Association Between Maternal Dietary Isoflavone Intake During Pregnancy and Childhood Allergic Rhinoconjunctivitis: The Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
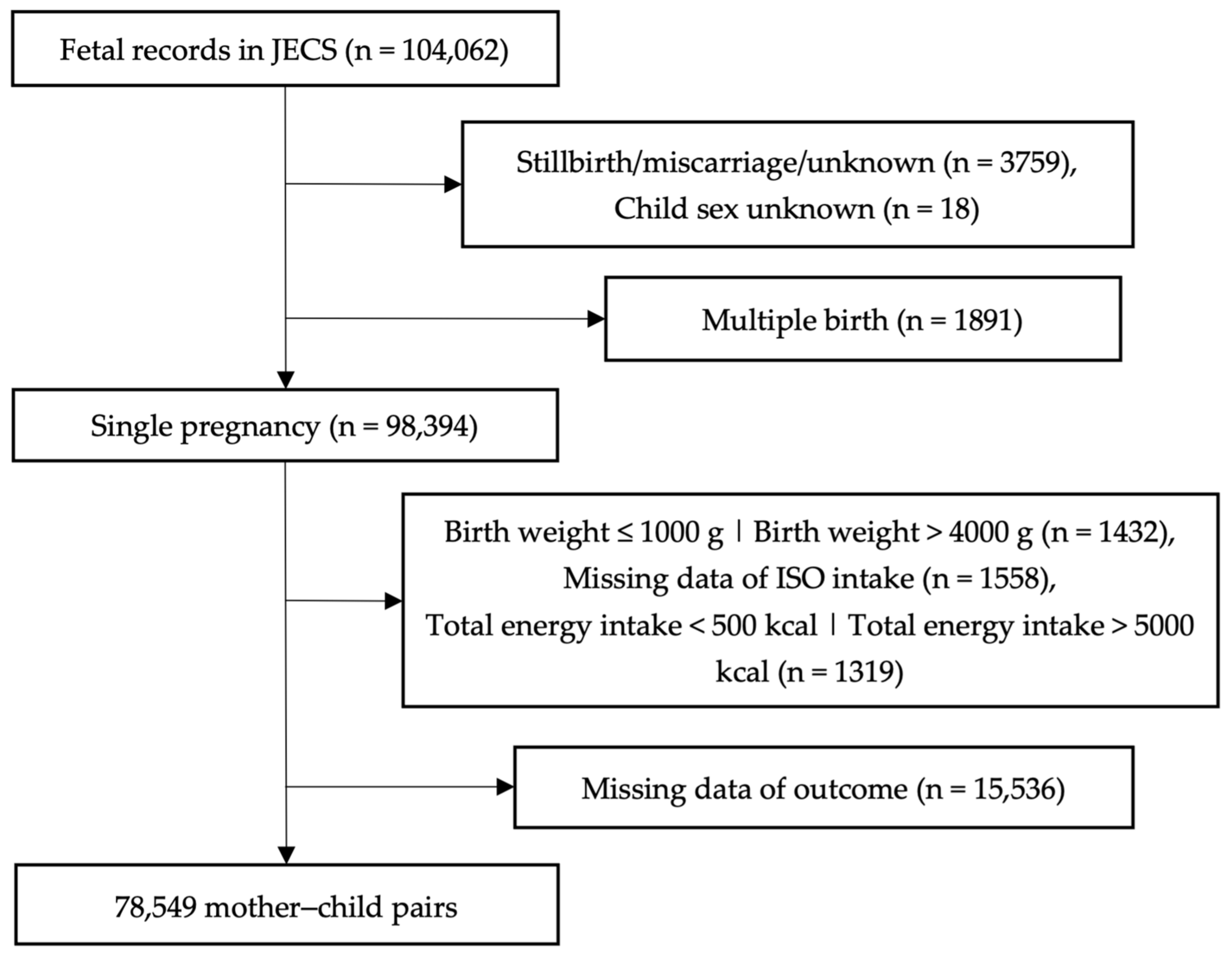
2.2. Study Population
2.3. Exposure
2.4. Outcome
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Association Between Maternal ISO Intake and Childhood Allergic Rhinoconjunctivitis
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aOR | Adjusted odds ratio |
| BMI | Body mass index |
| CI | Confidence interval |
| cOR | Crude odds ratio |
| DAI | Daidzein |
| GEN | Genistein |
| FFQ | Food frequency questionnaire |
| JECS | Japan Environment and Children’s Study |
| ISO | Isoflavone |
References
- Asher, M.; Montefort, S.; Bjorksten, B.; Lai, C.; Strachan, D.; Weiland, S.; Williams, H.; Group, I. phase three study Worldwide Time Trends in the Prevalence of Symptoms of Asthma, Allergic Rhinoconjunctivitis, and Eczema in Childhood: ISAAC Phases One and Three Repeat Multicountry Cross-Sectional Surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Pak, K.; Saito-Abe, M.; Yang, L.; Sato, M.; Irahara, M.; Mezawa, H.; Sasaki, H.; Nishizato, M.; Ishitsuka, K.; et al. Allergy and Immunology in Young Children of Japan: The JECS Cohort. World Allergy Organ. J. 2020, 13, 100479. [Google Scholar] [CrossRef]
- Iordache, A.; Boruga, M.; Mușat, O.; Jipa, D.A.; Tătaru, C.P.; Mușat, G.C. Relationship between Allergic Rhinitis and Allergic Conjunctivitis (Allergic Rhinoconjunctivitis)—Review. Rom. J. Ophthalmol. 2022, 66, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F.; Montejo, J.M. Allergic Rhinitis in Children and Adolescents. Pediatr. Clin. N. Am. 2019, 66, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.G.; Al-Reefy, H.; Fox, A.T.; Hopkins, C. Allergic Rhinitis in Children. BMJ 2014, 349, g4153. [Google Scholar] [CrossRef][Green Version]
- Scadding, G.K. Allergic Rhinitis in Children. Paediatr. Child Health 2008, 18, 323–328. [Google Scholar] [CrossRef][Green Version]
- Michaëlsson, J.; Mold, J.E.; McCune, J.M.; Nixon, D.F. Regulation of T Cell Responses in the Developing Human Fetus. J. Immunol. 2006, 176, 5741–5748. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Solvason, N.; Kearney, J.F. The Human Fetal Omentum: A Site of B Cell Generation. J. Exp. Med. 1992, 175, 397–404. [Google Scholar] [CrossRef]
- Ygberg, S.; Nilsson, A. The Developing Immune System—From Foetus to Toddler. Acta Paediatr. 2012, 101, 120–127. [Google Scholar] [CrossRef]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Halfon, N.; Larson, K.; Lu, M.; Tullis, E.; Russ, S. Lifecourse Health Development: Past, Present and Future. Matern. Child Health J. 2014, 18, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Stoody, E.E.; Spahn, J.M.; Casavale, K.O. The Pregnancy and Birth to 24 Months Project: A Series of Systematic Reviews on Diet and Health. Am. J. Clin. Nutr. 2019, 109 (Suppl. S7), 685S–697S. [Google Scholar] [CrossRef]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal Dietary Intake in Pregnancy and Lactation and Allergic Disease Outcomes in Offspring. Pediatr. Allergy Immunol. 2017, 28, 135–143. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Ahonen, S.; Kaila, M.; Erkkola, M.; Haapala, A.M.; Kronberg-Kippilä, C.; Veijola, R.; Ilonen, J.; Simell, O.; Knip, M.; et al. Maternal Diet during Pregnancy and Allergic Sensitization in the Offspring by 5 Yrs of Age: A Prospective Cohort Study. Pediatr. Allergy Immunol. 2010, 21, 29–37. [Google Scholar] [CrossRef]
- Baïz, N.; Just, J.; Chastang, J.; Forhan, A.; De Lauzon-Guillain, B.; Magnier, A.M.; Annesi-Maesano, I. Maternal Diet before and during Pregnancy and Risk of Asthma and Allergic Rhinitis in Children. Allergy Asthma Clin. Immunol. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Erkkola, M.; Kaila, M.; Nwaru, B.I.; Kronberg-Kippilä, C.; Ahonen, S.; Nevalainen, J.; Veijola, R.; Pekkanen, J.; Ilonen, J.; Simell, O.; et al. Maternal Vitamin D Intake during Pregnancy Is Inversely Associated with Asthma and Allergic Rhinitis in 5-Year-Old Children. Clin. Exp. Allergy 2009, 39, 875–882. [Google Scholar] [CrossRef]
- Ahonen, S.; Nwaru, B.I.; Erkkola, M.; Lumia, M.; Kronberg- Kippilä, C.; Ahonen, S.; Kaila, M.; Ilonen, J.; Simell, O.; Knip, M.; et al. Maternal Intake of Fatty Acids during Pregnancy and Allergies in the Offspring. Br. J. Nutr. 2012, 108, 720–732. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone Metabolites and Their in Vitro Dual Functions: They Can Act as an Estrogenic Agonist or Antagonist Depending on the Estrogen Concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Piskula, M.K.; Yamakoshi, J.; Iwai, Y. Daidzein and Genistein but Not Their Glucosides Are Absorbed from the Rat Stomach. FEBS Lett. 1999, 447, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, Distribution, Metabolism, and Excretion of Isoflavonoids after Soy Intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Todaka, E.; Sakurai, K.; Fukata, H.; Miyagawa, H.; Uzuki, M.; Omori, M.; Osada, H.; Ikezuki, Y.; Tsutsumi, O.; Iguchi, T.; et al. Fetal Exposure to Phytoestrogens—The Difference in Phytoestrogen Status between Mother and Fetus. Environ. Res. 2005, 99, 195–203. [Google Scholar] [CrossRef]
- Guo, T.L.; White, K.L.; Brown, R.D.; Delclos, K.B.; Newbold, R.R.; Weis, C.; Germolec, D.R.; McCay, J.A. Genistein Modulates Splenic Natural Killer Cell Activity, Antibody-Forming Cell Response, and Phenotypic Marker Expression in F0 and F1 Generations of Sprague-Dawley Rats. Toxicol. Appl. Pharmacol. 2002, 181, 219–227. [Google Scholar] [CrossRef]
- Klein, S.L.; Wisniewski, A.B.; Marson, A.L.; Glass, G.E.; Gearhart, J.P. Early Exposure to Genistein Exerts Long-Lasting Effects on the Endocrine and Immune Systems in Rats. Mol. Med. 2002, 8, 742–749. [Google Scholar] [CrossRef]
- Burris, R.L.; Vick, S.C.; Popovic, B.; Fraungruber, P.E.; Nagarajan, S. Maternal Exposure to Soy Diet Reduces Atheroma in Hyperlipidemic F1 Offspring Mice by Promoting Macrophage and T Cell Anti-Inflammatory Responses. Atherosclerosis 2020, 313, 26–34. [Google Scholar] [CrossRef]
- Ebaid, H.M.; Elgawish, R.A.R.; Abdelrazek, H.M.A.; Gaffer, G.; Tag, H.M. Prenatal Exposure to Soy Isoflavones Altered the Immunological Parameters in Female Rats. Int. J. Toxicol. 2016, 35, 274–283. [Google Scholar] [CrossRef]
- Sakai, T.; Kogiso, M.; Mitsuya, K.; Komatsu, T.; Yamamoto, S. Genistein Enhances Antigen-Specific Cytokine Production in Female DO11.10 Transgenic Mice. J. Nutr. Sci. Vitaminol. (Tokyo). 2006, 52, 327–332. [Google Scholar] [CrossRef]
- Guo, T.L.; Auttachoat, W.; Chi, R.P. Genistein Enhancement of Respiratory Allergen Trimellitic Anhydride-Induced IgE Production by Adult B6C3F1 Mice Following In Utero and Postnatal Exposure. Toxicol. Sci. 2005, 87, 399–408. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and Study Design of the Japan Environment and Children’s Study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Wang, H.J.; Murphy, P.A. Isoflavone Content in Commercial Soybean Foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Masilamani, M.; Wei, J.; Sampson, H.A. Regulation of the Immune Response by Soybean Isoflavones. Immunol. Res. 2012, 54, 95–110. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision Medicine and Phenotypes, Endotypes, Genotypes, Regiotypes, and Theratypes of Allergic Diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust Causal Inference Using Directed Acyclic Graphs: The R Package “Dagitty. ” Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Natale, A.; Fiori, F.; Parpinel, M.; Pelucchi, C.; Negri, E.; La Vecchia, C.; Rossi, M. Dietary Isoflavones Intake and Gastric Cancer. Nutr. 2024, 16, 2771. [Google Scholar] [CrossRef]
- Adgent, M.A.; Daniels, J.L.; Rogan, W.J.; Adair, L.; Edwards, L.J.; Westreich, D.; Maisonet, M.; Marcus, M. Early-Life Soy Exposure and Age at Menarche. Paediatr. Perinat. Epidemiol. 2012, 26, 163–175. [Google Scholar] [CrossRef]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Che, H.; Yang, S.; Chen, C. Estrogen and Estrogen Receptor Signaling Promotes Allergic Immune Responses: Effects on Immune Cells, Cytokines, and Inflammatory Factors Involved in Allergy. Allergol. Immunopathol. 2019, 47, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Hamano, N.; Terada, N.; Maesako, K.I.; Hohki, G.; Ito, T.; Yamashita, T.; Konno, A. Effect of Female Hormones on the Production of IL-4 and IL-13 from Peripheral Blood Mononuclear Cells. Acta Oto-Laryngologica 1998, 537, 27–31. [Google Scholar] [CrossRef]
- Prescott, S.L.; Macaubas, C.; Holt, B.J.; Smallacombe, T.B.; Loh, R.; Sly, P.D.; Holt, P.G. Transplacental Priming of the Human Immune System to Environmental Allergens: Universal Skewing of Initial T Cell Responses Toward the Th2 Cytokine Profile. J. Immunol. 1998, 160, 4730–4737. [Google Scholar] [CrossRef]
- Prescott, S.L.; Macaubas, C.; Smallacombe, T.; Holt, B.J.; Sly, P.D.; Holt, P.G. Development of Allergen-Specific T-Cell Memory in Atopic and Normal Children. Lancet 1999, 353, 196–200. [Google Scholar] [CrossRef]
- Martino, D.J.; Prescott, S.L. Silent Mysteries: Epigenetic Paradigms Could Hold the Key to Conquering the Epidemic of Allergy and Immune Disease. Allergy Eur. J. Allergy Clin. Immunol. 2010, 65, 7–15. [Google Scholar] [CrossRef]
- Harrison, R.; Phillippi, P.; Swan, K.; Henson, M. Effect of Genistein on Steroid Hormone Production in the Pregnant Rhesus Monkey. Proc. Soc. Exp. Biol. Med. 1999, 222, 78–84. [Google Scholar] [CrossRef]
- Guo, T.L.; Meng, A.H. In Utero Exposure to Genistein Enhanced Intranasal House Dust Mite Allergen-Induced Respiratory Sensitization in Young Adult B6C3F1 Mice. Toxicol. Lett. 2016, 253, 17–26. [Google Scholar] [CrossRef]
- Baraniuk, J.N. Pathogenesis of Allergic Rhinitis. Am. J. Rhinol. 1997, 11, 245. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic Rhinitis. Nat. Rev. Dis. Prim. 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Hamano, N.; Terada, N.; Maesako, K.; Numata, T.; Konno, A. Effect of Sex Hormones on Eosinophilic Inflammation in Nasal Mucosa. Allergy Asthma Proc. 1998, 19, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Mahallei, M.; Pormohammad, A.; Varshochi, M.; Ganbarov, K.; Zeinalzadeh, E.; Yousefi, B.; Bastami, M.; Tanomand, A.; Mahmood, S.S.; et al. Microbial Balance in the Intestinal Microbiota and Its Association with Diabetes, Obesity and Allergic Disease. Microb. Pathog. 2019, 127, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nanan, R.; Macia, L.; Tan, J.; Sominsky, L.; Quinn, T.P.; O’Hely, M.; Ponsonby, A.L.; Tang, M.L.K.; Collier, F.; et al. The Maternal Gut Microbiome during Pregnancy and Offspring Allergy and Asthma. J. Allergy Clin. Immunol. 2021, 148, 669–678. [Google Scholar] [CrossRef]
- Vuillermin, P.J.; Macia, L.; Nanan, R.; Tang, M.L.K.; Collier, F.; Brix, S. The Maternal Microbiome during Pregnancy and Allergic Disease in the Offspring. Semin. Immunopathol. 2017, 39, 669–675. [Google Scholar] [CrossRef]
- Ghimire, S.; Cady, N.M.; Lehman, P.; Peterson, S.R.; Shahi, S.K.; Rashid, F.; Giri, S.; Mangalam, A.K. Dietary Isoflavones Alter Gut Microbiota and Lipopolysaccharide Biosynthesis to Reduce Inflammation. Gut Microbes 2022, 14, 2127446. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.; Liang, Z.; Xu, H.; Du, P.; Li, A.; Meng, Y.; Reshetnik, E.I.; Liu, L.; Li, C. The Bioavailability of Soy Isoflavones in Vitro and Their Effects on Gut Microbiota in the Simulator of the Human Intestinal Microbial Ecosystem. Food Res. Int. 2022, 152, 110868. [Google Scholar] [CrossRef]
- Ying, X.; Qi, X.; Yin, Y.; Wang, H.; Zhang, H.; Jiang, H.; Yang, L.; Wu, J. Allergens Sensitization among Children with Allergic Diseases in Shanghai, China: Age and Sex Difference. Respir. Res. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Hong, S.; Son, D.K.; Lim, W.R.; Kim, S.H.; Kim, H.; Yum, H.Y.; Kwon, H. The Prevalence of Atopic Dermatitis, Asthma, and Allergic Rhinitis and the Comorbidity of Allergic Diseases in Children. Environ. Health Toxicol. 2012, 27, e2012006. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef]
- Alur, P. Sex Differences in Nutrition, Growth, and Metabolism in Preterm Infants. Front. Pediatr. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Zhou, A.; Chang, H.; Huo, W.; Zhang, B.; Hu, J.; Xia, W.; Chen, Z.; Xiong, C.; Zhang, Y.; Wang, Y.; et al. Prenatal Exposure to Bisphenol A and Risk of Allergic Diseases in Early Life. Pediatr. Res. 2017, 81, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Gilkeson, G. Estrogen Receptors in Immunity and Autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Grant, W.M.; McMullen, C.W.; Laves, K.S. High Fetal Estrogen Concentrations: Correlation with Increased Adult Sexual Activity and Decreased Aggression in Male Mice. Science 1983, 220, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, J.; Foster, W.G.; Kinniburgh, D.W. Phytoestrogens in Human Pregnancy. Obstet. Gynecol. Int. 2012, 2012, 850313. [Google Scholar] [CrossRef]
- Favari, C.; Rinaldi de Alvarenga, J.F.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors Driving the Inter-Individual Variability in the Metabolism and Bioavailability of (Poly)Phenolic Metabolites: A Systematic Review of Human Studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef]
- Hernández-Prieto, D.; Fernández, P.S.; Agulló, V.; García-Viguera, C.; Egea, J.A. Bioactive Compounds in Plasma as a Function of Sex and Sweetener Resulting from a Maqui-Lemon Beverage Consumption Using Statistical and Machine Learning Techniques. Int. J. Mol. Sci. 2023, 24, 2140. [Google Scholar] [CrossRef]
- Hernández-Prieto, D.; Garre, A.; Agulló, V.; García-Viguera, C.; Egea, J.A. Differences Due to Sex and Sweetener on the Bioavailability of (Poly)Phenols in Urine Samples: A Machine Learning Approach. Metabolites 2023, 13, 653. [Google Scholar] [CrossRef]
- Lu, L.J.W.; Anderson, K.E. Sex and Long-Term Soy Diets Affect the Metabolism and Excretion of Soy Isoflavones in Humans. Am. J. Clin. Nutr. 1998, 68, 1500S–1504S. [Google Scholar] [CrossRef]
- Bedard, D.; Shatenstein, B.; Nadon, S. Underreporting of Energy Intake from a Self-Administered Food-Frequency Questionnaire Completed by Adults in Montreal. Public Health Nutr. 2004, 7, 675–681. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women: Results from 3 Prospective Cohort Studies. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian Adult Soy Protein and Isoflavone Intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Travis, R.C.; Allen, N.E.; Appleby, P.N.; Spencer, E.A.; Roddam, A.W.; Key, T.J. A Prospective Study of Vegetarianism and Isoflavone Intake in Relation to Breast Cancer Risk in British Women. Int. J. Cancer 2008, 122, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Marriott, L.; Poole, J.; Crozier, S.; Borland, S.; Lawrence, W.; Law, C.; Godfrey, K.; Cooper, C.; Inskip, H. Dietary Patterns in Infancy: The Importance of Maternal and Family Influences on Feeding Practice. Br. J. Nutr. 2007, 98, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 3, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Croft, K.D.; Puddey, I.B.; Mori, T.A.; Beilin, L.J. Soybean Isoflavonoids and Their Metabolic Products Inhibit in Vitro Lipoprotein Oxidation in Serum. J. Nutr. Biochem. 1996, 7, 664–669. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seur, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal Ovarian Activation Has Effects in Estrogen Target Tissues in Infant Girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef]
| Quartile for ISO Intake | |||||
|---|---|---|---|---|---|
| Total | Q1 | Q2 | Q3 | Q4 | |
| n = 78,549 | n = 19,638 | n = 19,647 | n = 19,630 | n = 19,634 | |
| ISO (median [IQR]), mg/day | 23.36 [13.35–39.65] | 7.95 [5.0–10.7] | 17.91 [15.6–20.5] | 30.02 [26.5–34.5] | 55.93 [46.6–70.7] |
| Maternal age, mean (SD), years | 31.0 (4.9) | 30.6 (5.1) | 31.3 (4.9) | 31.7 (4.8) | 32.1 (4.7) |
| Child sex, female (%) | 38,400 (48.9) | 9579 (48.8) | 9634 (49.0) | 9538 (48.6) | 9649 (49.1) |
| Maternal educational level, >12 years, n (%) | 51,843 (66.2) | 11,989 (61.3) | 12,891 (65.8) | 13,301 (68.0) | 13,662 (69.8) |
| Maternal smoking status, n (%) | |||||
| Never smoker | 46,925 (60.1) | 11,314 (58.0) | 11,897 (60.9) | 11,807 (60.5) | 11,907 (61.1) |
| Ex-smoker who quit before pregnancy | 18,691 (24.0) | 4352 (22.3) | 4543 (23.3) | 4827 (24.8) | 4969 (25.5) |
| Ex-smoker who quit during pregnancy | 9581 (12.3) | 2854 (14.6) | 2364 (12.1) | 2268 (11.6) | 2095 (10.8) |
| Current smoker | 2824 (3.6) | 980 (5.0) | 726 (3.7) | 601 (3.1) | 517 (2.7) |
| Pre-pregnancy BMI (kg/m2), n (%) | |||||
| <18.5 kg/m2 | 12,788 (16.3) | 3180 (16.2) | 3301 (16.8) | 3176 (16.2) | 3131 (16.0) |
| ≥18.5 and <25 kg/m2 | 58,075 (74.0) | 14,288 (72.8) | 14,413 (73.4) | 14,657 (74.7) | 14,717 (75.0) |
| ≥25 kg/m2 | 7646 (9.7) | 2155 (11.0) | 1923 (9.8) | 1791 (9.1) | 1777 (9.1) |
| Maternal history of allergies, yes, n (%) | 45,639 (58.3) | 11,132 (56.9) | 11,416 (58.3) | 11,588 (59.2) | 11,503 (58.8) |
| Blood folic acid concentration (median [IQR]) | 6.0 [4.2–10.0] | 5.3 [3.7–8.6] | 5.8 [4.1–9.5] | 6.2 [4.3–10.0] | 6.9 [4.7–11.5] |
| Total energy intake (median [IQR]) | 1618 [1315–2005] | 1375 [1110–1692] | 1540 [1285–1859] | 1685 [1408–2028] | 1909 [1568–2350] |
| Fruit intake, g/day (median [IQR]) | 148.7 [95.5–223.4] | 102.5 [62.5–156.8] | 134.0 [90.6–193.2] | 163.3 [113.3–231.9] | 208.1 [142.3–303.7] |
| Vegetable intake, g/day (median [IQR]) | 108.5 [48.3–192.0] | 76.8 [27.2–147.8] | 100.5 [46.5–174.2] | 118.5 [56.8–198.1] | 145.7 [71.9–247.8] |
| Nutritional condition up to 4 months of age, n (%) | |||||
| Breastfeeding | 33,596 (43.7) | 7992 (41.7) | 8377 (43.5) | 8472 (44.1) | 8755 (45.5) |
| Formula milk | 1696 (2.2) | 543 (2.8) | 397 (2.1) | 375 (2.0) | 381 (2.0) |
| Mixed | 41,588 (54.1) | 10,618 (55.4) | 10,493 (54.5) | 10,376 (54.0) | 10,101 (52.5) |
| Allergic rhinoconjunctivitis at the age of 3 years, n (%) | 4298 (5.5) | 1029 (5.2) | 1063 (5.4) | 1068 (5.4) | 1138 (5.8) |
| ISO Intake | Total | Male Children | Female Children | |||
|---|---|---|---|---|---|---|
| cOR (95% CI) | aOR (95% CI) | cOR (95% CI) | aOR (95% CI) | cOR (95% CI) | aOR (95% CI) | |
| Q1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 1.03 (0.95–1.13) | 1.03 (0.94–1.13) | 0.94 (0.84–1.06) | 0.92 (0.81–1.03) | 1.17 (1.03–1.34) | 1.22 (1.06–1.40) |
| Q3 | 1.04 (0.95–1.14) | 1.04 (0.94–1.14) | 0.99 (0.88–1.11) | 0.95 (0.84–1.07) | 1.11 (0.97–1.28) | 1.17 (1.01–1.35) |
| Q4 | 1.11 (1.02–1.21) | 1.09 (0.99–1.20) | 1.06 (0.95–1.19) | 1.00 (0.88–1.13) | 1.19 (1.04–1.36) | 1.24 (1.07–1.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Hisada, A.; Yamamoto, M.; Takatani, R.; Konno, Y.; Mori, C.; Sakurai, K.; The Japan Environment and Children’s Study (JECS) Group. Association Between Maternal Dietary Isoflavone Intake During Pregnancy and Childhood Allergic Rhinoconjunctivitis: The Japan Environment and Children’s Study. Nutrients 2025, 17, 769. https://doi.org/10.3390/nu17050769
Yang G, Hisada A, Yamamoto M, Takatani R, Konno Y, Mori C, Sakurai K, The Japan Environment and Children’s Study (JECS) Group. Association Between Maternal Dietary Isoflavone Intake During Pregnancy and Childhood Allergic Rhinoconjunctivitis: The Japan Environment and Children’s Study. Nutrients. 2025; 17(5):769. https://doi.org/10.3390/nu17050769
Chicago/Turabian StyleYang, Gui, Aya Hisada, Midori Yamamoto, Rieko Takatani, Yuki Konno, Chisato Mori, Kenichi Sakurai, and The Japan Environment and Children’s Study (JECS) Group. 2025. "Association Between Maternal Dietary Isoflavone Intake During Pregnancy and Childhood Allergic Rhinoconjunctivitis: The Japan Environment and Children’s Study" Nutrients 17, no. 5: 769. https://doi.org/10.3390/nu17050769
APA StyleYang, G., Hisada, A., Yamamoto, M., Takatani, R., Konno, Y., Mori, C., Sakurai, K., & The Japan Environment and Children’s Study (JECS) Group. (2025). Association Between Maternal Dietary Isoflavone Intake During Pregnancy and Childhood Allergic Rhinoconjunctivitis: The Japan Environment and Children’s Study. Nutrients, 17(5), 769. https://doi.org/10.3390/nu17050769







