Optimizing Nutritional Management Before and After Bariatric Surgery: A Comprehensive Guide for Sustained Weight Loss and Metabolic Health
Abstract
1. Introduction
2. Methodology
3. Preoperative Nutritional Management
3.1. Nutritional Evaluation and Screening
3.2. Preoperative Dietary Optimization
3.3. Managing Comorbid Conditions
3.4. Pre-Surgical Exercise Recommendations
4. Postoperative Nutritional Management
4.1. Immediate Postoperative Phase
4.2. Long-Term Nutritional Strategies
4.3. Protein Intake
4.4. Preventing Common Postoperative Complications
4.5. Long-Term Follow-Up and Monitoring
5. Nutritional Challenges and Considerations for Specific Bariatric Procedures
5.1. Roux-en-Y Gastric Bypass (RYGB)
5.2. Sleeve Gastrectomy and Adjustable Gastric Banding
5.3. Biliopancreatic Diversion with Duodenal Switch (BPD/DS)
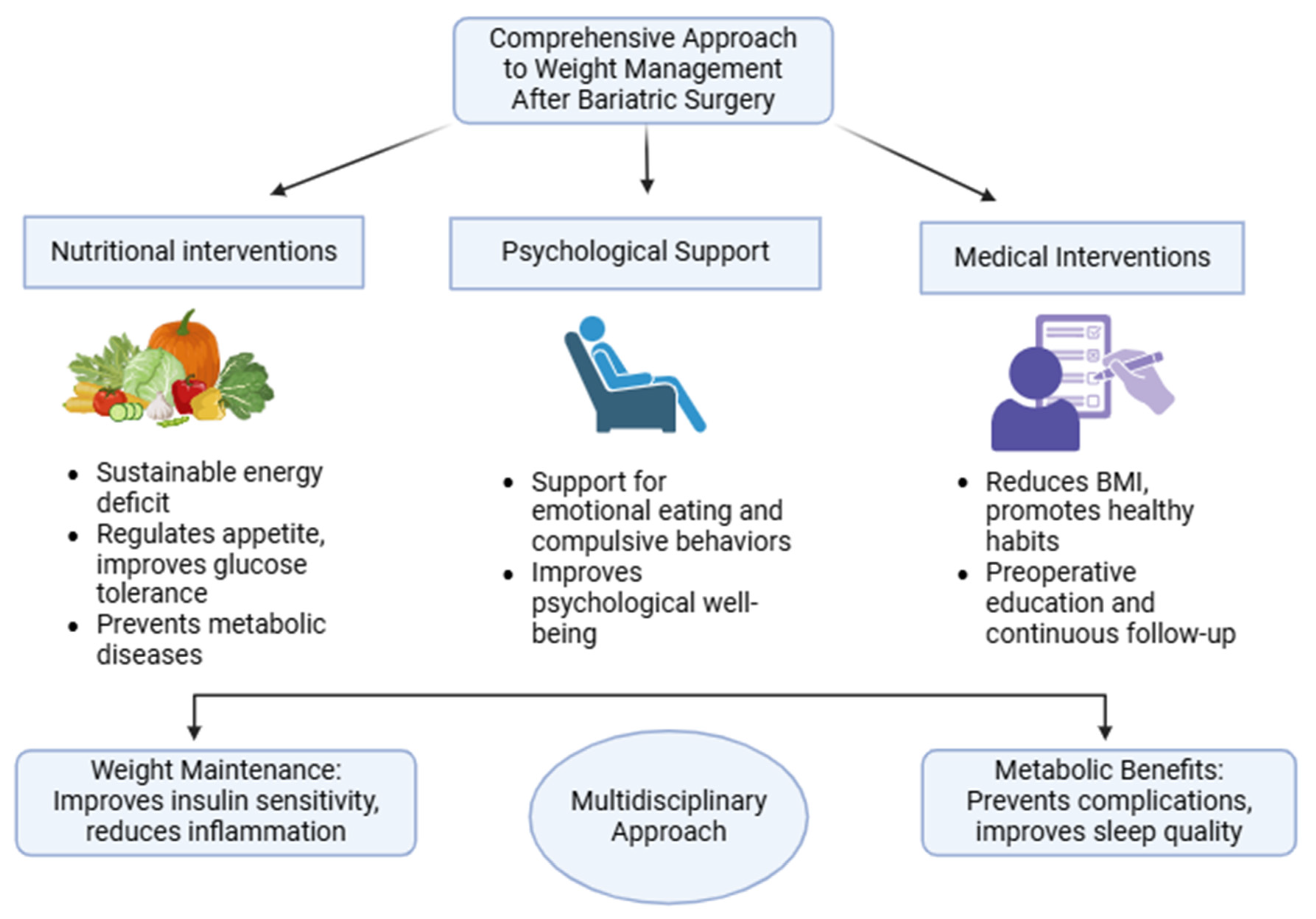
6. Role of Nutrition in Long-Term Weight Maintenance and Metabolic Health
6.1. Importance of Adherence to Nutritional Recommendations
6.2. Role of Physical Activity
6.3. Addressing Weight Regain
7. Future Directions and Research Gaps
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23 (Suppl. S1), 3–16. [Google Scholar] [CrossRef]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Frias-Toral, E.; Garcia-Velasquez, E.; de Los Angeles Carignano, M.; Rodriguez-Veintimilla, D.; Alvarado-Aguilera, I.; Bautista-Litardo, N. Polycystic ovary syndrome and obesity: Clinical aspects and nutritional management. Minerva Endocrinol. 2022, 47, 215–241. [Google Scholar] [CrossRef] [PubMed]
- Chapela, S.P.; Simancas-Racines, A.; Ceriani, F.; Martinuzzi, A.L.N.; Russo, M.P.; Zambrano, A.K.; Simancas-Racines, D.; Verde, L.; Muscogiuri, G.; Katsanos, C.S.; et al. Obesity and Obesity-Related Thyroid Dysfunction: Any Potential Role for the Very Low-Calorie Ketogenic Diet (VLCKD)? Curr. Nutr. Rep. 2024, 13, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Lanza, G.; Ferri, R.; Caraci, F.; Cano, S.S.; Elio, I.; Micek, A.; Castellano, S.; Grosso, G. Relation between dietary inflammatory potential and sleep features: Systematic review of observational studies. Med. J. Nutrition Metab. 2024, 17, 1–14. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the american heart association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Verde, L.; Frias-Toral, E.; Cardenas, D. Editorial: Environmental factors implicated in obesity. Front. Nutr. 2023, 10, 1171507. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H. Epidemiology of obesity in the Western Hemisphere. J. Clin. Endocrinol. Metab. 2008, 93, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Rosi, A.; Scazzina, F.; Frias-Toral, E.; Abdelkarim, O.; Aly, M.; Zambrano-Villacres, R.; Pons, J.; Vázquez-Araújo, L.; Sumalla Cano, S.; et al. Youth Healthy Eating Index (YHEI) and Diet Adequacy in Relation to Country-Specific National Dietary Recommendations in Children and Adolescents in Five Mediterranean Countries from the DELICIOUS Project. Nutrients 2024, 16, 3907. [Google Scholar] [CrossRef]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Luhar, S.; Timæus, I.M.; Jones, R.; Cunningham, S.; Patel, S.A.; Kinra, S.; Clarke, L.; Houben, R. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS ONE 2020, 15, e0229438. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Okati-Aliabad, H.; Ansari-Moghaddam, A.; Kargar, S.; Jabbari, N. Prevalence of Obesity and Overweight among Adults in the Middle East Countries from 2000 to 2020: A Systematic Review and Meta-Analysis. J. Obes. 2022, 2022, 8074837. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.-J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef]
- Guerreiro, V.; Neves, J.S.; Salazar, D.; Ferreira, M.J.; Oliveira, S.C.; Souteiro, P.; Pedro, J.; Magalhães, D.; Varela, A.; Belo, S.; et al. Long-Term Weight Loss and Metabolic Syndrome Remission after Bariatric Surgery: The Effect of Sex, Age, Metabolic Parameters and Surgical Technique—A 4-Year Follow-Up Study. Obes. Facts 2019, 12, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Marchetti, C.; Monami, M.; Mannucci, E.; Cresci, B. Efficacy and effects of bariatric surgery in the treatment of obesity: Network meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Spivak, H.; Sakran, N.; Dicker, D.; Rubin, M.; Raz, I.; Shohat, T.; Blumenfeld, O. Different effects of bariatric surgical procedures on dyslipidemia: A registry-based analysis. Surg. Obes. Relat. Dis. 2017, 13, 1189–1194. [Google Scholar] [CrossRef]
- Cunha, F.M.; Oliveira, J.; Preto, J.; Saavedra, A.; Costa, M.M.; Magalhães, D.; Lau, E.; Bettencourt-Silva, R.; Freitas, P.; Varela, A.; et al. The effect of bariatric surgery type on lipid profile: An age, sex, body mass index and excess weight loss matched study. Obes. Surg. 2016, 26, 1041–1047. [Google Scholar] [CrossRef]
- Brown, A.M.; Yang, J.; Zhang, X.; Docimo, S.; Pryo, A.D.; Spaniolas, K. Bariatric surgery lowers the risk of major cardiovascular events. Ann. Surg. 2022, 276, e417–e424. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Guo, Y.; Liu, C.-Q.; Huang, Z.-P.; Sheng, Y.; Zou, D.-J. Effects of bariatric surgery on glycemic and lipid metabolism, surgical complication and quality of life in adolescents with obesity: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2017, 13, 2037–2055. [Google Scholar] [CrossRef] [PubMed]
- Shoar, S.; Mahmoudzadeh, H.; Naderan, M.; Bagheri-Hariri, S.; Wong, C.; Parizi, A.S.; Shoar, N. Long-Term Outcome of Bariatric Surgery in Morbidly Obese Adolescents: A Systematic Review and Meta-Analysis of 950 Patients with a Minimum of 3 years Follow-Up. Obes. Surg. 2017, 27, 3110–3117. [Google Scholar] [CrossRef]
- Chang, S.-H.; Stoll, C.R.T.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Garb, J.; Welch, G.; Zagarins, S.; Kuhn, J.; Romanelli, J. Bariatric surgery for the treatment of morbid obesity: A meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes. Surg. 2009, 19, 1447–1455. [Google Scholar] [CrossRef]
- Carrano, F.M.; Iossa, A.; Di Lorenzo, N.; Silecchia, G.; Kontouli, K.-M.; Mavridis, D.; Alarçon, I.; Felsenreich, D.M.; Sanchez-Cordero, S.; Di Vincenzo, A.; et al. EAES Bariatric Surgery Guidelines Group EAES rapid guideline: Systematic review, network meta-analysis, CINeMA and GRADE assessment, and European consensus on bariatric surgery-extension 2022. Surg. Endosc. 2022, 36, 1709–1725. [Google Scholar] [CrossRef]
- Wiggins, T.; Guidozzi, N.; Welbourn, R.; Ahmed, A.R.; Markar, S.R. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003206. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Nayyar, A.; Herbella, F.A.M.; Patti, M.G. Preoperative evaluation in bariatric surgery. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 925–929. [Google Scholar] [CrossRef]
- Flore, G.; Deledda, A.; Fosci, M.; Lombardo, M.; Moroni, E.; Pintus, S.; Velluzzi, F.; Fantola, G. Perioperative Nutritional Management in Enhanced Recovery after Bariatric Surgery. Int. J. Environ. Res. Public Health 2023, 20, 6899. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Ciobârcă, D.M.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crişan, G. Nutritional status prior to bariatric surgery for severe obesity: A review. Med. Pharm. Rep. 2022, 95, 24–30. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Weiss, R.; Sherf-Dagan, S.; Nabulsi, N.; Maayani, A.; Khalaileh, A.; Abed, S.; Brodie, R.; Harari, R.; Mintz, Y.; et al. Nutritional Deficiencies in Patients with Severe Obesity before Bariatric Surgery: What Should Be the Focus During the Preoperative Assessment? J. Acad. Nutr. Diet. 2020, 120, 874–884. [Google Scholar] [CrossRef]
- Benalcazar, D.A.; Cascella, M. Obesity surgery preoperative assessment and preparation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Capuozzo, V.; Barbarisi, A. Micronutrient deficiencies in patients candidate for bariatric surgery: A prospective, preoperative trial of screening, diagnosis, and treatment. Int. J. Vitam. Nutr. Res. 2015, 85, 340–347. [Google Scholar] [CrossRef]
- Pellegrini, M.; Rahimi, F.; Boschetti, S.; Devecchi, A.; De Francesco, A.; Mancino, M.V.; Toppino, M.; Morino, M.; Fanni, G.; Ponzo, V.; et al. Pre-operative micronutrient deficiencies in patients with severe obesity candidates for bariatric surgery. J. Endocrinol. Investig. 2021, 44, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Gjuladin-Hellon, T.; Davies, I.G.; Penson, P.; Amiri Baghbadorani, R. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 161–180. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American society for metabolic and bariatric surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Chapela, S.P.; Martinuzzi, A.L.N.; Llobera, N.D.; Ceriani, F.; Gonzalez, V.; Montalvan, M.; Verde, L.; Frias-Toral, E. Obesity and micronutrients deficit, when and how to suplement. Food Agric. Immunol. 2024, 35, 2381725. [Google Scholar] [CrossRef]
- Barrea, L.; Frias-Toral, E.; Pugliese, G.; Garcia-Velasquez, E.; DE Los Angeles Carignano, M.; Savastano, S.; Colao, A.; Muscogiuri, G. Vitamin D in obesity and obesity-related diseases: An overview. Minerva Endocrinol. 2021, 46, 177–192. [Google Scholar] [CrossRef]
- Pilone, V.; Tramontano, S.; Cutolo, C.; Marchese, F.; Pagano, A.M.; Di Spirito, F.; Schiavo, L. Clinical factors correlated with vitamin D deficiency in patients with obesity scheduled for bariatric surgery: A single center experience. Int. J. Vitam. Nutr. Res. 2020, 90, 346–352. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron metabolism in obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Romano, M.; Pieretti, G.; Schneck, A.-S.; Iannelli, A. Correcting micronutrient deficiencies before sleeve gastrectomy may be useful in preventing early postoperative micronutrient deficiencies. Int. J. Vitam. Nutr. Res. 2019, 89, 22–28. [Google Scholar] [CrossRef]
- Kuruba, R.; Koche, L.S.; Murr, M.M. Preoperative assessment and perioperative care of patients undergoing bariatric surgery. Med. Clin. N. Am. 2007, 91, 339–351. [Google Scholar] [CrossRef]
- Glazer, S.; Biertho, L. Bariatric Surgery: Selection & Preoperative Workup. In Canadian Adult Obesity Clinical Practice Guidelines, 1st ed.; Obesity Canada: Edmonton, AB, Canada, 2020; p. 10. [Google Scholar]
- Schiavo, L.; Sans, A.; Scalera, G.; Barbarisi, A.; Iannelli, A. Why preoperative weight loss in preparation for bariatric surgery is important. Obes. Surg. 2016, 26, 2790–2792. [Google Scholar] [CrossRef]
- Sarno, G.; Calabrese, P.; Frias-Toral, E.; Ceriani, F.; Fuchs-Tarlovsky, V.; Spagnuolo, M.; Cucalón, G.; Córdova, L.Á.; Schiavo, L.; Pilone, V. The relationship between preoperative weight loss and intra and post-bariatric surgery complications: An appraisal of the current preoperative nutritional strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 10230–10238. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Grassi, D.; Cicero, A.F.G.; Bagnato, C.; Paolini, B.; Muscogiuri, G. A New Nomenclature for the Very Low-Calorie Ketogenic Diet (VLCKD): Very Low-Energy Ketogenic Therapy (VLEKT). Ketodiets and Nutraceuticals Expert Panels: “KetoNut”, Italian Society of Nutraceuticals (SINut) and the Italian Association of Dietetics and Clinical Nutrition (ADI). Curr. Nutr. Rep. 2024, 13, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Medina, S.; Aragón, C.; Sánchez, Á.; Vázquez, C. Preoperative diets: LCD, VLCD, and commercial supplements. In Nutrition and bariatric surgery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 35–44. ISBN 9780128229224. [Google Scholar]
- Dang, J.T.; Mocanu, V.; Park, H.; Laffin, M.; Hotte, N.; Karmali, S.; Birch, D.W.; Madsen, K.L. Roux-en-Y gastric bypass and sleeve gastrectomy induce substantial and persistent changes in microbial communities and metabolic pathways. Gut Microbes 2022, 14, 2050636. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef]
- Chierici, A.; Alromayan, M.; De Fatico, S.; Drai, C.; Vinci, D.; Anty, R.; Schiavo, L.; Iannelli, A. Is bariatric surgery safer before, during, or after liver transplantation? A systematic review and meta-analysis. J. Liver Transplant. 2023, 9, 100139. [Google Scholar] [CrossRef]
- Bettini, S.; Belligoli, A.; Fabris, R.; Busetto, L. Diet approach before and after bariatric surgery. Rev. Endocr. Metab. Disord. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Sergio, R.; De Sena, G.; Pilone, V.; Barbarisi, A. Clinical impact of Mediterranean-enriched-protein diet on liver size, visceral fat, fat mass, and fat-free mass in patients undergoing sleeve gastrectomy. Surg. Obes. Relat. Dis. 2015, 11, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Zubiaga, L.; Ruiz-Tovar, J. Impact of preoperative nutritional intervention on comorbidities: Type 2 diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. In Nutrition and Bariatric Surgery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–61. ISBN 9780128229224. [Google Scholar]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Quagliariello, V.; Iannelli, A.; Barbarisi, A. A Comparative Study Examining the Impact of a Protein-Enriched Vs Normal Protein Postoperative Diet on Body Composition and Resting Metabolic Rate in Obese Patients after Sleeve Gastrectomy. Obes. Surg. 2017, 27, 881–888. [Google Scholar] [CrossRef]
- Iannelli, A.; Treacy, P.; Sebastianelli, L.; Schiavo, L.; Martini, F. Perioperative complications of sleeve gastrectomy: Review of the literature. J. Minim. Access Surg. 2019, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kloock, S.; Ziegler, C.G.; Dischinger, U. Obesity and its comorbidities, current treatment options and future perspectives: Challenging bariatric surgery? Pharmacol. Ther. 2023, 251, 108549. [Google Scholar] [CrossRef]
- Barrea, L.; Vetrani, C.; Verde, L.; Frias-Toral, E.; Ceriani, F.; Cernea, S.; Docimo, A.; Graziadio, C.; Tripathy, D.; Savastano, S.; et al. Comprehensive Approach to Medical Nutrition Therapy in Patients with Type 2 Diabetes Mellitus: From Diet to Bioactive Compounds. Antioxidants 2023, 12, 904. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; Napolitano, B.; Laudisio, D.; Aprano, S.; Ceriani, F.; Savastano, S.; Colao, A.; Muscogiuri, G. Is there a relationship between the ketogenic diet and sleep disorders? Int. J. Food Sci. Nutr. 2022, 73, 285–295. [Google Scholar] [CrossRef]
- Pugliese, G.; Barrea, L.; Laudisio, D.; Salzano, C.; Aprano, S.; Colao, A.; Savastano, S.; Muscogiuri, G. Sleep apnea, obesity, and disturbed glucose homeostasis: Epidemiologic evidence, biologic insights, and therapeutic strategies. Curr. Obes. Rep. 2020, 9, 30–38. [Google Scholar] [CrossRef]
- Tian, P.; Fu, J.; Liu, Y.; Li, M.; Liu, J.; Liu, J.; Zhang, Z.; Zhang, P. Unveiling the hidden pathologies: Preoperative endoscopic findings in patients with obesity undergoing bariatric surgery. BMC Surg. 2024, 24, 215. [Google Scholar] [CrossRef]
- Abilés, V.; Rodríguez-Ruiz, S.; Abilés, J.; Mellado, C.; García, A.; Pérez de la Cruz, A.; Fernández-Santaella, M.C. Psychological characteristics of morbidly obese candidates for bariatric surgery. Obes. Surg. 2010, 20, 161–167. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Allison, K.C.; Wadden, T.A.; Ashare, R.; Spitzer, J.C.; McCuen-Wurst, C.; LaGrotte, C.; Williams, N.N.; Edwards, M.; Tewksbury, C.; et al. Psychopathology, disordered eating, and impulsivity as predictors of outcomes of bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Barbuti, M.; D’Alessandro, G.; Weiss, F.; Calderone, A.; Santini, F.; Perugi, G.; Maremmani, I. The Impact of Negative Emotional Dysregulation on the Outcome of Bariatric Surgery in Patients with Severe Obesity: An Observational One-Year Follow-Up Study. J. Clin. Med. 2024, 13, 5158. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Iannelli, A. The role of the nutritionist in a multidisciplinary bariatric surgery team. Obes. Surg. 2019, 29, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Schmidt, J.; Mautner Molina, C.; Kalazich Rosales, M.; Muñoz, M.; Ruiz-Uribe, M.; Fuentes Leal, F.; Monrroy Uarac, M.; Cárcamo Ibaceta, C.; Fazakerley, D.J.; Larance, M.; et al. Moderate-intensity constant or high-intensity interval training? Metabolic effects on candidates to undergo bariatric surgery. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1681–1691. [Google Scholar] [CrossRef]
- Tabesh, M.R.; Eghtesadi, M.; Abolhasani, M.; Maleklou, F.; Ejtehadi, F.; Alizadeh, Z. Nutrition, Physical Activity, and Prescription of Supplements in Pre- and Post-bariatric Surgery Patients: An Updated Comprehensive Practical Guideline. Obes. Surg. 2023, 33, 2557–2572. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Gallagher, J.W.; Neiberg, R.H.; Eagleton, E.B.; DeLany, J.P.; Lang, W.; Punchai, S.; Gourash, W.; Jakicic, J.M. Bariatric Surgery vs Lifestyle Intervention for Diabetes Treatment: 5-Year Outcomes from a Randomized Trial. J. Clin. Endocrinol. Metab. 2020, 105, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.; Gonsalves, C.; de Almeida, B.P.; Cavazzotto, T.G.; da Silva, M.P. Do patients with obesity undergoing bariatric surgery modify their objectively measured physical activity? A systematic review and meta-analysis. Int. J. Obes. 2024, 48, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, N.; Chomiuk, T.; Mamcarz, A.; Śliż, D. Physical Activity before and After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2024, 23, 1–12. [Google Scholar] [CrossRef]
- O’Kane, M.; Parretti, H.M.; Pinkney, J.; Welbourn, R.; Hughes, C.A.; Mok, J.; Walker, N.; Thomas, D.; Devin, J.; Coulman, K.D.; et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes. Rev. 2020, 21, e13087. [Google Scholar] [CrossRef] [PubMed]
- Sauerland, S.; Angrisani, L.; Belachew, M.; Chevallier, J.M.; Favretti, F.; Finer, N.; Fingerhut, A.; Garcia Caballero, M.; Guisado Macias, J.A.; Mittermair, R.; et al. European Association for Endoscopic Surgery Obesity surgery: Evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg. Endosc. 2005, 19, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Palacio, A.; Vargas, P.; Ghiardo, D.; Rios, M.J.; Vera, G.; Vergara, C.; Gabarroche, R.; Rubilar, J.; Reyes, A. Primer consenso chileno de nutricionistas en cirugía bariátrica. Rev. Chil. Nutr. 2019, 46, 61–72. [Google Scholar] [CrossRef]
- Yadlapati, S.; Sánchez-Luna, S.A.; Gromski, M.A.; Mulki, R. Managing the bariatric surgery patient: Presurgery and postsurgery considerations. Gastrointest. Endosc. Clin. N. Am. 2024, 34, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional recommendations for adult bariatric surgery patients: Clinical practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by american association of clinical endocrinologists/american college of endocrinology, the obesity society, american society for metabolic & bariatric surgery, obesity medicine association, and american society of anesthesiologists—Executive summary. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Leahy, C.R.; Luning, A. Review of nutritional guidelines for patients undergoing bariatric surgery. AORN J. 2015, 102, 153–160. [Google Scholar] [CrossRef]
- Steenackers, N.; Van der Schueren, B.; Augustijns, P.; Vanuytsel, T.; Matthys, C. Development and complications of nutritional deficiencies after bariatric surgery. Nutr. Res. Rev. 2023, 36, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rey, M.; Castellar-Visbal, L.; Acevedo-Vergara, K.; Vargas-Manotas, J.; Rivera-Porras, D.; Londoño-Juliao, G.; Castillo-Guerrero, B.; Perdomo-Jiménez, M.-C.; Bermúdez, V. The Weight of Bariatric Surgery: Wernicke-Korsakoff Syndrome after Vertical Sleeve Gastrectomy-A Case Series. J. Pers. Med. 2024, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.; Da Prato, G.; Gremes, V.; Valletta, F.; Bariani, M.; Zenti, M.G. A case of wernicke’s encephalopathy after sleeve gastrectomy. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Sousa, J.P.; Santos-Sousa, H.; Vieira, S.; Nunes, R.; Nogueiro, J.; Pereira, A.; Resende, F.; Costa-Pinho, A.; Preto, J.; Sousa-Pinto, B.; et al. Assessing Nutritional Deficiencies in Bariatric Surgery Patients: A Comparative Study of Roux-en-Y Gastric Bypass versus Sleeve Gastrectomy. J. Pers. Med. 2024, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Kaberi-Otarod, J.; Still, C.D.; Wood, G.C.; Benotti, P.N. Iron Treatment in Patients with Iron Deficiency Before and After Metabolic and Bariatric Surgery: A Narrative Review. Nutrients 2024, 16, 3350. [Google Scholar] [CrossRef]
- Kamal, F.A.; Fernet, L.Y.; Rodriguez, M.; Kamal, F.; Da Silva, N.K.; Kamal, O.A.; Ayala Aguilar, A.; Arruarana, V.S.; Martinez Ramirez, M. Nutritional Deficiencies Before and After Bariatric Surgery in Low- and High-Income Countries: Prevention and Treatment. Cureus 2024, 16, e55062. [Google Scholar] [CrossRef]
- Mohn, E.S.; Kern, H.J.; Saltzman, E.; Mitmesser, S.H.; McKay, D.L. Evidence of Drug-Nutrient Interactions with Chronic Use of Commonly Prescribed Medications: An Update. Pharmaceutics 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Allied Health Sciences Section Ad Hoc Nutrition Committee; Aills, L.; Blankenship, J.; Buffington, C.; Furtado, M.; Parrott, J. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surg. Obes. Relat. Dis. 2008, 4, S73–S108. [Google Scholar] [CrossRef] [PubMed]
- Romeijn, M.M.; Holthuijsen, D.D.B.; Kolen, A.M.; Janssen, L.; Schep, G.; van Dielen, F.M.H.; Leclercq, W.K.G. The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: A systematic review. Nutr. J. 2021, 20, 27. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Miller, S.L.; Miller, K.B. Optimal protein intake in the elderly. Clin. Nutr. 2008, 27, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Arts, J.; Karamanolis, G.; Laurenius, A.; Siquini, W.; Suzuki, H.; Ukleja, A.; Van Beek, A.; Vanuytsel, T.; Bor, S.; et al. International consensus on the diagnosis and management of dumping syndrome. Nat. Rev. Endocrinol. 2020, 16, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Gastaldelli, A.; D’Alessio, D.A. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J. Clin. Endocrinol. Metab. 2014, 99, 2008–2017. [Google Scholar] [CrossRef]
- Bjørklund, G.; Semenova, Y.; Pivina, L.; Costea, D.-O. Follow-up after bariatric surgery: A review. Nutrition 2020, 78, 110831. [Google Scholar] [CrossRef]
- van Meijeren, J.; Timmer, I.; Brandts, H.; Janssen, I.; Boer, H. de Evaluation of carbohydrate restriction as primary treatment for post-gastric bypass hypoglycemia. Surg. Obes. Relat. Dis. 2017, 13, 404–410. [Google Scholar] [CrossRef]
- Mantziari, S.; Abboretti, F.; Favre, L.; Thomopoulos, T.; Barigou, M.; Demartines, N.; Suter, M. Protein malnutrition after Roux-en-Y gastric bypass: A challenging case and scoping review of the literature. Surg. Obes. Relat. Dis. 2023, 19, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.-H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Yasumatsu, K.; Inoue, M.; Iwata, S.; Yoshida, R.; Shigemura, N.; Yanagawa, Y.; Drucker, D.J.; Margolskee, R.F.; Ninomiya, Y. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015, 29, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Haemer, M.A.; Fox, C.K. Lifestyle and pharmacologic management before and after bariatric surgery. Semin. Pediatr. Surg. 2020, 29, 150889. [Google Scholar] [CrossRef]
- Franken, R.J.; Lyyjynen, H.S.; Nienhuijs, S.W.; Våge, V.; van de Laar, A.W. Adding Evidence to an Evidence-Based Classification for Recurrent Weight Gain after Bariatric and Metabolic Surgery from a Norwegian National Registry. Obes. Surg. 2024, 34, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, B.; Ouellette-Kuntz, H.; Patel, S.V.; Anvari, M.; Zevin, B. Severe nutritional complications after bariatric surgery in Ontario adults: A population-based descriptive study. Surg. Obes. Relat. Dis. 2020, 16, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lin, S.; Guan, W. Choice of bariatric and metabolic surgical procedures. Zhonghua Wei Chang Wai Ke Za Zhi 2017, 20, 388–392. [Google Scholar] [PubMed]
- Thibault, R.; Huber, O.; Azagury, D.E.; Pichard, C. Twelve key nutritional issues in bariatric surgery. Clin. Nutr. 2016, 35, 12–17. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Chiappetta, S.; Parmar, C.; Shikora, S.A.; Prager, G.; LaMasters, T.; Ponce, J.; Kow, L.; Nimeri, A.; Kothari, S.N.; et al. Current recommendations for procedure selection in class I and II obesity developed by an expert modified Delphi consensus. Sci. Rep. 2024, 14, 3445. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Landecho, M.F.; Valentí, V.; Moncada, R.; Frühbeck, G. Clinical perspectives, eligibility, and success criteria for bariatric/metabolic surgery. Adv. Exp. Med. Biol. 2024, 1460, 677–695. [Google Scholar] [CrossRef]
- Thibault, R.; Pichard, C. Overview on nutritional issues in bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 484–490. [Google Scholar] [CrossRef]
- Yu, A.T.; Gross, A.; Park, K.; Harvey, E.J. Wernicke encephalopathy after bariatric surgery: A literature review. Obes. Surg. 2023, 33, 3621–3627. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Thorell, A.; Rutkowski, W.; Haas, S.L.; Arnelo, U.; Martin, L.; Löhr, J.-M. Pancreatic Exocrine Insufficiency after Bariatric Surgery. Nutrients 2017, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Kow, L.; Aminian, A.; Kaplan, L.M.; Nimeri, A.; Prager, G.; Behrens, E.; White, K.P.; Shikora, S. IFSO Experts Panel IFSO Consensus on Definitions and Clinical Practice Guidelines for Obesity Management-an International Delphi Study. Obes. Surg. 2024, 34, 30–42. [Google Scholar] [CrossRef]
- Honka, H.; Salehi, M. Postprandial hypoglycemia after gastric bypass surgery: From pathogenesis to diagnosis and treatment. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 295–302. [Google Scholar] [CrossRef]
- Buser, A.; Joray, C.; Schiavon, M.; Kosinski, C.; Minder, B.; Nakas, C.T.; Man, C.D.; Muka, T.; Herzig, D.; Bally, L. Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on β-Cell Function at 1 Year After Surgery: A Systematic Review. J. Clin. Endocrinol. Metab. 2022, 107, 3182–3197. [Google Scholar] [CrossRef] [PubMed]
- Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity. Nutrients 2017, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Billeter, A.T.; Müller-Stich, B.P. Comment on: Metabolic comparison of one anastomosis gastric bypass, single-anastomosis duodenal-switch, Roux-en-Y gastric bypass, and vertical sleeve gastrectomy in rat. Surg. Obes. Relat. Dis. 2018, 14, 1867–1868. [Google Scholar] [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric surgery in obesity: Effects on gut microbiota and micronutrient status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.S.; da Silva, T.S.; Leal, P.R.F.; Lopes, K.G.; Kraemer-Aguiar, L.G. Early Changes in Eating Behavior Patterns and Their Relationship with Weight Outcomes in Patients Undergoing Bariatric Surgery. Nutrients 2024, 16, 3868. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.; Pucci, A.; Batterham, R.L. Roux-en-Y gastric bypass: Effects on feeding behavior and underlying mechanisms. J. Clin. Investig. 2015, 125, 939–948. [Google Scholar] [CrossRef]
- Carriel-Mancilla, J.; Suárez, R.; Frias-Toral, E.; Bautista-Valarezo, E.; Andrade Zambrano, T.; Andrade García, A.; Muñoz Jaramillo, R.; Ferrín, M.; Martin, J.; Cardoso Ramos, A.; et al. Short-medium term complications of bariatric surgery: A pilot study. Minerva Endocrinol. 2024, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borbély, Y.; Plebani, A.; Kröll, D.; Ghisla, S.; Nett, P.C. Exocrine Pancreatic Insufficiency after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2016, 12, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Coupaye, M.; Puchaux, K.; Bogard, C.; Msika, S.; Jouet, P.; Clerici, C.; Larger, E.; Ledoux, S. Nutritional consequences of adjustable gastric banding and gastric bypass: A 1-year prospective study. Obes. Surg. 2009, 19, 56–65. [Google Scholar] [CrossRef]
- Ledoux, S.; Msika, S.; Moussa, F.; Larger, E.; Boudou, P.; Salomon, L.; Roy, C.; Clerici, C. Comparison of nutritional consequences of conventional therapy of obesity, adjustable gastric banding, and gastric bypass. Obes. Surg. 2006, 16, 1041–1049. [Google Scholar] [CrossRef]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef] [PubMed]
- Giusti, V.; Suter, M.; Héraïef, E.; Gaillard, R.C.; Burckhardt, P. Effects of laparoscopic gastric banding on body composition, metabolic profile and nutritional status of obese women: 12-months follow-up. Obes. Surg. 2004, 14, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.H.; Ren, C.J.; Siegel, N.; Williams, T.; Barr, D.; Wolfe, B.; Dolan, K.; Fielding, G.A. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J. Gastrointest. Surg. 2004, 8, 48–55; discussion 54. [Google Scholar] [CrossRef]
- Biertho, L.; Biron, S.; Hould, F.-S.; Lebel, S.; Marceau, S.; Marceau, P. Is biliopancreatic diversion with duodenal switch indicated for patients with body mass index <50 kg/m2? Surg. Obes. Relat. Dis. 2010, 6, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Nett, P.; Borbély, Y.; Kröll, D. Micronutrient Supplementation after Biliopancreatic Diversion with Duodenal Switch in the Long Term. Obes. Surg. 2016, 26, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.F.d.C.; de Carvalho, R.B.; Chaim, E.A.; Cazzo, E. Brown bowel syndrome: A rare malnutrition-related complication of bariatric surgery. Nutr. Hosp. 2019, 36, 743–747. [Google Scholar] [CrossRef]
- Strain, G.W.; Torghabeh, M.H.; Gagner, M.; Ebel, F.; Dakin, G.F.; Abelson, J.S.; Connolly, D.; Pomp, A. The Impact of Biliopancreatic Diversion with Duodenal Switch (BPD/DS) Over 9 Years. Obes. Surg. 2017, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.; Al-Najim, W.; le Roux, C.W. Health benefits beyond the scale: The role of diet and nutrition during weight loss programmes. Nutrients 2024, 16, 3585. [Google Scholar] [CrossRef]
- Spetz, K.; Svedjeholm, S.; Roos, S.; Grehn, S.; Olbers, T.; Andersson, E. Adherence to vitamin and mineral supplementation after bariatric surgery—A two-year cohort study. Obes. Res. Clin. Pract. 2022, 16, 407–412. [Google Scholar] [CrossRef]
- Sherf-Dagan, S.; Biton, R.; Ribeiro, R.; Kessler, Y.; Raziel, A.; Rossoni, C.; Kais, H.; Bragança, R.; Santos, Z.; Goitein, D.; et al. Nutritional and lifestyle behaviors reported following one anastomosis gastric bypass based on a multicenter study. Nutrients 2023, 15, 1515. [Google Scholar] [CrossRef]
- Montastier, E.; Chalret du Rieu, M.; Tuyeras, G.; Ritz, P. Long-term nutritional follow-up post bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 388–393. [Google Scholar] [CrossRef]
- Ledoux, S.; Calabrese, D.; Bogard, C.; Dupré, T.; Castel, B.; Msika, S.; Larger, E.; Coupaye, M. Long-term evolution of nutritional deficiencies after gastric bypass: An assessment according to compliance to medical care. Ann. Surg. 2014, 259, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Smelt, H.J.M.; Pouwels, S.; Smulders, J.F.; Hazebroek, E.J. Patient adherence to multivitamin supplementation after bariatric surgery: A narrative review. J. Nutr. Sci. 2020, 9, e46. [Google Scholar] [CrossRef]
- Elks, W.; Rooks, A.; Schulte, S.; Batra, K.; Burke, J.; Jain, V. Resistance training in patients after metabolic and bariatric surgery: Protocol for a systematic review. JMIR Res. Protoc. 2023, 12, e49513. [Google Scholar] [CrossRef]
- Sundgot-Borgen, C.; Bond, D.S.; Sniehotta, F.F.; Kvalem, I.L.; Hansen, B.H.; Bergh, I.; Rø, Ø.; Mala, T. Associations of changes in physical activity and sedentary time with weight recurrence after bariatric surgery: A 5-year prospective study. Int. J. Obes. 2023, 47, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 35, 4069–4084. [Google Scholar] [CrossRef]
- Romagna, E.C.; Lopes, K.G.; Mattos, D.M.F.; Farinatti, P.; Kraemer-Aguiar, L.G. Physical Activity Level, Sedentary Time, and Weight Regain After Bariatric Surgery in Patients Without Regular Medical Follow-up: A Cross-Sectional Study. Obes. Surg. 2021, 31, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Kaouk, L.; Hsu, A.T.; Tanuseputro, P.; Jessri, M. Modifiable factors associated with weight regain after bariatric surgery: A scoping review. F1000Research 2019, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training before and after bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. S4), e13296. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodlo, M.; Shakibazadeh, E.; Barzin, M.; Salimi, Y.; Mokhtari, Z.; Yaseri, M. Covariates of a healthy diet and physical activity self-management one year after Bariatric surgery: A cross-sectional study. PLoS ONE 2023, 18, e0287137. [Google Scholar] [CrossRef] [PubMed]
- Launius, K.N.; Herb Neff, K.M.; Schuh, L.M.; Saules, K.K.; Creel, D.B.; Inman, M.M. Long-term Engagement in Physical Activity Among Bariatric Surgery Patients: Associations with Treatment Outcomes at 5-Year Follow-up. Obes. Surg. 2023, 33, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.S.; Vieira, F.T.; Lamarca, F.; Lima, R.M.; Carvalho, K.M.B.; Dutra, E.S. Resistance Training Improves Muscle Strength and Function, Regardless of Protein Supplementation, in the Mid- to Long-Term Period after Gastric Bypass. Nutrients 2021, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Ceyhan, M.; Schönenberger, K.A.; Stanga, Z.; Reber, E. Nutrient and fluid requirements in post-bariatric patients performing physical activity: A systematic review. Nutrition 2022, 97, 111577. [Google Scholar] [CrossRef]
- Cadena-Obando, D.; Ramírez-Rentería, C.; Ferreira-Hermosillo, A.; Albarrán-Sanchez, A.; Sosa-Eroza, E.; Molina-Ayala, M.; Espinosa-Cárdenas, E. Are there really any predictive factors for a successful weight loss after bariatric surgery? BMC Endocr. Disord. 2020, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps-a Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Quinto, G.; Bettini, S.; Neunhaeuserer, D.; Battista, F.; Milan, G.; Gasperetti, A.; Vecchiato, M.; Vettor, R.; Ermolao, A.; Busetto, L. Down-staging of obesity one year after bariatric surgery: A new proposal of Edmonton obesity staging system. Front. Endocrinol. 2023, 14, 1147171. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, E.O.; Liem, R.S.L.; Nienhuijs, S.W.; Greve, J.W.M.; Marang-van de Mheen, P.J. Dutch Audit for Treatment of Obesity Research Group Weight recurrence after Sleeve Gastrectomy versus Roux-en-Y gastric bypass: A propensity score matched nationwide analysis. Surg. Endosc. 2023, 37, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.J.; Priya, P.; Mahawar, K.K.; Shah, S. Indian Bariatric Surgery Outcome Reporting (IBSOR) Group Weight Regain After Bariatric Surgery—A Multicentre Study of 9617 Patients from Indian Bariatric Surgery Outcome Reporting Group. Obes. Surg. 2019, 29, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- King, W.C.; Hinerman, A.S.; Courcoulas, A.P. Weight regain after bariatric surgery: A systematic literature review and comparison across studies using a large reference sample. Surg. Obes. Relat. Dis. 2020, 16, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Berber, L.C.L.; Melendez-Araújo, M.S.; Nakano, E.Y.; de Carvalho, K.M.B.; Dutra, E.S. Grazing Behavior Hinders Weight Loss in Long-Term Post Bariatric Surgery: A Cross-Sectional Study. Obes. Surg. 2021, 31, 4076–4082. [Google Scholar] [CrossRef]
- Reynoso, C. Factores asociados a reganancia de peso en pacientes con cirugía bariátrica con al menos 5 años de cirugía. RFEM 2020, 15, 0086–0090. [Google Scholar] [CrossRef]
- Endevelt, R.; Ben-Assuli, O.; Klain, E.; Zelber-Sagi, S. The role of dietician follow-up in the success of bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 963–968. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Zuk, J.B.; Khavaran, H. Plant bioactive compounds from Mediterranean diet improve risk factors for metabolic syndrome. Int. J. Food Sci. Nutr. 2023, 74, 403–423. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Yu, X.; Pu, H.; Voss, M. Overview of anti-inflammatory diets and their promising effects on non-communicable diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.; Baikady, R.R.; Clevenger, B.; Butcher, A.; Abeysiri, S.; Chau, M.; Macdougall, I.C.; Murphy, G.; Swinson, R.; Collier, T.; et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): A randomised, double-blind, controlled trial. Lancet 2020, 396, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, F.; Di Stefano, C.; Baratta, R.; Pulvirenti, A.; Mastrandrea, G.; Piazza, L.; Guccione, F.; Navarra, G.; Frittitta, L. Efficacy of High-dose Liraglutide 3.0 mg in Patients with Poor Response to Bariatric Surgery: Real-world Experience and Updated Meta-analysis. Obes. Surg. 2024, 34, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Noria, S.F.; Shelby, R.D.; Atkins, K.D.; Nguyen, N.T.; Gadde, K.M. Weight regain after bariatric surgery: Scope of the problem, causes, prevention, and treatment. Curr. Diab. Rep. 2023, 23, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Dicker, D.; Sagy, Y.W.; Ramot, N.; Battat, E.; Greenland, P.; Arbel, R.; Lavie, G.; Reges, O. Bariatric Metabolic Surgery vs Glucagon-Like Peptide-1 Receptor Agonists and Mortality. JAMA Netw. Open 2024, 7, e2415392. [Google Scholar] [CrossRef]
- Murvelashvili, N.; Xie, L.; Schellinger, J.N.; Mathew, M.S.; Marroquin, E.M.; Lingvay, I.; Messiah, S.E.; Almandoz, J.P. Effectiveness of semaglutide versus liraglutide for treating post-metabolic and bariatric surgery weight recurrence. Obesity 2023, 31, 1280–1289. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Pinhel, M.A.S.; Nonino, C.B. Bariatric surgery and precision nutrition. Nutrients 2017, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Boswell, L.; Jiménez, A.; Ortega, E.; Pané, A.; Hollanda, A.d.; Moizé, V.; Andreu, A.; Ibarzabal, A.; Flores, L.; Vidal, J. Genetic background influences weight-loss trajectories on the mid-term after bariatric surgery. Int. J. Obes. 2019, 43, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Aasbrenn, M.; Schnurr, T.M.; Have, C.T.; Svendstrup, M.; Hansen, D.L.; Worm, D.; Balslev-Harder, M.; Hollensted, M.; Grarup, N.; Burgdorf, K.S.; et al. Genetic determinants of weight loss after bariatric surgery. Obes. Surg. 2019, 29, 2554–2561. [Google Scholar] [CrossRef]
- Cooiman, M.I.; Aarts, E.O.; Janssen, I.M.C.; Hazebroek, E.J.; Berends, F.J. Weight loss, remission of comorbidities, and quality of life after bariatric surgery in young adult patients. Obes. Surg. 2019, 29, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Kaur, J.; Singh, J.; Rasane, P.; Sharma, K.; Bhadariya, V.; Kaur, S.; Kumar, V. Genetics, nutrition, and health: A new frontier in disease prevention. J. Am. Nutr. Assoc. 2024, 43, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Bravo, E.; Pacheco-Orozco, R.A.; Payán-Gómez, C.; López-Rippe, J. Abordaje nutrigenómico de la obesidad: ¿dónde estamos? Rev. Nutr. Clin. Metab. 2021, 4, 25–34. [Google Scholar] [CrossRef]
- Novais, P.F.S.; Weber, T.K.; Lemke, N.; Verlengia, R.; Crisp, A.H.; Rasera-Junior, I.; de Oliveira, M.R.M. Gene polymorphisms as a predictor of body weight loss after Roux-en-Y gastric bypass surgery among obese women. Obes. Res. Clin. Pract. 2016, 10, 724–727. [Google Scholar] [CrossRef]
- Resende, C.M.M.; Durso, D.F.; Borges, K.B.G.; Pereira, R.M.; Rodrigues, G.K.D.; Rodrigues, K.F.; Silva, J.L.P.; Rodrigues, E.C.; Franco, G.R.; Alvarez-Leite, J.I. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes in women submitted to bariatric surgery over 60 months. Clin. Nutr. 2018, 37, 1286–1292. [Google Scholar] [CrossRef]
- Duarte, A.C.S.; da Silva, N.R.; Santos Gonçalves, V.S.; Corgosinho, F.C.; de Carvalho, K.M.B.; Horst, M.A. The influence of single nucleotide polymorphisms on body weight trajectory after bariatric surgery: A systematic review. Curr. Obes. Rep. 2023, 12, 280–307. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; de Oliveira, A.P.R.P.; Brochado, M.J.F.; Pinhel, M.A.S.; de Oliveira, B.A.P.; Marchini, J.S.; Dos Santos, J.E.; Salgado, W.; Cury, N.M.; de Araújo, L.F.; et al. The Ala55Val and -866G>A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition 2017, 33, 326–330. [Google Scholar] [CrossRef]
- Seip, R.L.; Papasavas, P.; Stone, A.; Thompson, S.; Ng, J.; Tishler, D.S.; Ruaño, G. Comparative physiogenomic analyses of weight loss in response to 2 modes of bariatric surgery: Demonstration with candidate neuropsychiatric and cardiometabolic genes. Surg. Obes. Relat. Dis. 2016, 12, 369–377. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Cantarero-Cuenca, A.; González-Jiménez, A.; Linares-Pineda, T.; Peña-Montero, N.; Ocaña-Wilhelmi, L.; Tinahones, F.J.; Morcillo, S. Epigenetic Marks as Predictors of Metabolic Response to Bariatric Surgery: Validation from an Epigenome Wide Association Study. Int. J. Mol. Sci. 2023, 24, 14778. [Google Scholar] [CrossRef] [PubMed]
- de Toro-Martín, J.; Guénard, F.; Tchernof, A.; Pérusse, L.; Marceau, S.; Vohl, M.-C. Polygenic risk score for predicting weight loss after bariatric surgery. JCI Insight 2018, 3, e122011. [Google Scholar] [CrossRef] [PubMed]
- Faenza, M.; Benincasa, G.; Docimo, L.; Nicoletti, G.F.; Napoli, C. Clinical epigenetics and restoring of metabolic health in severely obese patients undergoing batriatric and metabolic surgery. Updates Surg. 2022, 74, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pinhel, M.A.d.S.; Noronha, N.Y.; Nicoletti, C.F.; de Oliveira, B.A.P.; Cortes-Oliveira, C.; Pinhanelli, V.C.; Salgado Junior, W.; Machry, A.J.; da Silva Junior, W.A.; Souza, D.R.S.; et al. Changes in global transcriptional profiling of women following obesity surgery bypass. Obes. Surg. 2018, 28, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Galyean, S.; Sawant, D.; Shin, A.C. Immunometabolism, Micronutrients, and Bariatric Surgery: The Use of Transcriptomics and Microbiota-Targeted Therapies. Mediators Inflamm. 2020, 2020, 8862034. [Google Scholar] [CrossRef]
- Galyean, S.; Sawant, D.; Shin, A.C. Personalized Nutrition for Management of Micronutrient Deficiency-Literature Review in Non-bariatric Populations and Possible Utility in Bariatric Cohort. Obes. Surg. 2020, 30, 3570–3582. [Google Scholar] [CrossRef] [PubMed]

| Key Points | |
|---|---|
| (A) Nutritional Evaluation and Screening [35,40,50,51] |
|
| Common Deficiencies |
|
| Preoperative Medical Evaluation |
|
| Preoperative Imaging |
|
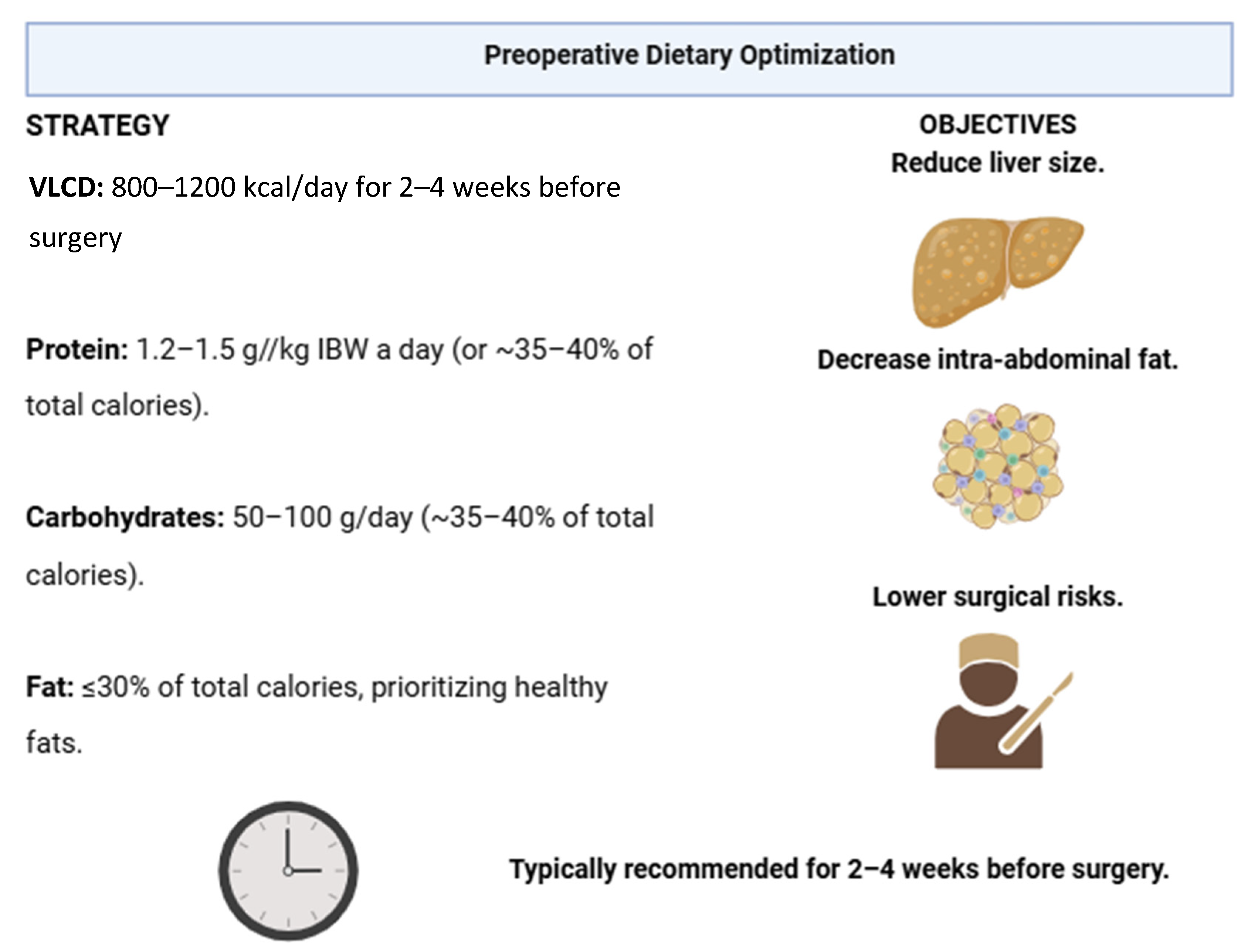
| (B) Preoperative Dietary Optimization [36,40,55] | Importance: Weight loss improves metabolic profile, reduces liver size, and enhances technical feasibility of BS.
|
Diets common recommendations:
| |
| Micronutrient Supplementation [78] |
|
| Macronutrient Guidelines | Proteins: 1.2–1.5 g/kg of ideal body weight; supports muscle maintenance, healing, and immunity. |
| Carbohydrates: 40–50% of intake; focus on complex carbs to avoid fatty liver. | |
| Fats: 20–30% of intake; prioritize healthy fats and minimize trans and saturated fats. | |
| Micronutrients: Adequate intake of iron, calcium, vitamin D, B vitamins is critical. | |
| Duration: | Typically recommended for 2–4 weeks before surgery.
|
| (C) Managing Comorbid Conditions [35,50,69,70,79] | Hypertension: Continue antihypertensive medication; individualize insulin and diuretic management |
| OSA: Screen all patients, consider polysomnography for diagnosis. | |
| Gastrointestinal (GI) Pathologies: Preoperative EGD helps detect GI disorders and H. pylori. | |
| Mental health: Essential to address depression and anxiety. | |
| Team Coordination | Multidisciplinary care involving surgeons, endocrinologists, nutritionists, psychologists, and other professionals optimizes outcomes. |
| (D) re-Surgical Exercise Recommendations [73,74,77] |
|
| Length | Diet Type | Suggestions |
|---|---|---|
| 0 to 3 days | Clear liquids [58,61] |
|
| From 7 to 14 days | Low-fat full liquid diet [58,61] |
|
| From day 7 to 14 and for 1 to 2 weeks according to evolution and tolerance. | High-protein, low-fat, and moderate carbohydrate diet (“purée”) [58,61] |
|
| Approximately week 4 and for 1 to 2 weeks according to evolution and tolerance. | Hypocaloric-hyperproteic solid diet (soft foods) [58,61] |
|
| Recommendation | Description |
|---|---|
| Personalized Interventions | Adopt personalized nutritional strategies focusing on high-quality foods such as fruits, vegetables, and lean proteins [132]. |
| Regulated Energy Deficit | Maintain a controlled energy deficit to prevent weight gain, while avoiding nutritional deficiencies [132]. |
| Balanced and High-Quality Diet | Promote balanced diets, such as the Mediterranean or DASH diets, that provide cardiometabolic benefits [132,157,158]. |
| Micronutrient Supplementation | Supplement key nutrients (vitamins B12, D, and iron) to prevent deficiencies, especially in younger patients or those with mental health disorders [133,135,136,161] |
| Separation of Liquids and Solids | Maintain the separation between liquids and solids during meals to improve satiety and prevent overeating [134]. |
| Prioritize Protein-Rich Foods | Favor protein-rich foods to improve satiety, preserve muscle mass, and control appetite [132,142]. |
| Incorporate Fiber in the Diet | Increase fiber intake to improve metabolic health, control appetite, and reduce visceral fat [159]. |
| Adherence to Continuous Medical Monitoring | Maintain regular follow-up consultations with medical and nutritional guidance to prevent metabolic complications and reinforce healthy habits [135,136]. |
| Behavioral Interventions | Integrate behavioral strategies such as cognitive-behavioral therapy to improve adherence and address psychological factors contributing to weight gain [132,140]. |
| Preoperative Education | Promote nutritional education before surgery to ensure sustainable eating habits and prevent long-term weight gain [132,135]. |
| Monitoring Eating Habits | Continuously monitor and evaluate eating and psychological habits, such as emotional eating or grazing behaviors, to address factors contributing to weight regain [140]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frias-Toral, E.; Chapela, S.; Gonzalez, V.; Martinuzzi, A.; Locatelli, J.; Llobera, N.; Manrique, E.; Sarno, G.; Mingo, M.; Marchese, F.; et al. Optimizing Nutritional Management Before and After Bariatric Surgery: A Comprehensive Guide for Sustained Weight Loss and Metabolic Health. Nutrients 2025, 17, 688. https://doi.org/10.3390/nu17040688
Frias-Toral E, Chapela S, Gonzalez V, Martinuzzi A, Locatelli J, Llobera N, Manrique E, Sarno G, Mingo M, Marchese F, et al. Optimizing Nutritional Management Before and After Bariatric Surgery: A Comprehensive Guide for Sustained Weight Loss and Metabolic Health. Nutrients. 2025; 17(4):688. https://doi.org/10.3390/nu17040688
Chicago/Turabian StyleFrias-Toral, Evelyn, Sebastián Chapela, Victoria Gonzalez, Andres Martinuzzi, Julieta Locatelli, Natalia Llobera, Ezequiel Manrique, Gerardo Sarno, Monica Mingo, Federica Marchese, and et al. 2025. "Optimizing Nutritional Management Before and After Bariatric Surgery: A Comprehensive Guide for Sustained Weight Loss and Metabolic Health" Nutrients 17, no. 4: 688. https://doi.org/10.3390/nu17040688
APA StyleFrias-Toral, E., Chapela, S., Gonzalez, V., Martinuzzi, A., Locatelli, J., Llobera, N., Manrique, E., Sarno, G., Mingo, M., Marchese, F., Cuomo, R., Romaniello, L., Perna, M., Giordano, A., Santella, B., & Schiavo, L. (2025). Optimizing Nutritional Management Before and After Bariatric Surgery: A Comprehensive Guide for Sustained Weight Loss and Metabolic Health. Nutrients, 17(4), 688. https://doi.org/10.3390/nu17040688








