. [Google Scholar] [CrossRef] [PubMed]


| Maternal Variable | n = 27 |
|---|---|
| Age, years | 32.3 ± 4.4 |
| Weight gain during pregnancy, kg | 11.5 ± 7.2 |
| Weight at 5 months pp, kg | 68.8 ± 15.5 |
| Height, cm | 162.3 ± 6.5 |
| Pre-gestational BMI, kg/m2 | 25.5 ± 5.2 |
| BMI at 5 months pp, kg/m2 | 26.0 ± 5.2 |
| Underweight | 1 (3.7) |
| Normal weight | 12 (44.4) |
| Overweight | 9 (33.3) |
| Obese | 5 (18.5) |
| Primiparous | 13 (48) |
| Vaginal delivery | 15 (56) |
| Infant Variable | |
| Gender, female | 14 (51.8) |
| Gestational age, weeks | 38.9 ± 1.2 |
| Weight at birth, kg | 3.3 ± 0.4 |
| Length at birth, cm | 50.1 ± 1.8 |
| Weight at 5.5 months, kg | 7.5 ± 1.3 |
| Length at 5.5 months, cm | 65.4 ± 3.1 |
| Weight at 12 months, kg * | 9.5 ± 1.5 |
| Length at 12 months, cm * | 75.2 ± 3.5 |
| WLZ at birth | −0.3 ± 1.1 |
| WLZ at 5.5 months | 0.3 ± 1.3 |
| WLZ at 12 months * | 0.1 ± 1.2 |
| Fed breast milk and expressed milk | 14 (51.8) |
| Breastfed at 12 months of age * | 23 (92) |
| Age of introduction of solid foods, months * | 6.04 ± 0.4 |
| Component | Mean ± SD |
|---|---|
| Protein (mg/mL) | 14.2 ± 4.6 |
| Lipids (mg/mL) | 47.7 ± 15.2 |
| Lactose (mg/mL) | 64.6 ± 11.7 |
| HMOs (mg/mL) | 14.8 ± 9.4 |
| Component | Maternal BMI | |||
|---|---|---|---|---|
| Pre-Gestational | p Value | At 5 Months pp | p Value | |
| Protein (mg/mL) | −0.42 | 0.02 | −0.38 | 0.05 |
| Lipids (mg/mL) | −0.19 | 0.35 | −0.22 | 0.26 |
| Lactose (mg/mL) | 0.26 | 0.19 | 0.20 | 0.30 |
| HMOs (mg/mL) | −0.34 | 0.09 | −0.33 | 0.10 |
| Breast Milk Component | Infant Growth 0–5.5 Months | ||
|---|---|---|---|
| SWG (n = 8) | NWG (n = 14) | RWG (n = 5) | |
| Protein (mg/mL) | 10.7 ± 3.9 a | 16.6 ± 4.3 b | 12.9 ± 1.1 ab |
| Lipids (mg/mL) | 46.9 ± 14.6 a | 49.1 ± 17 a | 44.9 ± 12.6 a |
| Lactose (mg/mL) | 64.8 ± 17.4 a | 64.1 ± 9.7 a | 65.5 ± 7.3 a |
| HMOs (mg/mL) | 18.0 ± 12.1 a | 15.1 ± 8.9 a | 9.4 ± 3.9 a |
| Taxa Relative Abundance | Breast Milk Component (mg/mL) | |||
|---|---|---|---|---|
| Lipids | Proteins | Lactose | HMOs | |
| Ruminococcus gnavus group | 0.25 (0.44) | −0.04 (0.89) | −0.23 (0.49) | 0.32 (0.36) |
| Bifidobacterium | 0.32 (0.12) | 0.18 (0.39) | 0.18 (0.38) | −0.21 (0.34) |
| Escherichia-Shigella | 0.22 (0.31) | −0.04 (0.87) | 0.01 (0.97) | 0.26 (0.29) |
| Veillonella | −0.50 (0.02) | −0.33 (0.16) | 0.23 (0.33) | −0.18 (0.45) |
| Bacteroides | 0.23 (0.44) | 0.15 (0.61) | 0.08 (0.77) | −0.19 (0.55) |
| Enterobacteriaceae | −0.16 (0.64) | 0.21 (0.51) | 0.28 (0.39) | −0.48 (0.13) |
| Veillonellaceae | −0.45 (0.055) | −0.29 (0.21) | 0.19 (0.41) | −0.18 (0.46) |
| Lachnospiraceae | 0.27 (0.46) | −0.41 (0.70) | 0.63 (0.06) | −0.75 (0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arguelles-Lopez, A.; Aguayo-Patrón, S.V.; Calderón de la Barca, A.M. Breastfeeding Shapes the Gut Microbiota and Its Structure Is Associated with Weight Gain Trajectories in Mexican Infants. Nutrients 2025, 17, 826. https://doi.org/10.3390/nu17050826
Arguelles-Lopez A, Aguayo-Patrón SV, Calderón de la Barca AM. Breastfeeding Shapes the Gut Microbiota and Its Structure Is Associated with Weight Gain Trajectories in Mexican Infants. Nutrients. 2025; 17(5):826. https://doi.org/10.3390/nu17050826
Chicago/Turabian StyleArguelles-Lopez, Alejandra, Sandra V. Aguayo-Patrón, and Ana M. Calderón de la Barca. 2025. "Breastfeeding Shapes the Gut Microbiota and Its Structure Is Associated with Weight Gain Trajectories in Mexican Infants" Nutrients 17, no. 5: 826. https://doi.org/10.3390/nu17050826
APA StyleArguelles-Lopez, A., Aguayo-Patrón, S. V., & Calderón de la Barca, A. M. (2025). Breastfeeding Shapes the Gut Microbiota and Its Structure Is Associated with Weight Gain Trajectories in Mexican Infants. Nutrients, 17(5), 826. https://doi.org/10.3390/nu17050826






