Evidence-Based Nutritional Recommendations for Maintaining or Restoring Nutritional Status in Patients with Amyotrophic Lateral Sclerosis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Research Strategy
2.2. Selection Criteria
2.3. Outcome Measures
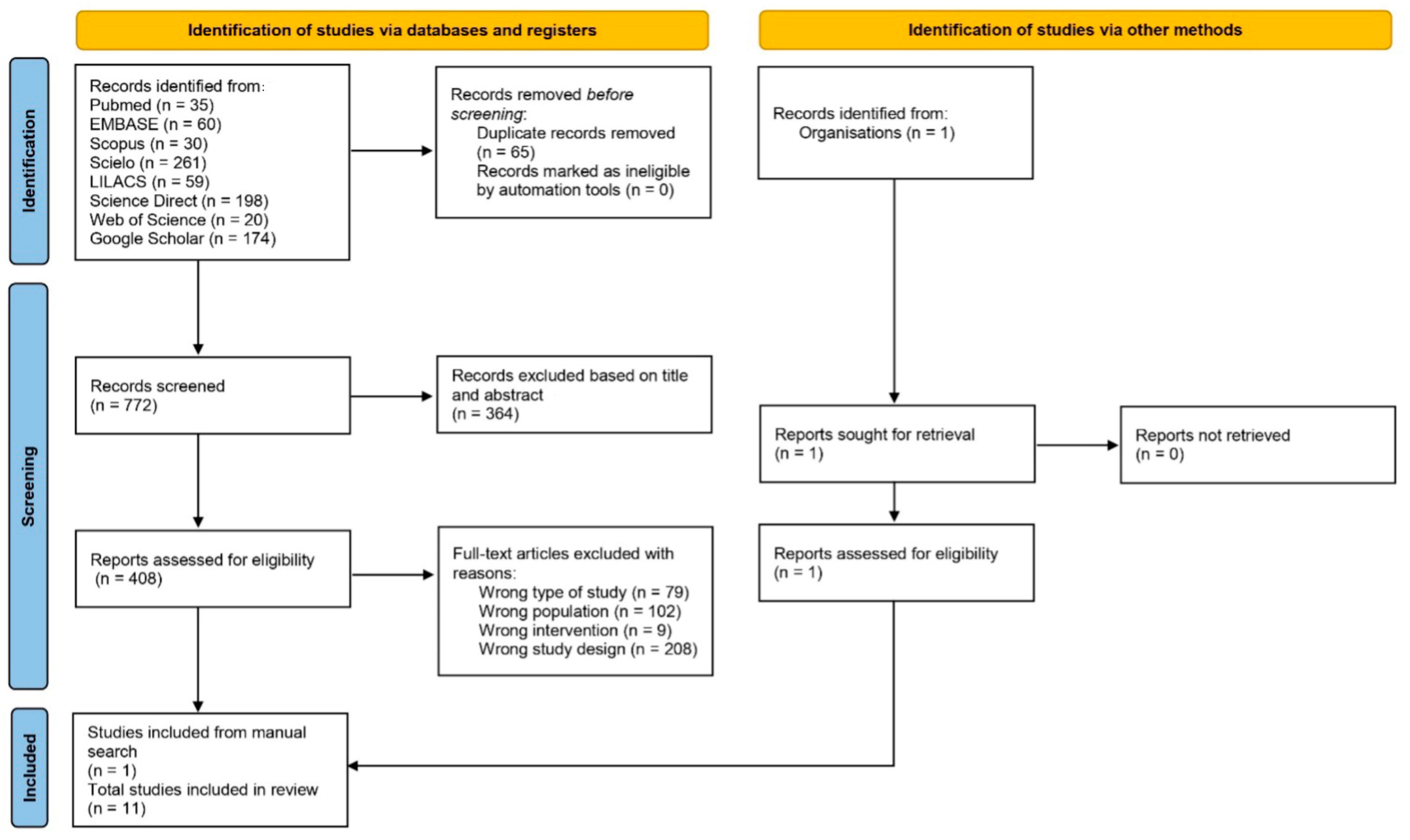
2.4. Study Selection
2.5. Data Extraction
2.6. Assessment of Guideline Quality
2.7. Data Synthesis
3. Results
3.1. Research Resources and Guidelines
3.2. Quality of Guidelines
3.3. Summary of Recommendations
3.4. Nutritional Evaluation
3.5. Dysphagia
3.6. Energy
3.7. Protein
3.8. Supplementation
3.9. Percutaneous Endoscopic Gastrostomy (PEG)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J. Revisiting the concept of amyotrophic lateral sclerosis as a multisystems disorder of limited phenotypic expression. Curr. Opin. Neurol. 2017, 30, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2020, 267, 944–953. [Google Scholar] [CrossRef]
- GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Cox, P.A.; Banack, S.A.; Lecusay, P.D.; Garamszegis, S.P.; Hagan, M.J.; Powell, J.T.; Metcalf, J.S.; Palmour, R.M.; Beierschmitt, A.; et al. l-Serine Reduces Spinal Cord Pathology in a Vervet Model of Preclinical ALS/MND. J. Neuropathol. Exp. Neurol. 2020, 79, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bai, Z.; Qin, X.; Cheng, Y. Aberrations in Oxidative Stress Markers in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 1712323. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Kushta, I.; Molfino, A.; Inghilleri, M.; Sabatelli, M.; Rossi Fanelli, F. Nutritional and metabolic support in patients with amyotrophic lateral sclerosis. Nutrition 2012, 28, 959–966. [Google Scholar] [CrossRef]
- Piquet, M.A. Approche nutritionnelle des patients atteints de Sclérose Latérale Amyotrophique [Nutritional approach for patients with amyotrophic lateral sclerosis]. Rev. Neurol. 2006, 162, 177–187. [Google Scholar] [CrossRef]
- Marin, B.; Desport, J.C.; Kajeu, P.; Jesus, P.; Nicolaud, B.; Nicol, M.; Preux, P.M.; Couratier, P. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 628–634. [Google Scholar] [CrossRef]
- Roubeau, V.; Blasco, H.; Maillot, F.; Corcia, P.; Praline, J. Nutritional assessment of amyotrophic lateral sclerosis in routine pratice: Value of weighing and bioelectrical impedance analysis. Muscle Nerve 2015, 51, 479–484. [Google Scholar] [CrossRef]
- Andersen, P.M.; Abrahams, S.; Borasio, G.D.; de Carvalho, M.; Chio, A.; Van Damme, P.; Hardiman, O.; Kollewe, K.; Morrison, K.E.; Petri, S.; et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—Revised report of an EFNS task force. Eur. J. Neurol. 2012, 19, 360–375. [Google Scholar]
- Almeida, C.S.; Stanich, P.; Salvioni, C.C.; Diccini, S. Assessment and nutrition education in patients with amyotrophic lateral sclerosis. Arq. Neuropsiquiatr. 2016, 74, 902–908. [Google Scholar] [CrossRef][Green Version]
- Prado, L.G.R.; Bicalho, I.C.S.; Vidigal-Lopes, M.; Prado, V.G.R.; Gomez, R.S.; Souza, L.C.; Teixeira, A.L. Depression and anxiety in a case series of amyotrophic lateral sclerosis: Frequency and association with clinical features. Einstein 2017, 15, 58–60. [Google Scholar] [CrossRef][Green Version]
- Campos, C.F.; Gromicho, M.; Uysal, H.; Grosskreutz, J.; Kuzma-Kozakiewicz, M.; Pinto, S.; Petri, S.; de Carvalho, M. Family history of neurodegenerative disorders in patients with amyotrophic lateral sclerosis: Population-based case-control study. J. Neurol. Neurosurg. Psychiatry 2020, 91, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Bogaert, E.; Dewil, M.; Hersmus, N.; Kiraly, D.; Scheveneels, W.; Bockx, I.; Braeken, D.; Verpoorten, N.; Verhoeven, K.; et al. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc. Natl. Acad. Sci. USA 2007, 104, 14825–14830. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Florez, I.D.; McNair, S.A.; Vella, E.T.; Yao, X. Clinical Practice Guidelines: Tools to Support High Quality Patient Care. Semin. Nucl. Med. 2019, 49, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kredo, T.; Bernhardsson, S.; Machingaidze, S.; Young, T.; Louw, Q.; Ochodo, E.; Grimmer, K. Guide to clinical practice guidelines: The current state of play. Int. J. Qual. Health Care 2016, 28, 122–128. [Google Scholar] [CrossRef]
- Ghorob, A.; Bodenheimer, T. Sharing the care to improve access to primary care. N. Engl. J. Med. 2012, 366, 1955–1957. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.M.; Russell, I.T. Effect of clinical guidelines on medical practice: A systematic review of rigorous evaluations. Lancet 1993, 342, 1317–1322. [Google Scholar] [CrossRef]
- Vilar, M.D.C.; Coutinho, K.M.D.; Vale, S.H.L.; Medeiros, G.C.B.S.; Piuvezam, G.; Leite-Lais, L.; Brandao-Neto, J. Nutritional therapy in amyotrophic lateral sclerosis: Protocol for a systematic review and meta-analysis. BMJ 2022, 12, e064086. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- AGREE Collaboration. AGREE: Advancing the Science of Practice Guidelines. Available online: https://www.agreetrust.org/ (accessed on 12 October 2024).
- Heffernan, C.; Jenkinson, C.; Holmes, T.; Feder, G.; Kupfer, R.; Leigh, P.N.; McGowan, S.; Rio, A.; Sidhu, P. Nutritional management in MND/ALS patients: An evidence based review. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Jackson, C.E.; Kasarskis, E.J.; England, J.D.; Forshew, D.; Johnston, W.; Kalra, S.; Katz, J.S.; Mitsumoto, H.; Rosenfeld, J.; et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009, 73, 1218–1226. [Google Scholar] [CrossRef]
- The EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis; Andersen, P.M.; Abrahams, S.; Borasio, G.D.; de Carvalho, M.; Chio, A.; Van Damme, P.; Hardiman, O.; Kollewe, K.; Morrison, K.E.; et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)-revised report of an EFNS task force. Eur. J. Neurol. 2012, 19, 360–375. [Google Scholar]
- Oliver, D.; Radunovic, A.; Allen, A.; McDermott, C. The development of the UK National Institute of Health and Care Excellence evidence-based clinical guidelines on motor neurone disease. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jésus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef]
- Shoesmith, C.; Abrahao, A.; Benstead, T.; Chum, M.; Dupre, N.; Izenberg, A.; Johnston, W.; Kalra, S.; Leddin, D.; O’Connell, C.; et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. CMAJ 2020, 192, E1453–E1468. [Google Scholar] [CrossRef]
- Brasil—Ministerio da Saude. Protocolo Clínico e Diretrizes Terapêuticas da Esclerose Lateral Amiotrofica. Available online: https://www.gov.br/conitec/pt-br/midias/protocolos/publicacoes_ms/20210713_publicacao_ela.pdf (accessed on 4 October 2024).
- Boostani, R.; Olfati, N.; Shamshiri, H.; Salimi, Z.; Fatehi, F.; Hedjazi, S.A.; Fakharian, A.; Ghasemi, M.; Okhovat, A.A.; Basiri, K.; et al. Iranian clinical practice guideline for amyotrophic lateral sclerosis. Front. Neurol. 2023, 14, 1154579. [Google Scholar] [CrossRef] [PubMed]
- Petri, S.; Grehl, T.; Grosskreutz, J.; Hecht, M.; Hermann, A.; Jesse, S.; Lingor, P.; Löscher, W.; Maier, A.; Schoser, B.; et al. Guideline “Motor neuron diseases” of the German Society of Neurology (Deutsche Gesellschaft für Neurologie). Neurol. Res. Prática 2023, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Urushitani, M.; Warita, H.; Atsuta, N.; Izumi, Y.; Kano, O.; Shimizu, T.; Nakayama, Y.; Narita, Y.; Nodera, H.; Fujita, T.; et al. The clinical practice guideline for the management of amyotrophic lateral sclerosis in Japan-update 2023. Rinsho Shinkeigaku 2024, 64, 252–271. [Google Scholar] [CrossRef]
- Van Damme, P.; Al-Chalabi, A.; Andersen, P.M.; Chiò, A.; Couratier, P.; De Carvalho, M.; Hardiman, O.; Kuźma-Kozakiewicz, M.; Ludolph, A.; McDermott, C.J.; et al. European Academy of Neurology (EAN) guideline on the management of amyotrophic lateral sclerosis in collaboration with European Reference Network for Neuromuscular Diseases (ERN EURO-NMD). Eur. J. Neurol. 2024, 31, e16264. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Zarotti, N.; Beever, D.; Bradburn, M.; Norman, P.; Coates, E.; Stavroulakis, T.; White, D.; McGeachan, A.; Williams, I.; et al. The nutritional management of people living with amyotrophic lateral sclerosis: A national survey of dietitians. J. Hum. Nutr. Diet. 2021, 34, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, E.; Grosso, G.; Nieves, J.W.; Zanghì, A.; Factor-Litvak, P.; Mitsumoto, H. Metabolic Abnormalities, Dietary Risk Factors and Nutritional Management in Amyotrophic Lateral Sclerosis. Nutrients 2021, 13, 2273. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.N.A.; Vale, S.H.L.; Alves, C.X.; Castro, J.L.; Dourado Junior, M.E.T.; Leite, L.D. Protocolo diferenciado para Terapia Nutricional na Esclerose Lateral Amiotrófica. Rev. Bras. Ciências Saúde 2014, 18, 79–86. [Google Scholar] [CrossRef]
- Mariani, L.; Ruoppolo, G.; Cilfone, A.; Cocchi, C.; Preziosi Standoli, J.; Longo, L.; Ceccanti, M.; Greco, A.; Inghilleri, M. Progression of Oropharyngeal Dysphagia in Amyotrophic Lateral Sclerosis: A Retrospective Cohort Study. Dysphagia 2022, 37, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.B.; Oda, A.L. Intervenção Fonoaudiológica nas Disfagias Orofaríngeas nas Doenças Neuromusculares. In Reabilitação em Doenças Neuromusculares: Guia Terapêutico Prático; Oliveira, A.S.B., Oda, A.L., Eds.; Atheneu: Sao Paulo, Brazil, 2014. [Google Scholar]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Essat, M.; Coates, E.; Clowes, M.; Beever, D.; Hackney, G.; White, S.; Stavroulakis, T.; Halliday, V.; McDermott, C.; HighCALS Group. Understanding the current nutritional management for people with amyotrophic lateral sclerosis—A mapping review. Clin. Nutr. ESPEN 2022, 49, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Matamala, J.M.; Moreno-Roco, J.; Acosta, I.; Hughes, R.; Lillo, P.; Casar, J.C.; Earle, N. Multidisciplinary care and therapeutic advances in amyotrophic lateral sclerosis. Rev. Med. Chil. 2022, 150, 1633–1646. [Google Scholar] [CrossRef]
- Del Olmo García Ma, D.; Virgili Casas, N.; Cantón Blanco, A.; Lozano Fuster, F.M.; Wanden-Berghe, C.; Avilés, V.; Ashbaugh Enguídanos, R.; Ferrero López, I.; Molina Soria, J.B.; Montejo González, J.C.; et al. Nutritional management of amyotrophic lateral sclerosis: Summary of recommendations. Nutr. Hosp. 2018, 35, 1243–1251. [Google Scholar]
- Traynor, B.J.; Alexander, M.; Corr, B.; Hardiman, O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: A population based study, 1996–2000. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1258–1261. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) [Ref] | Country, Region or Society | Language | Nutrition Topics Addressed | AGREE II |
|---|---|---|---|---|
| Heffernan et al. (2004) [25] | United Kingdom | English | Nutritional status, Dysphagia, Energy, Supplementation, PEG | 5 |
| Miller et al. (2009) [26] | American Academy of Neurology | English | Supplementation, PEG | 6 |
| Andersen et al. (2012) [27] | European Federation of Neurological Society | English | Dysphagia, PEG | 5 |
| Oliver et al. (2017) [28] | National Institute for Health and Care Excellence | English | Nutritional status, Dysphagia, PEG | 5 |
| Burgos et al. (2018) [29] | European Society for Clinical Nutrition and Metabolism | English | Nutritional status, Dysphagia, Energy, Protein, Supplementation, PEG | 6 |
| Shoesmith et al. (2020) [30] | Canada | English | Nutritional status, Dysphagia, Energy, PEG | 7 |
| BRASIL (2021) [31] | Brazil | Portuguese | PEG | 6 |
| Boostani et al. (2023) [32] | Iran | English | Nutritional status, Dysphagia, Supplementation, PEG | 5 |
| Petri et al. (2023) [33] | German Society of Neurology | German | Dysphagia, Energy, PEG | 4 |
| Urushitani et al. (2023) [34] | Japan | Japanese | Nutritional status, Dysphagia, Energy, Supplementation, PEG | 6 |
| Van Damme et al. (2024) [35] | European Academy of Neurology | English | Nutritional status, Dysphagia, PEG | 5 |
| Authors (Year) [Ref] | Dysphagia | Nutrition Evaluation | Supplementation | Energy | Protein | PEG |
|---|---|---|---|---|---|---|
| Heffernan et al. (2004) [25] | Multidisciplinary therapy and continuous assessment. Modification of food consistency and monitoring of swallowing. Safe feeding techniques (e.g., chin tuck). Education for patients and caregivers on swallowing and feeding techniques. Involvement of occupational or physical therapy | NR | Vitamins and minerals should be obtained through the diet rather than supplements | Energy needs should be regularly monitored, and intake should be adjusted to meet requirements as the disease progresses | NR | PEG helps stabilize weight, prevent malnutrition, and maintain long-term nutritional status. Individualized approach is required for the timing of PEG placement. PEG is not recommended in the advanced stages of the disease. PRG is an alternative when PEG is not indicated. NGT feeding is considered for short-term use |
| Miller et al. (2009) [26] | NR | NR | Creatine (5–10 g/day): Does not slow disease progression. Vitamin E: High doses should not be considered as a treatment, and there is no recommendation for low doses | NR | NR | PEG stabilizes body weight when oral feeding is no longer sufficient. There is insufficient evidence to support or refute the optimal timing for PEG insertion. PEG may help prolong survival |
| Andersen et al. (2012) [27] | Referral to a nutritionist and a speech therapist can provide guidance on appropriate swallowing techniques | The evaluation of nutritional status, including body weight measurement, should be performed at each visit | NR | NR | NR | When considering PEG insertion, consider bulbar symptoms, malnutrition (weight loss > 10%), respiratory function, and the patient’s overall health. Early insertion of PEG is recommended, and patients and caregivers should be made aware of the possible risks and benefits. PRG is a suitable alternative to PEG. NGT is considered for short-term use. Home parenteral nutrition is indicated in advanced stages |
| Oliver et al. (2017) [28] | The patient’s ability to eat and drink should be assessed. Assistance should be provided during feeding. The consistency of food and liquids should be modified as needed. Guidance on proper positioning during meals should also be given | The patient’s weight, nutritional status, and swallowing ability should be assessed | NR | NR | NR | PEG should be discussed early and regularly. When indicated, it should be performed promptly without delay |
| Burgos et al. (2018) [29] | Break up and enrich meals to provide additional energy or address nutrient deficiencies. Assess the need for oral nutritional supplementation in cases of progressive weight loss | Complete nutritional assessment should include BMI, weight loss, and lipid status. If available, body composition analysis using DEXA or BIA should be performed. Ongoing monitoring of nutritional status should focus on BMI and weight changes over time. Weight gain is recommended for patients with BMI < 25.0 kg/m2. For patients with BMI between 25 and 35 kg/m2, weight stabilization should be targeted. For patients with BMI > 35 kg/m2, weight loss is recommended to enhance both passive and active mobilization | Nutritional supplementation is recommended when a patient’s nutritional needs are not fully met through diet alone | Energy requirements for non-ventilated patients should be estimated at 30 kcal/kg of body weight, considering physical activity and weight changes. For patients with non-invasive ventilation, energy needs range from 25 to 30 kcal/kg of body weight, or can be calculated using the Harris–Benedict equation | There are insufficient data to make specific recommendations. However, factors such as age, kidney function, and level of stress should be considered when determining nutritional needs | Discussions about PEG should occur early and be revisited regularly. PEG should be considered in the presence of dysphagia, prolonged meal duration, weight loss, impaired respiratory function, choking risk, and indivudual’s preferences. It is recommended to consider PEG before substantial weight loss and worsening respiratory function. Patients and caregivers should be fully informed about possible risks and benefits. Enteral nutrition should be prioritized, with parenteral nutrition considered only when enteral feeding is not viable. Home parenteral nutrition is generally not indicated |
| Shoesmith et al. (2020) [30] | Swallowing function should be regularly assessed and monitored | Weight and BMI should be monitored every three months or as clinically indicated | NR | High-calorie diets may help improve nutritional indicators and enhance survival. High-calorie, high-carbohydrate diets may be more beneficial than high-calorie, high-fat diets | NR | Factors to indicate PEG are heightened risk of aspiration despite changes in diet texture or compensatory strategies, weight loss of ≥5–10%, a reduction of ≥1 point in usual/baseline BMI, BMI < 18.5, or TDEE exceeding daily energy intake. Patients should be informed of the risks and benefits PEG should be performed within four weeks after a shared decision between the healthcare team and the patient. The nutritionist should regularly monitor the prescribed enteral feeding. NGT is not recommended for long-term use, and parenteral nutrition should be considered only in exceptional cases |
| BRASIL (2021) [31] | NR | NR | NR | NR | NR | PEG helps stabilize or increase body weight, prolong survival, and improve quality of life |
| Boostani et al. (2023) [32] | An initial speech and language pathology consultation should be conducted. Strategies for modifying food consistency, implementing postural adjustments, and using swallowing maneuvers should be applied. PEG should be considered in more severe stages of dysphagia, and high-viscosity liquids should be handled with caution. Oral hygiene should be maintained throughout the day | A regular nutritional assessment should be conducted by a nutritionist every 3 months. It is recommended to assess nutritional history, perform a clinical examination evaluating swallowing, weight, and BMI. The assessment of body composition and energy expenditure may be considered on an individual basis | Nutritional counseling should include food fortification, oral nutritional supplementation, and the potential need for early enteral nutrition, such as PEG. When weight loss, fatigue, or effortful eating is present, food fortification is recommended. Oral nutritional supplementation is advised for patients with unmet energy needs | NR | NR | PEG should be considered in cases of worsening speech or changes in food consistency, weight loss of 10%, BMI < 18.5 kg/m2, risk of bronchoaspiration, or if FVC > 50%. PEG and NGT have similar efficacy in maintaining food intake, but PEG is superior in quality of life measures |
| Petri et al. (2023) [33] | The speech and language pathologist plays a key role in detecting clinically inapparent dysphagia and adjusting treatment accordingly. Regular monitoring for dysphagia is recommended | Patients should be regularly monitored for weight loss | In situations involving patient discomfort, weight loss, dehydration, and risk of aspiration, the nutritional counseling may include the prescription of high-calorie liquid nutrition | A high-calorie diet can be beneficial, especially in patients experiencing rapid disease progression | NR | PEG is recommended for patients with advanced dysphagia and significant weight loss |
| Urushitani et al. (2023) [34] | Swallowing function should be monitored regularly. Screening tests for dysphagia include repetitive saliva swallowing, modified water swallowing, and cervical auscultation. Swallowing rehabilitation (direct and indirect) by a multidisciplinary team is essential. Expiratory strength training is recommended to maintain swallowing function. In cases of risk of choking and/or aspiration, PEG should be considered. Surgical intervention for aspiration prevention may also serve as an alternative approach | Weigh monitoring is important, as weight loss and reduction rate of weight are independent prognostic factors for short survival | NR | The formulas for calculating the ideal energy intake for early-stage Japanese patients are: (1) TEE = 1.68 × BEE + 11.8 × ALSFRS-R − 690, and (2) REE = 1.000251 × BEE + 313.3507 × TV − 112.036. A high-calorie, high-fat diet is recommended to maintain body weight and prolong survival. In patients using NIV, energy intake should be restricted. For patients in a fully blocked state, the daily energy requirement is less than 800 kcal/day | NR | PEG should be considered when there is a risk of aspiration and progressive weight loss. Parenteral nutrition may be considered when other feeding routes are not viable. Indications for PEG include a >10% reduction in premorbid weight, early dysphagia, reduced and delayed food intake, and preserved respiratory function (FVC ≥ 50%). PEG should ideally be performed before arterial carbon dioxide pressure increases. Patients with low FVC or those on NIV can undergo PEG with respiratory support. PRG is not widely used in Japan |
| Van Damme et al. (2024) [35] | If there is weight loss or swallowing difficulties, consult a nutritionist, speech therapist, and/or occupational therapist. Consider the composition of food, food consistency, frequency of meals, intake and consistency of liquids, risk of choking, use of utensils, and optimal positioning and seating | Identify the causes of weight loss and reduced food and fluid intake, such as swallowing difficulties, respiratory failure, depression, loss of appetite, muscle atrophy, and upper limb weakness | Consider the use of dietary supplements in cases of weight loss or swallowing difficulties | NR | NR | Discuss PEG at an early stage and at regular intervals. Explain the benefits of early insertion and the risks associated with late insertion. In patients with respiratory failure, the use of NIV during PEG insertion is recommended. When PEG is indicated, it should be performed without delay. Consider NGT while awaiting PEG insertion. Engage family members and/or caregivers in discussions about PEG. If PEG is not feasible, consider TPN feeding as an option |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho Vilar, M.D.; Coutinho, K.M.D.; de Lima Vale, S.H.; Dourado Junior, M.E.T.; de Medeiros, G.C.B.S.; Piuvezam, G.; Brandao-Neto, J.; Leite-Lais, L. Evidence-Based Nutritional Recommendations for Maintaining or Restoring Nutritional Status in Patients with Amyotrophic Lateral Sclerosis: A Systematic Review. Nutrients 2025, 17, 782. https://doi.org/10.3390/nu17050782
de Carvalho Vilar MD, Coutinho KMD, de Lima Vale SH, Dourado Junior MET, de Medeiros GCBS, Piuvezam G, Brandao-Neto J, Leite-Lais L. Evidence-Based Nutritional Recommendations for Maintaining or Restoring Nutritional Status in Patients with Amyotrophic Lateral Sclerosis: A Systematic Review. Nutrients. 2025; 17(5):782. https://doi.org/10.3390/nu17050782
Chicago/Turabian Stylede Carvalho Vilar, Mariana Dantas, Karla Monica Dantas Coutinho, Sancha Helena de Lima Vale, Mario Emilio Teixeira Dourado Junior, Gidyenne Christine Bandeira Silva de Medeiros, Grasiela Piuvezam, Jose Brandao-Neto, and Lucia Leite-Lais. 2025. "Evidence-Based Nutritional Recommendations for Maintaining or Restoring Nutritional Status in Patients with Amyotrophic Lateral Sclerosis: A Systematic Review" Nutrients 17, no. 5: 782. https://doi.org/10.3390/nu17050782
APA Stylede Carvalho Vilar, M. D., Coutinho, K. M. D., de Lima Vale, S. H., Dourado Junior, M. E. T., de Medeiros, G. C. B. S., Piuvezam, G., Brandao-Neto, J., & Leite-Lais, L. (2025). Evidence-Based Nutritional Recommendations for Maintaining or Restoring Nutritional Status in Patients with Amyotrophic Lateral Sclerosis: A Systematic Review. Nutrients, 17(5), 782. https://doi.org/10.3390/nu17050782







