The Preventive Impact of Chokeberry (Aronia melanocarpa L.) Extract Regarding the Disruption of Calcium and Phosphorus Homeostasis and Chosen Pathways of Its Regulation in an Animal Model of General Population Exposure to Cadmium
Abstract
1. Introduction
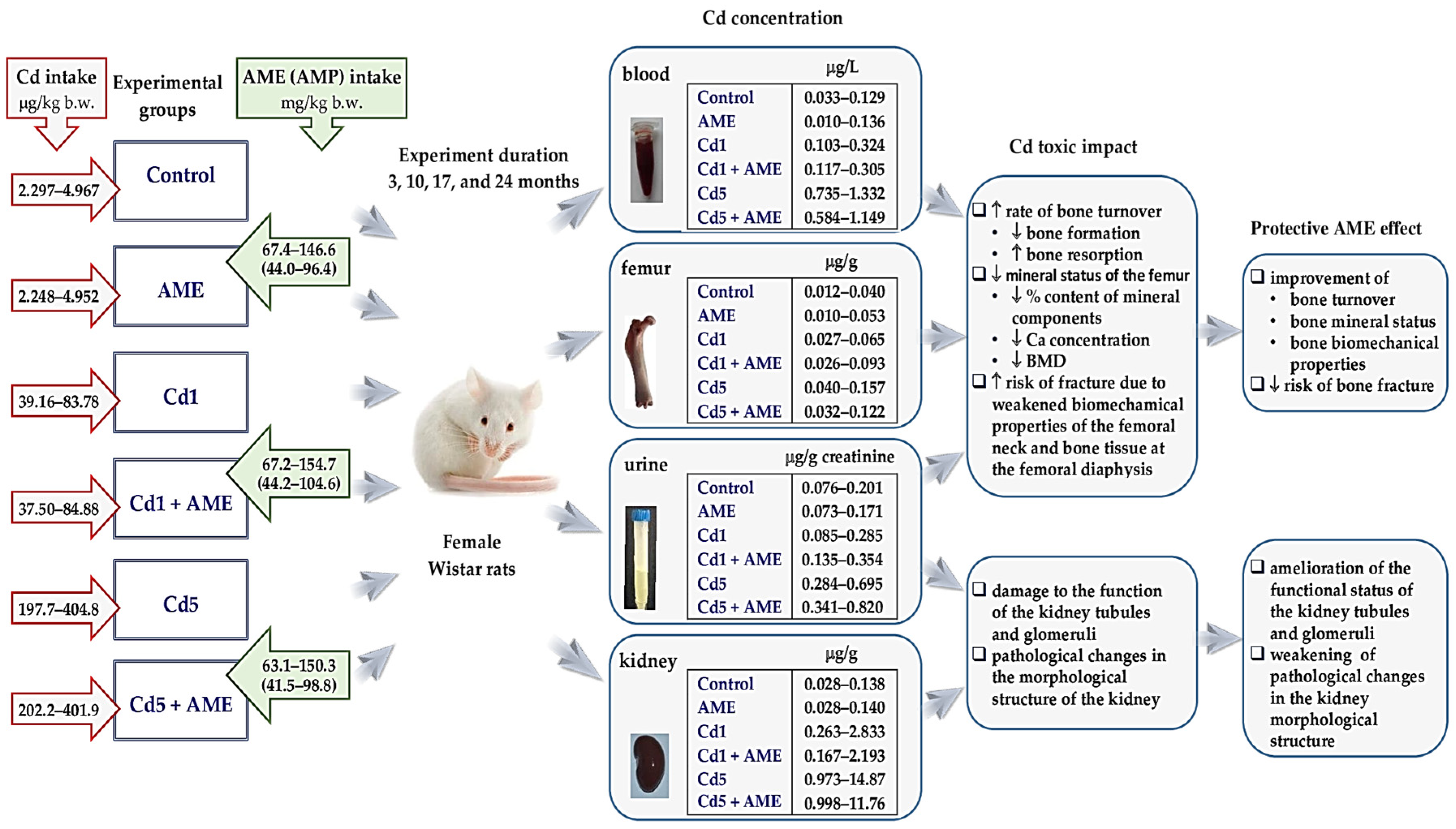
2. Materials and Methods
2.1. Laboratory Animals and Ethical Rules in Animal Research
2.2. Labofeed Diets with Added Cd
2.3. A. melanocarpa Berry Extract
2.4. Experimental Protocol
2.5. Analytical Procedures
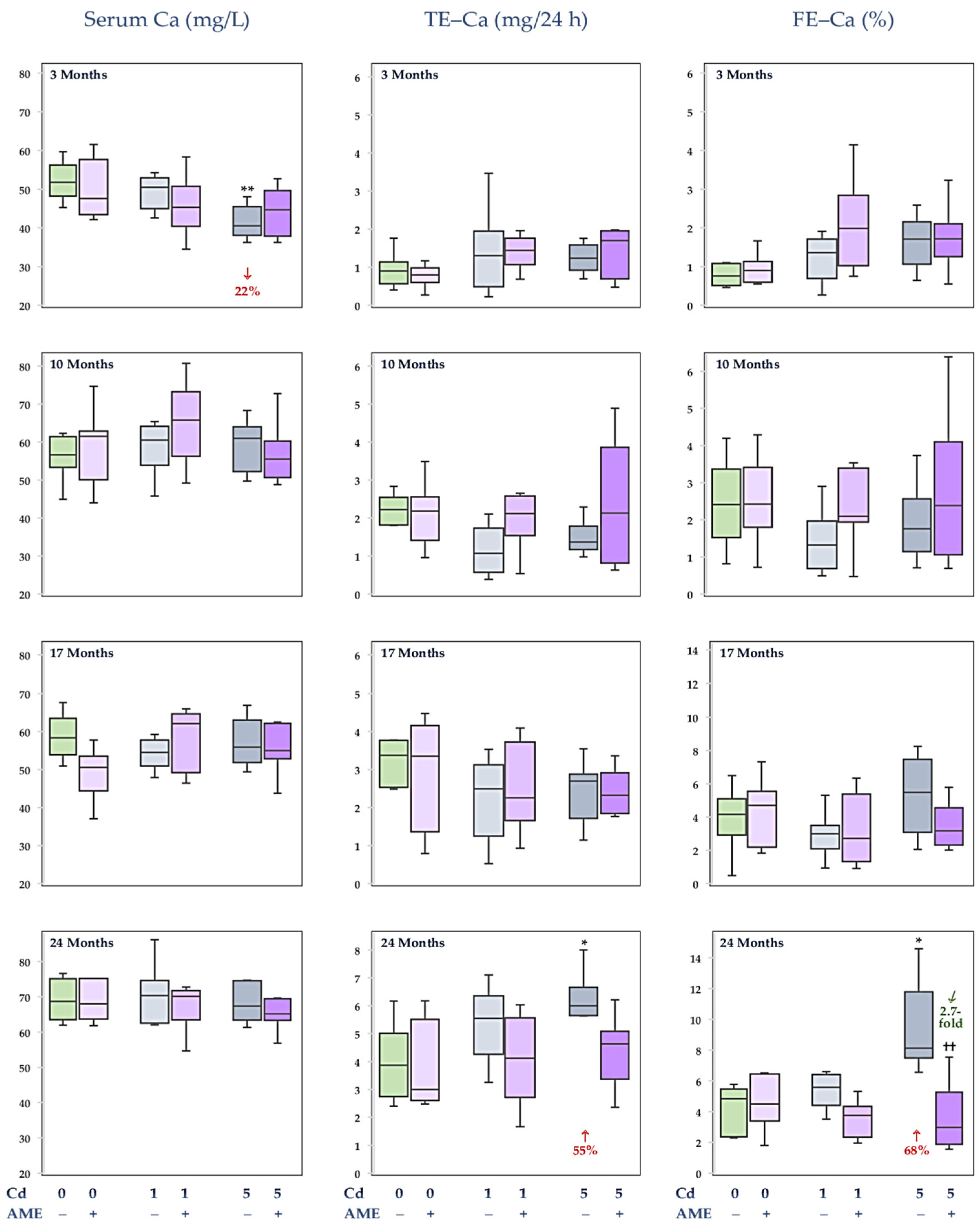
2.5.1. Measurement of the Concentration of Ca in the Serum and Urine and Evaluation of Its Fractional Excretion in the Urine
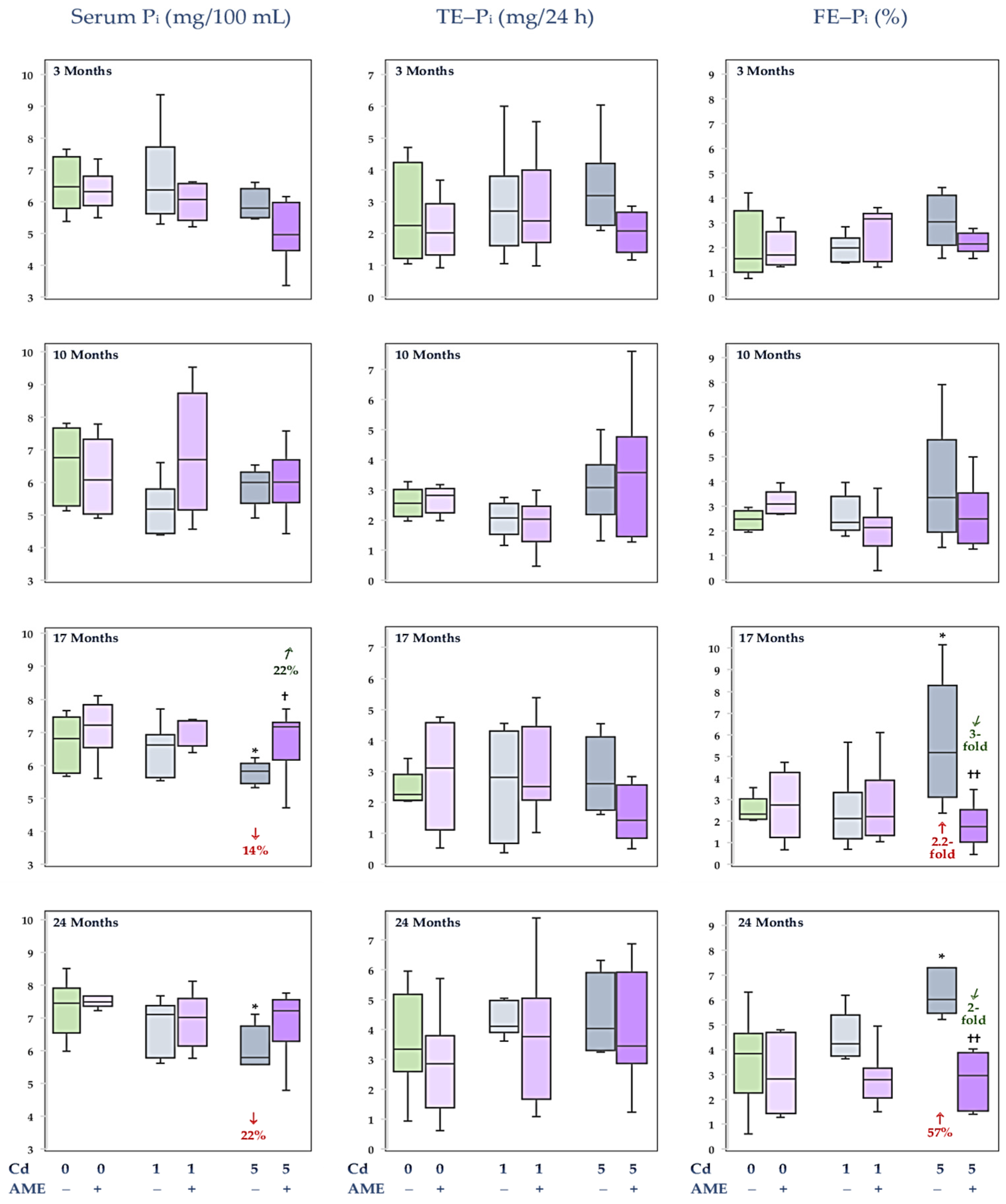
2.5.2. Measurement of the Concentration of Pi in the Serum and Urine and Evaluation of Its Fractional Excretion in the Urine
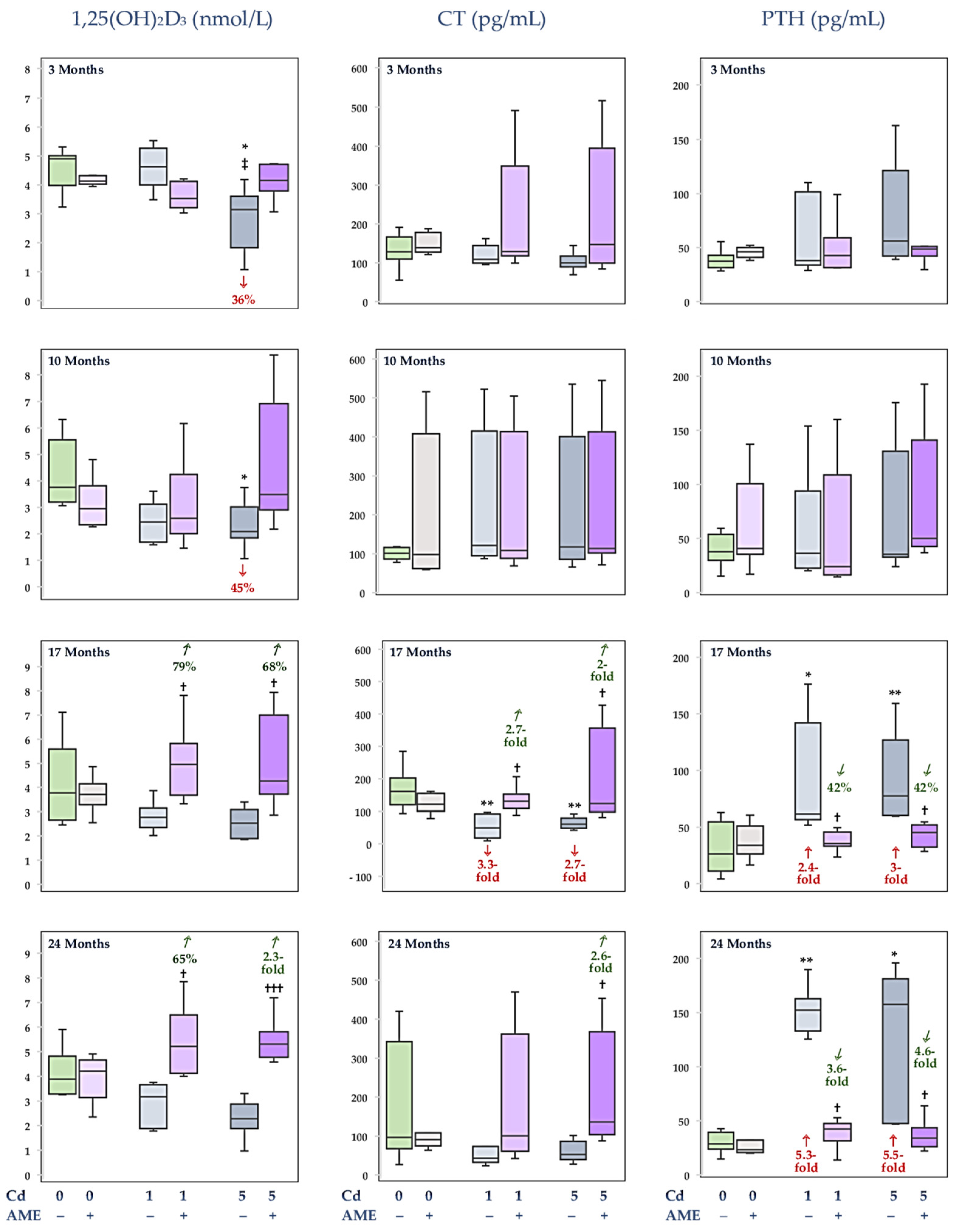
2.5.3. Quantification of Calciotropic Hormones in the Serum
2.5.4. Determination of Klotho Concentration in the Serum
2.5.5. Determination of 1alpha-OHase and 1,25(OH)2D3 Concentrations in the Kidney
2.6. Statistical Analysis
3. Results
3.1. The Impact of Exposure to Cd and/or Supplementation with AME on Ca Concentration in the Serum and Its Urinary Excretion
3.2. The Impact of Exposure to Cd and/or Supplementation with AME on Pi Concentration in the Serum and Its Urinary Excretion
3.3. The Impact of Exposure to Cd and/or Supplementation with AME on the Serum Concentrations of Calciotropic Hormones
3.4. The Impact of Exposure to Cd and/or Supplementation with AME on Klotho Concentration in the Serum
3.5. The Impact of Exposure to Cd and/or Supplementation with AME on the Concentrations of 1alpha-OHase and 1,25(OH)2D3 in the Kidney
3.6. Relationships Between Indices of Ca and Pi Metabolism and Markers of the Pathways of Its Regulation
3.7. Relationships Between the Determined Indices of the Metabolism of Ca and Pi and Markers of the Pathways of Its Regulation and Cd Content in the Body
3.8. Relationships Between the Determined Indices of the Metabolism of Ca and Pi and Markers of the Pathways of Its Regulation and Indices of the Bone and Kidney Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; Lane, N.E. Osteoporosis. Review of etiology, mechanisms, and approach to management in the aging population. Endocrinol. Metab. Clin. N. Am. 2023, 52, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Tański, W.; Kosiorowska, J.; Szymańska-Chabowska, A. Osteoporosis—Risk factors, pharmaceutical and non-pharmaceutical treatment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Kunioka, C.T.; Manso, M.C.; Carvalho, M. Association between environmental cadmium exposure and osteoporosis risk in postmenopausal women: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2023, 20, 485. [Google Scholar] [CrossRef]
- Buha, A.; Jugdaohsingh, R.; Matovica, V.; Bulata, Z.; Antonijevica, B.; Kernsc, J.G.; Goodshipd, A.; Hartd, A.; Powell, J.J. Bone mineral health is sensitively related to environmental cadmium exposure—Experimental and human data. Environ. Res. 2019, 176, 108539. [Google Scholar] [CrossRef] [PubMed]
- Malin Igra, A.; Vahter, M.; Raqib, R.; Kippler, M. Early-life cadmium exposure and bone-related biomarkers: A longitudinal study in children. Environ. Health Perspect. 2019, 127, 37003. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, T.; Zhao, M.; Fu, H.; Wang, J.; Xu, Q. Protective effects of moderate Ca supplementation against Cd-induced bone damage under different population-relevant doses in young female rats. Nutrients 2019, 11, 849. [Google Scholar] [CrossRef]
- Lei, Y.; Guo, M.; Xie, J.; Liu, X.; Li, X.; Wang, H.; Xu, Y.; Zheng, D. Relationship between blood cadmium levels and bone mineral density in adults: A cross-sectional study. Front. Endocrinol. 2024, 15, 1354577. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, A.; Cirovic, A.; Yimthiang, S.; Vesey, D.A.; Satarug, S. Modulation of adverse health effects of environmental cadmium exposure by zinc and its transporters. Biomolecules 2024, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef]
- Satarug, S. Is chronic kidney disease due to cadmium exposure inevitable and can it be reversed? Biomedicines 2024, 12, 718. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Harada, S.; Eitaki, Y.; Kurihara, A.; Kato, S.; Kuwabara, K.; Iida, M.; Hirata, A.; Sata, M.; Matsumoto, M.; et al. A population-based urinary and plasma metabolomics study of environmental exposure to cadmium. Environ. Health Prev. Med. 2024, 29, 22. [Google Scholar] [CrossRef]
- Verzelloni, P.; Urbano, T.; Wise, L.A.; Vinceti, M.; Filippini, T. Cadmium exposure and cardiovascular disease risk: A systematic review and dose-response meta-analysis. Environ. Pollut. 2024, 345, 123462. [Google Scholar] [CrossRef] [PubMed]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of cadmium neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.G.; Ahmed, K.; Zaidi, S.F.; Muhammad, J.S. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat. Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, W.; Zhu, C.; Yang, S.; Zhou, M.; Wang, B.; Wang, X.; Wang, D.; Ma, J.; Zhou, Y.; et al. Cadmium exposure, fasting blood glucose changes, and type 2 diabetes mellitus: A longitudinal prospective study in China. Environ. Res. 2021, 192, 110259. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols on cadmium accumulation in the body: A study in a rat model of human exposure to this metal. Curr. Drug Targets 2015, 16, 1470–1487. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Roszczenko, A.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. Biol. Interact. 2015, 229, 132–146. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Roszczenko, A.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Mężyńska, M. Protective effect of chokeberry (Aronia melanocarpa L.) extract against cadmium impact on the biomechanical properties of the femur: A study in a rat model of low and moderate lifetime women exposure to this heavy metal. Nutrients 2017, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Rogalska, J.; Roszczenko, A.; Gałażyn-Sidorczuk, M.; Tomczyk, M. The mechanism of the osteoprotective action of a polyphenol-rich Aronia melanocarpa extract during chronic exposure to cadmium is mediated by the oxidative defense system. Planta Med. 2016, 82, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Smereczański, N.M.; Brzóska, M.M.; Rogalska, J.; Hutsch, T. The protective potential of Aronia melanocarpa L. berry extract against cadmium-induced kidney damage: A study in an animal model of human environmental exposure to this toxic element. Int. J. Mol. Sci. 2023, 24, 11647. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Kozłowska, M.; Smereczański, N.M. The body status of manganese and activity of this element-dependent mitochondrial superoxide dismutase in a rat model of human exposure to cadmium and co-administration of Aronia melanocarpa L. extract. Nutrients 2022, 14, 4773. [Google Scholar] [CrossRef]
- Ruczaj, A.; Brzóska, M.M.; Rogalska, J. The protective impact of Aronia melanocarpa L. berries extract against prooxidative cadmium action in the brain—A study in an in vivo model of current environmental human exposure to this harmful element. Nutrients 2024, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.; Arif, H.; Tabassum, B.; Rehman, S.; Bajaj, P.; Sirohi, R.; Khan, M.F.A. An overview of the ameliorative efficacy of Catharanthus roseus extract against Cd2+ toxicity: Implications for human health and remediation strategies. Front. Public Health 2024, 12, 1327611. [Google Scholar] [CrossRef]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Mognetti, B.; Franco, F.; Castrignano, C.H.; Bovolin, P.; Berta, G.N. Mechanisms of phytoremediation by resveratrol against cadmium toxicity. Antioxidants 2024, 13, 782. [Google Scholar] [CrossRef]
- Sandbichler, A.M.; Höckner, M. Cadmium protection strategies—A hidden trade-off? Int. J. Mol. Sci. 2016, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Górka, I. Alleviating effect of α-lipoic acid and magnesium on cadmium-induced inflammatory processes, oxidative stress and bone metabolism disorders in Wistar rats. Int. J. Environ. Res. Public Health 2019, 16, 4483. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: Studies on a rat model of human environmental exposure. Toxicol. Sci. 2004, 82, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Effect of exposure to cadmium on the mineral status and mechanical properties of lumbar spine of male rats. Toxicol. Lett. 2005, 157, 161–172. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Effect of low-level lifetime exposure to cadmium on calciotropic hormones in aged female rats. Arch. Toxicol. 2005, 79, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M. Mechanism of bone mineralization. Cold Spring Harb. Perspect. Med. 2018, 8, a031229. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflugers Arch. Eur. J. Physiol. 2019, 471, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Chacar, F.C.; Kogika, M.M.; Zafalon, R.V.A.; Brunetto, M.A. Vitamin D metabolism and its role in mineral and bone disorders in chronic kidney disease in humans, dogs and cats. Metabolites 2020, 10, 499. [Google Scholar] [CrossRef]
- Chwalba, A.; Orłowska, J.; Słota, M.; Jeziorska, M.; Filipecka, K.; Bellanti, F.; Dobrakowski, M.; Kasperczyk, A.; Zalejska-Fiolka, J.; Kasperczyk, S. Effect of cadmium on oxidative stress indices and vitamin D concentrations in children. J. Clin. Med. 2023, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Pleić, N.; Gunjača, I.; Zemunik, T. Environmental factors that affect parathyroid hormone and calcitonin levels. Int. J. Mol. Sci. 2022, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Andreollo, N.A.; Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.L.; Wolf, M. Calcium and phosphate disorders: Core curriculum 2024. Am. J. Kidney Dis. 2024, 83, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Xing, C.; Hu, M.C. Alpha Klotho and phosphate homeostasis. J. Endocrinol. Investig. 2014, 37, 1121–1126. [Google Scholar] [CrossRef]
- Tan, S.J.; Smith, E.R.; Holt, S.G.; Hewitson, T.D.; Toussaint, N.D. Soluble Klotho may be a marker of phosphate reabsorption. Clin. Kidney J. 2017, 10, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Moe, O.W. Klotho: A novel regulator of calcium and phosphorus homeostasis. Flugers Arch. Eur. J. Physiol. 2011, 462, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Toro-Román, V.; Robles-Gil, M.C.; Munoz, D.; Bartolomé, I.; Grijota, F.J.; Maynar-Marino, M. Sex differences in cadmium and lead concentrations in different biological matrices in athletes. Relationship with iron status. Environ. Toxicol. Pharmacol. 2023, 99, 104107. [Google Scholar] [CrossRef]
- Lee, B.K.; Kim, Y. Sex-specific profiles of blood metal levels associated with metal–iron interactions. Saf. Health Work 2014, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, L.; Lv, X.; Liu, M.; Zhang, Y.; He, L.; Hao, J.; Xi, L.; Qu, H.; Shi, C.; et al. Intestinal segment and vitamin D3 concentration affect gene expression levels of calcium and phosphorus transporters in broiler chickens. J. Anim. Sci. Technol. 2023, 65, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, W.; Bai, L.; Li, H.; Wang, L.; Zou, X. Dietary cadmium chloride supplementation impairs renal function and bone metabolism in laying hens. Animals 2019, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lu, H.; Yin, Z.; Dai, S.; Jia, R.; Xu, J.; Song, X.; Li, L. Effects of mixed subchronic lead acetate and cadmium chloride on bone metabolism in rats. Int. J. Clin. Exp. Med. 2014, 7, 1378–1385. [Google Scholar]
- Delrue, C.; Speeckaert, M.M. Vitamin D and vitamin D-binding protein in health and disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef]
- Engström, A.; Skerving, S.; Lidfeldt, J.; Burgaz, A.; Lundh, T.; Samsioe, G.; Vahter, M.; Åkesson, A. Cadmium-induced bone effect is not mediated via low serum 1,25-dihydroxy vitamin D. Environ. Res. 2009, 109, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dai, Y.; Wang, Z.; Zhu, G.; Ding, X.; Jin, T. The association between serum vitamin D levels and renal tubular dysfunction in a general population exposed to cadmium in China. PLoS ONE 2018, 13, e0195682. [Google Scholar] [CrossRef]
- Niwano, Y.; Kohzaki, H.; Shirato, M.; Shishido, S.; Nakamura, K. Anti-osteoporotic mechanisms of polyphenols elucidated based on in vivo studies using ovariectomized animals. Antioxidants 2022, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Nicolin, V.; De Tommasi, N.; Nori, S.L.; Costantinides, F.; Berton, F.; Di Lenarda, R. Modulatory effects of plant polyphenols on bone remodeling: A prospective view from the bench to bedside. Front. Endocrinol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Xu, K.; Chen, X. Intake of dietary flavonoids in relation to bone loss among U.S. adults: A promising strategy for improving bone health. Food Funct. 2024, 15, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.K.; Maiz, M.; Cao, S.; Lachcik, P.J.; Peacock, M.; McCabe, G.P.; McCabe, L.D.; Cladis, D.P.; Jackson, G.S.; Ferruzzi, M.G.; et al. Moderate consumption of freeze-dried blueberry powder increased net bone calcium retention compared with no treatment in healthy postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2023, 118, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry juice antioxidants protect osteogenic activity against oxidative stress and improve long-term activation of the mineralization process in human osteoblast-like SaOS-2 cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef]
- Faienza, M.F.; Giardinelli, S.; Annicchiarico, A.; Chiarito, M.; Barile, B.; Corbo, F.; Brunetti, G. Nutraceuticals and functional foods: A comprehensive review of their role in bone health. Int. J. Mol. Sci. 2024, 25, 5873. [Google Scholar] [CrossRef]
- Hassan, J.K.; Sharrad, A.K.; Sheri, F.H. Effect of quercetin supplement on some bone mineralization biomarkers in diabetic type 2 patients. Adv. Pharmacol. Pharm. 2018, 6, 43–49. [Google Scholar] [CrossRef]
- Marchionatti, A.M.; Pacciaroni, A.; Tolosa de Talamoni, N.G. Effects of quercetin and menadione on intestinal calcium absorption and the underlying mechanisms. Comp. Biochem. Physiol. A 2013, 164, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Quercetin as an agent for protecting the bone: A review of the current evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.I.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.A.; Mohamed, E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Biol. Interact. 2017, 271, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Tang, C.H.; Ho, T.L.; Wang, W.L.; Yao, C.H. Chlorogenic acid prevents ovariectomized-induced bone loss by facilitating osteoblast functions and suppressing osteoclast formation. Aging 2024, 16, 4832. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Chen, L.; Chen, Y.; Zhang, Z.; Wang, X.; Zhou, B. Cyanidin-3-glucoside regulates osteoblast differentiation via the ERK1/2 signaling pathway. ACS Omega 2021, 6, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Cladis, D.P.; Debelo, H.; Lachcik, P.J.; Ferruzzi, M.G.; Weaver, C.M. Increasing doses of blueberry polyphenols alters colonic metabolism and calcium absorption in ovariectomized rats. Mol. Nutr. Food Res. 2020, 64, 2000031. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E. Chokeberry (A. melanocarpa (Michx.) Elliott)—A natural product for metabolic disorders? Nutrients 2022, 14, 2688. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, J.E.; Nam, Y.E.; Kim, W.S.; Lee, I.; Yim, S.V.; Kwon, O. Eight-week supplementation of Aronia berry extract promoted the glutathione defence system against acute aerobic exercise-induced oxidative load immediately and 30 min post-exercise in healthy adults: A double-blind, randomised controlled trial. J. Hum. Nutr. Diet 2023, 36, 1589–1599. [Google Scholar] [CrossRef]
- Milutinovic, M.; Velickovic Radovanovic, R.; Savikin, K.; Radenkovic, S.; Arvandi, M.; Pesic, M.; Kostic, M.; Miladinovic, B.; Brankovic, S.; Kitic, D. Chokeberry juice supplementation in type 2 diabetic patients—Impact on health status. J. Appl. Biomed. 2019, 17, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The effect of long-term Aronia melanocarpa extract supplementation on cognitive performance, mood, and vascular function: A randomized controlled trial in healthy, middle-aged individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, B.; Cieślicka, M.; Mieszkowski, J.; Kochanowicz, A.; Niespodziński, B.; Szwarc, A.; Waldziński, T.; Reczkowicz, J.; Piskorska, E.; Petr, M.; et al. Effect of supplementation with black chokeberry (Aronia melanocarpa) extract on inflammatory status and selected markers of iron metabolism in young football players: A randomized double-blind trial. Nutrients 2023, 15, 975. [Google Scholar] [CrossRef] [PubMed]


| Certified Reference Material | Reference Values | Noticed Values 1 | Recovery | Precision (CV) 2 |
|---|---|---|---|---|
| Trace Elements Serum L-1 LOT 0903106 (SERO AS, Billingstad, Norway) | 88.3–103.7 mg/L (mean 96.0 ± 7.7 mg/L) | 92.8 ± 2.7 mg/L | 97% | 2.9% |
| Trace Elements Urine L-2 LOT 1011645 (SeronormTM, Billingstad, Norway) | 71 mg/L | 67.3 ± 1.4 mg/L | 95% | 2.1% |
| Parameter | Serum | Urine | Kidney | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Pi | 1,25(OH)2D3 | CT | PTH | Klotho | TE–Ca | FE–Ca | TE–Pi | FE–Pi | 1alpha-OHase | 1,25(OH)2D3 | ||
| Serum | Ca | 0.206 a | 0.233 a | NS | NS | NS | 0.430 c | NS | NS | NS | NS | −0.474 c | |
| Pi | 0.210 a | NS | NS | NS | NS | 0.368 c | 0.412 c | NS | NS | NS | −0.447 c | ||
| 1,25(OH)2D3 | −0.239 a | 0.261a | 0.203 a | NS | NS | 0.233 a | NS | NS | NS | NS | −0.357 c | ||
| CT | −0.208 a | NS | 0.373 c | 0.320 b | NS | NS | −0.232 a | NS | NS | 0.227 a | NS | ||
| PTH | NS | NS | −0.413 c | −0.352 c | NS | NS | NS | NS | NS | NS | NS | ||
| Klotho | −0.263 b | NS | 0.375 c | 0.600 c | −0.476 c | NS | NS | NS | NS | NS | NS | ||
| Urine | TE–Ca | 0.549 c | 0.228 a | NS | −0.364 c | NS | −0.440 c | 0.815 c | 0.554 c | 0.441 c | 0.210 a | −0.610 c | |
| FE–Ca | 0.483 c | NS | −0.225 a | −0.423 c | 0.256 a | −0.557 c | 0.891 c | 0.287 b | 0.385 c | NS | −0.439 c | ||
| TE–Pi | 0.261 b | NS | NS | −0.346 c | 0.252 a | NS | 0.549 c | 0.468 c | 0.818 c | NS | −0.212 a | ||
| FE–Pi | 0.370 c | NS | −0.284 b | −0.389 c | 0.300 b | −0.366 c | 0.585 c | 0.699 c | 0.766 c | NS | NS | ||
| Kidney | 1alpha-OHase | NS | NS | 0.287 b | 0.199 a | −0.195 a | 0.192 c | NS | NS | NS | −0.204 a | NS | |
| 1,25(OH)2D3 | −0.501 c | NS | 0.438 c | 0.477 c | −0.382 c | 0.708 c | −0.575 c | −0.670 c | −0.305 b | −0.492 c | 0.369 c | ||
| Parameter | Cd in the Blood | Cd in the Kidney | Cd in the Urine | |||
|---|---|---|---|---|---|---|
| Without AME | With AME | Without AME | With AME | Without AME | With AME | |
| Serum Ca | NS | NS | NS | 0.249 a | NS | NS |
| TE–Ca | NS | NS | NS | 0.319 b | NS | NS |
| FE–Ca | 0.202 a | NS | 0.357 c | 0.207 a | NS | NS |
| Serum Pi | −0.315 b | NS | NS | NS | −0.272 b | NS |
| TE–Pi | NS | NS | 0.251 a | NS | 0.246 a | NS |
| FE–Pi | 0.378 c | NS | 0.482 c | NS | 0.397 c | NS |
| Serum 1,25(OH)2D3 | −0.556 c | 0.281 b | −0.594 c | 0.280 b | −0.406 c | 0.272 b |
| Serum CT | −0.415 c | NS | −0.472 c | NS | −0.332 b | NS |
| Serum PTH | 0.459 c | NS | 0.535 c | NS | 0.417 c | NS |
| Serum Klotho | −0.447 c | NS | −0.548 c | NS | −0.322 b | NS |
| Kidney 1alpha-OHase | −0.475 c | NS | −0.551 c | NS | −0.548 c | NS |
| Kidney 1,25(OH)2D3 | −0.444 c | −0.343 c | −0.594 c | −0.567 c | −0.322 b | −0.239 a |
| Parameter | Serum | Urine | Kidney | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Pi | 1,25(OH)2D3 | CT | PTH | Klotho | TE–Ca | FE–Ca | TE–Pi | FE–Pi | 1alpha-OHase | 1,25(OH)2D3 | ||
| Bone turnover | |||||||||||||
| Serum | OC | NS | NS | 0.372 c | NS | NS | NS | NS | NS | NS | NS | NS | 0.257 a |
| ALP | 0.317 b | NS | NS | NS | NS | NS | 0.357 c | 0.274 b | NS | NS | NS | −0.221 a | |
| CTX | −0.226 a | NS | NS | NS | 0.344 c | −0.300 b | NS | NS | NS | NS | NS | NS | |
| Bone tissue—distal femoral epiphysis | ALP | −0.674 c | NS | 0.383 c | 0.540 c | −0.309 b | 0.690 c | −0.677 c | −0.719 c | −0.298 b | −0.496 c | NS | 0.778 c |
| PC I | 0.445 c | 0.253 a | NS | NS | NS | −0.233 a | 0.408 c | 0.413 c | NS | NS | NS | −0.369 c | |
| OPG | −0.242 a | 0.271b | 0.445 c | 0.362 c | −0.357 c | 0.317 b | NS | NS | −0.218 a | −0.372 c | 0.347 c | 0.467 c | |
| sRANKL | NS | NS | −0.252 a | −0.460 c | 0.489 c | −0.621 c | 0.292 b | 0.407 c | 0.204 a | 0.251a | NS | −0.409 c | |
| sRANKL/OPG | NS | NS | −0.544 c | −0.466 c | 0.605 c | −0.598 c | 0.222 a | 0.353 c | 0.255 a | 0.398c | −0.360 c | −0.635 c | |
| Mineral status | |||||||||||||
| Femur | % mineral comp. 3 | −0.398 c | NS | NS | 0.380 c | −0.307 b | 0.477 c | −0.293 b | −0.385 c | NS | −0.354 c | 0.363 c | 0.615 c |
| Ca 3 | −0.312 b | NS | NS | 0.424 c | −0.306 b | 0.390 c | −0.273 b | −0.284 b | −0.304 b | −0.362 c | 0.272 b | 0.398 c | |
| BMD | NS | 0.308 b | 0.484 c | 0.218 a | −0.229 a | NS | 0.275 b | NS | NS | NS | 0.279 b | NS | |
| Bone biomechanical properties | |||||||||||||
| Femoral neck | Yield strength | NS | NS | 0.316 a | NS | −0.266 b | NS | NS | NS | −0.255 a | NS | NS | NS |
| Fracture strength | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Femoral diaphysis | Yield strength | 0.538 c | 0.278 b | NS | NS | NS | −0.282 b | 0.510 c | 0.487 c | NS | 0.239 a | NS | −0.427 c |
| Fracture strength | 0.608 c | 0.242 a | −0.249 a | −0.306 b | NS | −0.514 c | 0.590 c | 0.638 c | NS | 0.403 c | NS | −0.670 c | |
| Parameter | Serum | Urine | Kidney | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Pi | 1,25(OH)2D3 | CT | PTH | Klotho | TE–Ca | FE–Ca | TE–Pi | FE–Pi | 1alpha-OHase | 1,25(OH)2D3 | ||
| Bone turnover | |||||||||||||
| Serum | OC | NS | NS | NS | NS | NS | NS | 0.253 a | NS | NS | NS | 0.202 a | −0.276 b |
| ALP | 0.342 c | 0.321 b | 0.222 a | −0.230 a | −0.332 b | NS | 0.374 c | 0.250 a | NS | NS | NS | −0.572 c | |
| CTX | −0.369 c | −0.252 a | NS | NS | NS | NS | NS | −0.251 a | NS | NS | NS | NS | |
| Bone tissue—distal femoral epiphysis | ALP | −0.565 c | −0.434 c | −0.268 b | 0.216 a | 0.236 a | NS | −0.552 c | −0.486 c | NS | NS | −0.321 b | 0.620 c |
| PC I | 0.360 c | 0.319 b | NS | NS | NS | NS | 0.437 c | 0.412 c | NS | NS | NS | −0.460 c | |
| OPG | −0.311 b | 0.529 c | 0.250 a | NS | NS | NS | 0.438 c | 0.378 c | NS | NS | 0.341 c | −0.664 c | |
| sRANKL | −0.359 c | NS | NS | NS | NS | −0.241 a | NS | NS | NS | NS | NS | NS | |
| sRANKL/OPG | −0.491 c | −0.447 c | −0.218 a | NS | NS | NS | −0.451 c | −0.337 c | NS | NS | −0.214 a | 0.618 c | |
| Mineral status | |||||||||||||
| Femur | % mineral comp. 3 | −0.397 c | −0.236 a | −0.228 a | NS | 0.312 b | NS | −0.356 c | −0.252 a | NS | NS | NS | 0.660 c |
| Ca 3 | −0.262 a | NS | −0.230 a | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| BMD | NS | 0.300 b | NS | NS | −0.272 b | NS | 0.326 b | 0.320 b | NS | NS | 0.246 a | −0.331 b | |
| Bone biomechanical properties | |||||||||||||
| Femoral neck | Yield strength | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Fracture strength | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Femoral diaphysis | Yield strength | 0.468 c | 0.476 c | 0.301 b | NS | NS | NS | 0.605 c | 0.475 c | NS | NS | 0.273 b | −0.733 c |
| Fracture strength | 0.433 c | 0.495 c | 0.350 c | NS | NS | NS | 0.537 c | 0.417 c | NS | NS | 0.225 a | −0.774 c | |
| Parameter | Serum | Urine | Kidney | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Pi | 1,25(OH)2D3 | CT | PTH | Klotho | TE–Ca | FE–Ca | TE–Pi | FE–Pi | 1alpha-OHase | 1,25(OH)2D3 | ||
| Urine | KIM-1 | NS | NS | −0.433 c | −0.361 c | 0.523 c | −0.460 c | NS | 0.333 b | 0.244 a | 0.369 c | −0.272 b | −0.367 c |
| β2-MG | 0.433 c | NS | −0.438 c | −0.592 c | 0.399 c | −0.579 c | 0.581 c | 0.710 c | 0.453 c | 0.644 c | −0.312 b | −0.658 c | |
| NAG | 0.493 c | NS | −0.548 c | −0.452 c | 0.416 c | −0.451 c | 0.392 c | 0.489 c | 0.218 a | 0.471 c | −0.320 b | −0.608 c | |
| ACR | 0.359 c | NS | −0.420 c | −0.564 c | 0.467 c | −0.608 c | 0.531 c | 0.688 c | 0.530 c | 0.728 c | −0.256 a | −0.622 c | |
| PCR | 0.514 c | NS | −0.520 c | −0.469 c | 0.392 c | −0.540 c | 0.419 c | 0.561 c | 0.218 a | 0.500 c | −0.324 b | −0.677 c | |
| ALP | 0.420 c | NS | −0.623 c | −0.342 c | 0.356 c | −0.385 c | NS | 0.294 b | NS | 0.309 b | −0.313 b | −0.493 c | |
| Uric acid | NS | NS | NS | NS | NS | NS | 0.212 a | NS | 0.686 c | 0.494 c | NS | NS | |
| Urea | NS | NS | NS | −0.232 a | 0.221 a | NS | 0.340 c | 0.309 b | 0.602 c | 0.425 c | NS | NS | |
| Serum | Uric acid | NS | NS | NS | −0.225 a | 0.253 a | −0.454 c | 0.287 b | 0.319 b | NS | NS | NS | −0.278 b |
| Urea | NS | −0.220 a | −0.289 b | −0.233 a | 0.301 b | −0.221 a | NS | 0.329 b | 0.210 a | 0.402 c | −0.203 a | −0.338 c | |
| Creatinine clearance | −0.288 b | NS | 0.294 b | 0.225 a | NS | 0.475 c | −0.266 b | −0.577 c | NS | −0.495 c | NS | 0.519 c | |
| Parameter | Serum | Urine | Kidney | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Pi | 1,25(OH)2D3 | CT | PTH | Klotho | TE–Ca | FE–Ca | TE–Pi | FE–Pi | 1alpha-OHase | 1,25(OH)2D3 | ||
| Urine | KIM-1 | NS | 0.365 c | NS | NS | NS | NS | 0.373 c | 0.325 b | 0.375 c | 0.281 b | 0.308 b | −0.321 b |
| β2-MG | 0.300 b | 0.453 c | NS | NS | NS | NS | 0.524 c | 0.472 c | 0.437 c | 0.417 c | 0.223 a | −0.487 c | |
| NAG | 0.237 a | 0.313 b | NS | NS | NS | NS | 0.251 a | 0.212 a | NS | NS | NS | −0.389 c | |
| ACR | 0.356 c | 0.416 c | 0.229 a | NS | NS | NS | 0.388 c | 0.317 b | 0.272 b | 0.226 a | 0.286 b | −0.483 c | |
| PCR | 0.232 a | 0.211 a | NS | NS | NS | NS | NS | 0.247 a | NS | NS | NS | −0.305 b | |
| ALP | 0.346 c | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | −0.277 b | |
| Uric acid | NS | NS | NS | NS | NS | NS | 0.378 c | 0.262 a | 0.611 c | 0.579 c | NS | NS | |
| Urea | NS | NS | NS | NS | NS | NS | NS | NS | 0.554 c | 0.470 c | NS | NS | |
| Serum | Uric acid | NS | NS | 0.262 b | NS | NS | NS | NS | NS | NS | NS | 0.436 c | −0.245 a |
| Urea | NS | NS | −0.250 a | NS | NS | NS | 0.226 a | NS | NS | NS | NS | NS | |
| Creatinine clearance | NS | NS | NS | NS | NS | NS | NS | −0.349 c | 0.338 c | NS | NS | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Rogalska, J. The Preventive Impact of Chokeberry (Aronia melanocarpa L.) Extract Regarding the Disruption of Calcium and Phosphorus Homeostasis and Chosen Pathways of Its Regulation in an Animal Model of General Population Exposure to Cadmium. Nutrients 2025, 17, 702. https://doi.org/10.3390/nu17040702
Brzóska MM, Gałażyn-Sidorczuk M, Rogalska J. The Preventive Impact of Chokeberry (Aronia melanocarpa L.) Extract Regarding the Disruption of Calcium and Phosphorus Homeostasis and Chosen Pathways of Its Regulation in an Animal Model of General Population Exposure to Cadmium. Nutrients. 2025; 17(4):702. https://doi.org/10.3390/nu17040702
Chicago/Turabian StyleBrzóska, Małgorzata M., Małgorzata Gałażyn-Sidorczuk, and Joanna Rogalska. 2025. "The Preventive Impact of Chokeberry (Aronia melanocarpa L.) Extract Regarding the Disruption of Calcium and Phosphorus Homeostasis and Chosen Pathways of Its Regulation in an Animal Model of General Population Exposure to Cadmium" Nutrients 17, no. 4: 702. https://doi.org/10.3390/nu17040702
APA StyleBrzóska, M. M., Gałażyn-Sidorczuk, M., & Rogalska, J. (2025). The Preventive Impact of Chokeberry (Aronia melanocarpa L.) Extract Regarding the Disruption of Calcium and Phosphorus Homeostasis and Chosen Pathways of Its Regulation in an Animal Model of General Population Exposure to Cadmium. Nutrients, 17(4), 702. https://doi.org/10.3390/nu17040702






