A Randomized Phase II/III Trial Evaluating the Efficacy and Safety of 100 and 125 µg of Calcifediol Weekly Treatment of Severe Vitamin D Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Study Population
2.3. Endpoints and Assessments
2.4. Other Assessments
2.5. Statistical Evaluations
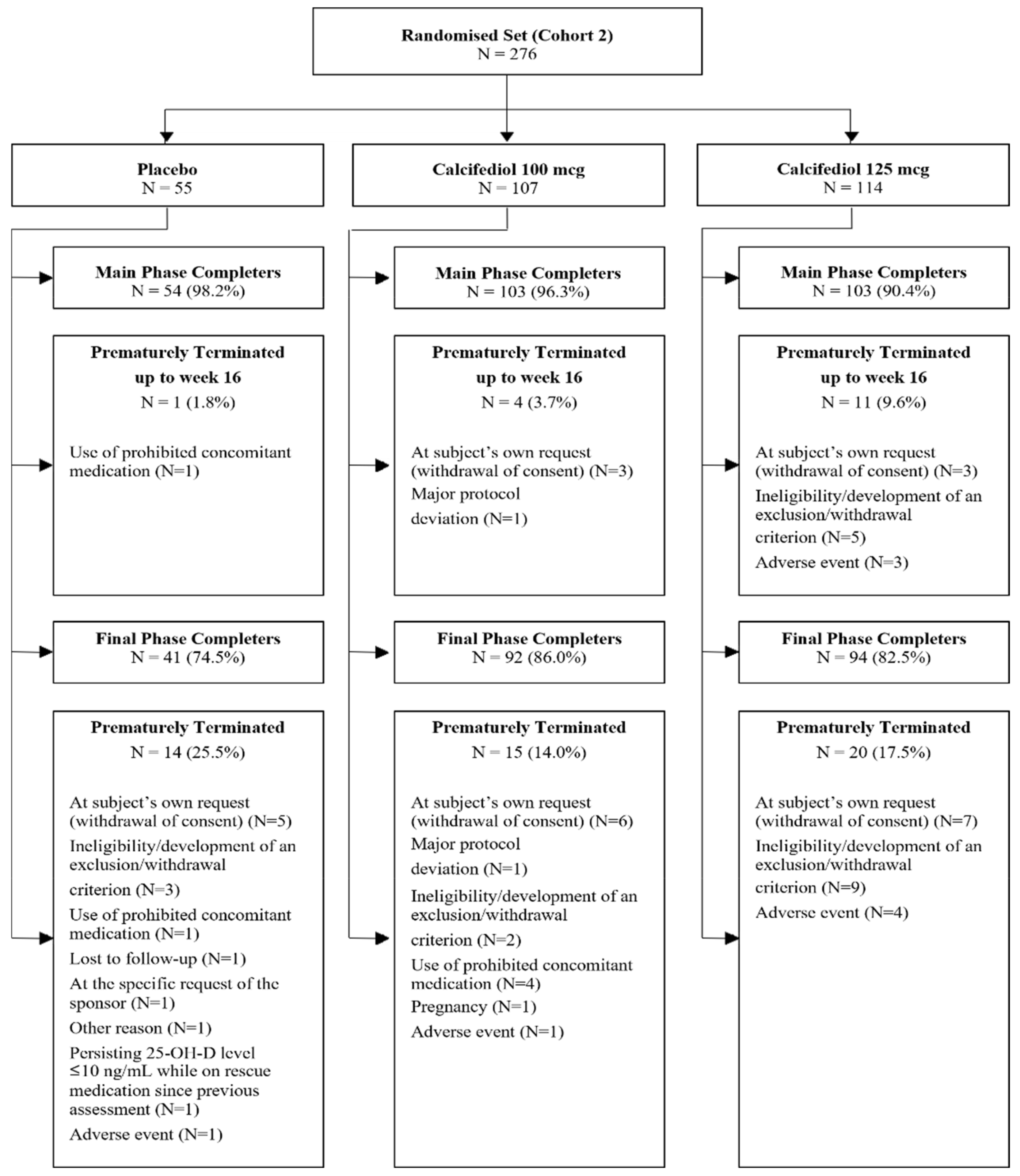
3. Results
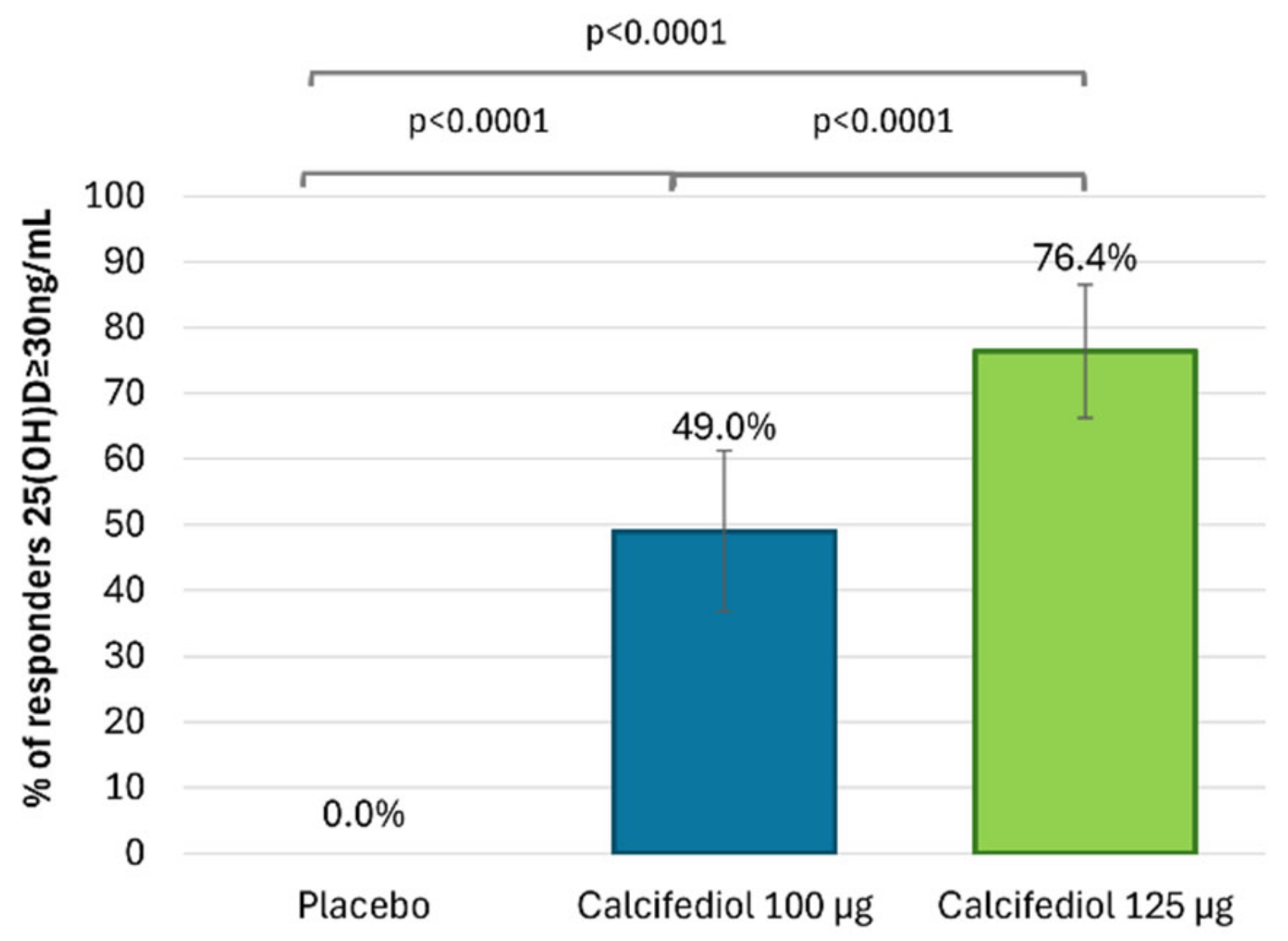
3.1. Efficacy of Calcifediol 100 µg and 125 µg After 16 Weeks of Treatment
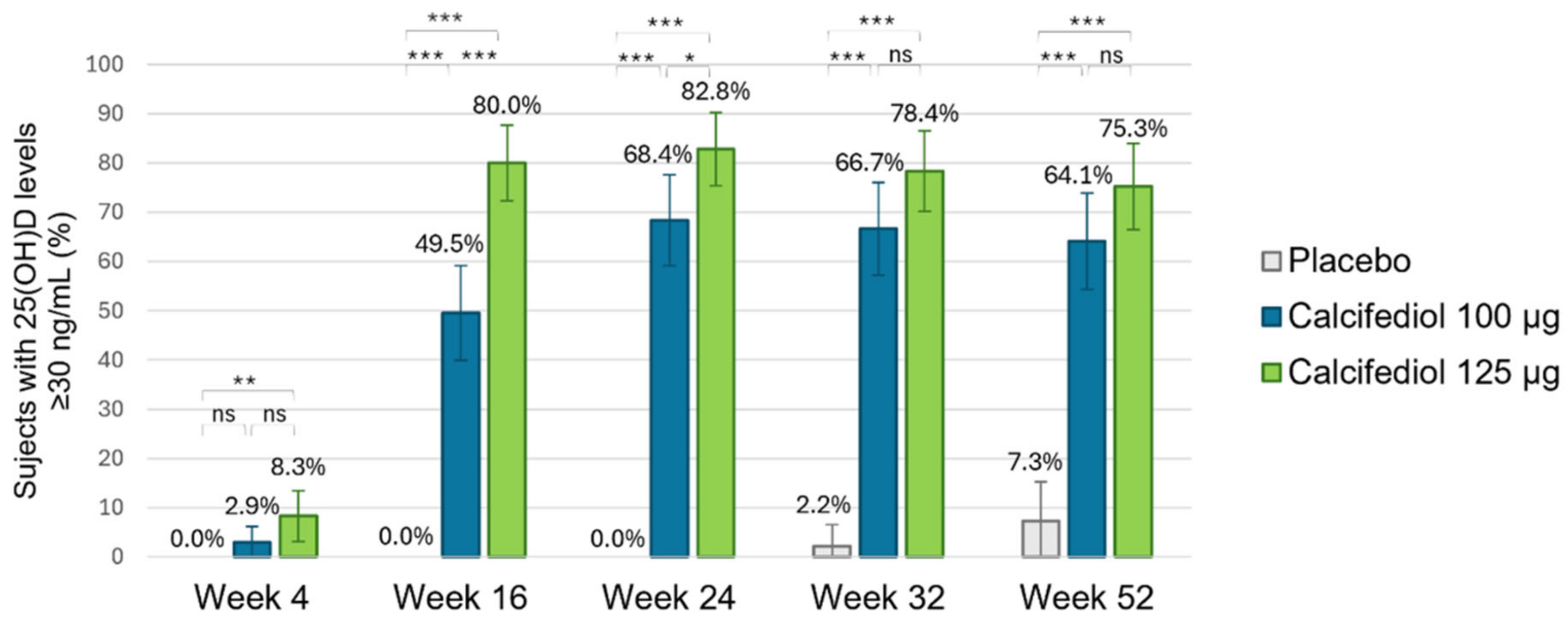
3.2. Efficacy of Calcifediol 100 µg and 125 µg over Time (Responders Rate)
3.3. The Proportion of Patients Who Achieved a Sustained Response
3.4. Subjects in Need of Rescue Medication
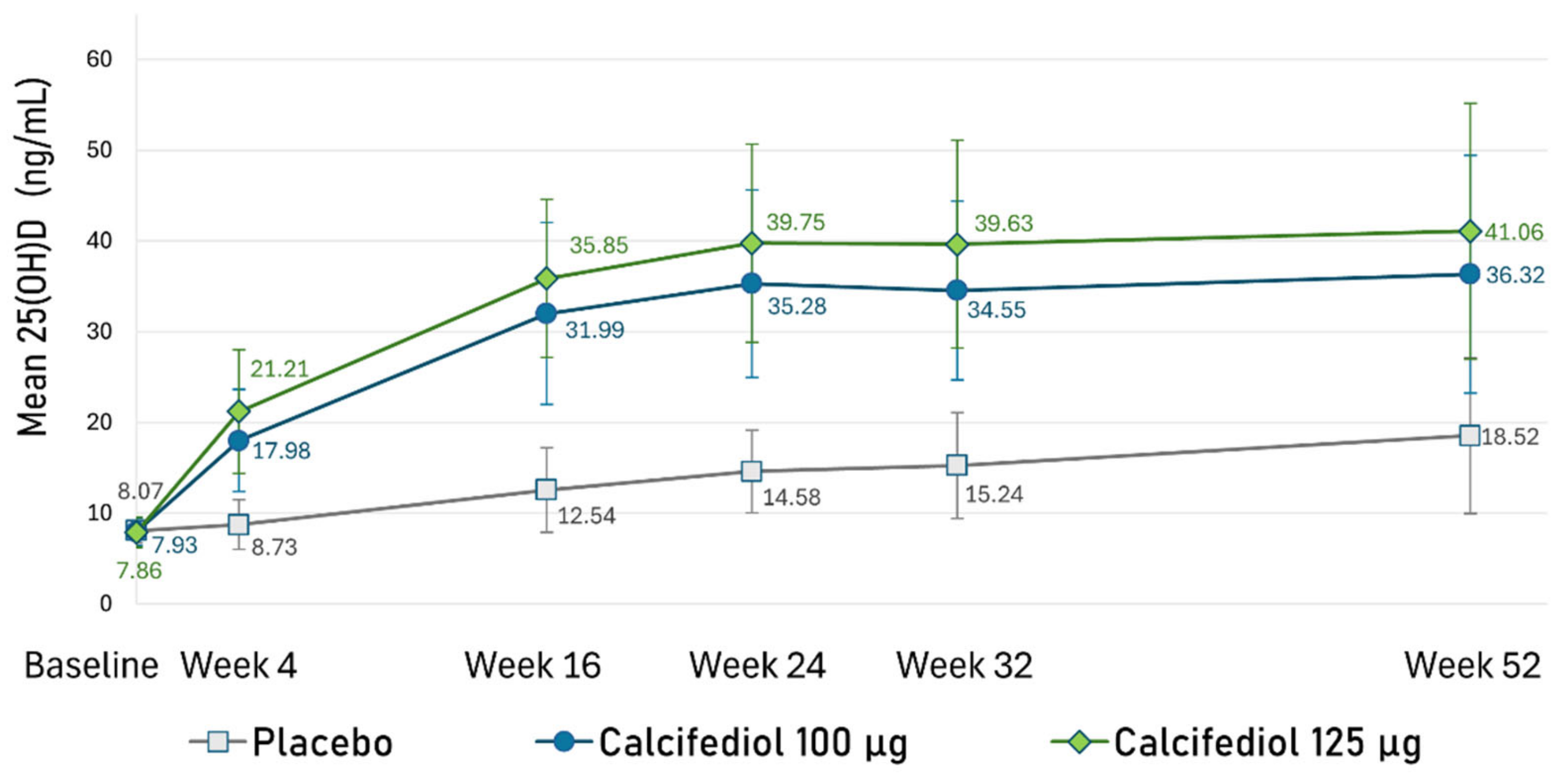
3.5. Evolution of 25(OH)D Concentration Throughout the Study
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Medica 2017, 27, 030501. [Google Scholar] [CrossRef] [PubMed]
- Pappa, H.M.; Bern, E.; Kamin, D.; Grand, R.J. Vitamin D status in gastrointestinal and liver disease. Curr. Opin. Gastroenterol. 2008, 24, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.L.; Sîrbe, C.; Benţa, G.; Mititelu, A.; Grama, A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10705. [Google Scholar] [CrossRef]
- Valero Zanuy, M.Á.; Hawkins Carranza, F. Metabolismo, fuentes endógenas y exógenas de vitamina D. Rev. Esp. Enfermedades Metab. Óseas 2007, 16, 63–70. [Google Scholar] [CrossRef]
- Albanes, D.; Mondul, A.M.; Yu, K.; Parisi, D.; Horst, R.L.; Virtamo, J.; Weinstein, S.J. Serum 25-Hydroxy Vitamin D and Prostate Cancer Risk in a Large Nested Case–Control Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1850–1860. [Google Scholar] [CrossRef]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association Between Vitamin D and Risk of Colorectal Cancer: A Systematic Review of Prospective Studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Zhao, S.; Qian, F.; Wan, Z.; Chen, X.; Pan, A.; Liu, G. Vitamin D and major chronic diseases. Trends Endocrinol. Metab. 2024, 35, 1050–1061. [Google Scholar] [CrossRef]
- Donati, S.; Marini, F.; Giusti, F.; Palmini, G.; Aurilia, C.; Falsetti, I.; Iantomasi, T.; Brandi, M.L. Calcifediol: Why, When, How Much? Pharmaceuticals 2023, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Optimal Serum 25-Hydroxyvitamin D Levels for Multiple Health Outcomes. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2014; pp. 500–525. Available online: http://link.springer.com/10.1007/978-1-4939-0437-2_28 (accessed on 27 June 2024).
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- Khaw, K.T.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Camargo, C.A.; Scragg, R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: Secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017, 5, 438–447. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Dueñas-Laita, A.; Gómez-Alonso, C.; Jódar, E.; Del Pino-Montes, J.; Brandi, M.L.; Cereto Castro, F.; Quesada-Gómez, J.M.; Gallego López, L.; Olmos Martínez, J.M.; et al. Long-Term Treatment and Effect of Discontinuation of Calcifediol in Postmenopausal Women with Vitamin D Deficiency: A Randomized Trial. J. Bone Miner. Res. 2020, 38, 471–479. [Google Scholar] [CrossRef]
- Navarro-Valverde, C.; Sosa-Henríquez, M.; Alhambra-Expósito, M.R.; Quesada-Gómez, J.M. Vitamin D3 and calcidiol are not equipotent. J. Steroid Biochem. Mol. Biol. 2016, 164, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Ingersoll, K.S.; Cohen, J. The impact of medication regimen factors on adherence to chronic treatment: A review of literature. J. Behav. Med. 2008, 31, 213–224. [Google Scholar] [CrossRef]
- Torres-Robles, A.; Benrimoj, S.I.; Gastelurrutia, M.A.; Martinez-Martinez, F.; Peiro, T.; Perez-Escamilla, B.; Rogers, K.; Valverde-Merino, I.; Varas-Doval, R.; Garcia-Cardenas, V. Effectiveness of a medication adherence management intervention in a community pharmacy setting: A cluster randomised controlled trial. BMJ Qual. Saf. 2022, 31, 105–115. [Google Scholar] [CrossRef]
- Jódar-Gimeno, E.; Pérez-Castrillón, J.L.; Nociar, J.; Lojka, M.; Nikolov, D.; Cereto-Castro, F.; Novković, S.; Tarantino, U.; Mehsen-Cetre, N.; Arranz, P.; et al. Efficacy and Safety of Weekly Calcifediol Formulations (75 and 100 µg) in Subjects with Vitamin D Deficiency: A Phase II/III Randomised Trial. Nutrients 2024, 16, 3796. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.M.; Buring, J.E.; et al. Association of Body Weight with Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Goyal, A.; Givler, A. Vitamin D Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK532266/ (accessed on 29 November 2024).
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Castrillon, J.L.; Usategui-Martín, R.; Pludowski, P. Treatment of Vitamin D Deficiency with Calcifediol: Efficacy and Safety Profile and Predictability of Efficacy. Nutrients 2022, 14, 1943. [Google Scholar] [CrossRef] [PubMed]
- Jodar, E.; Campusano, C.; De Jongh, R.T.; Holick, M.F. Calcifediol: A review of its pharmacological characteristics and clinical use in correcting vitamin D deficiency. Eur. J. Nutr. 2023, 62, 1579–1597. [Google Scholar] [CrossRef]
- Graeff-Armas, L.A.; Bendik, I.; Kunz, I.; Schoop, R.; Hull, S.; Beck, M. Supplemental 25-Hydroxycholecalciferol Is More Effective than Cholecalciferol in Raising Serum 25-Hydroxyvitamin D Concentrations in Older Adults. J. Nutr. 2020, 150, 73–81. [Google Scholar] [CrossRef]
- Vaes, A.M.M.; Tieland, M.; De Regt, M.F.; Wittwer, J.; Van Loon, L.J.C.; De Groot, L.C.P.G.M. Dose–response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: A randomized controlled trial in older adults. Clin. Nutr. 2018, 37, 808–814. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; López-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef]
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol Treatment and COVID-19–Related Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, e4017–e4027. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Stöcklin, E.; Sidelnikov, E.; Willett, W.C.; Edel, J.O.; Stähelin, H.B.; Wolfram, S.; Jetter, A.; Schwager, J.; et al. Oral supplementation with 25(OH)D3 versus vitamin D3: Effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J. Bone Miner. Res. 2012, 27, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Rotondo, C.; Cici, D.; Berardi, S.; Cantatore, F.P. Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study. Nutrients 2021, 13, 380. [Google Scholar] [CrossRef]
- Andújar-Espinosa, R.; Salinero-González, L.; Illán-Gómez, F.; Castilla-Martínez, M.; Hu-Yang, C.; Ruiz-López, F.J. Effect of vitamin D supplementation on asthma control in patients with vitamin D deficiency: The ACVID randomised clinical trial. Thorax 2021, 76, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Espinosa, R.; Aparicio-Vicente, M.; Ruiz-López, F.J.; Salinero-González, L. Influence of vitamin D supplementation on the quality of life of asthma patients: Findings from ACVID randomised clinical trial. Respir. Med. 2021, 185, 106484. [Google Scholar] [CrossRef]
- Diaz-Ruiz, R.; Poca, M.; Roman, E.; Panadero-Gomez, R.; Cuyàs, B.; Bañares, I.; Morales, A.; Puerto, M.; Lopez-Esteban, R.; Blazquez, E.; et al. Vitamin D Supplementation Is Associated with Inflammation Amelioration and Cognitive Improvement in Decompensated Patients with Cirrhosis. Nutrients 2025, 17, 226. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef]
- Guerra López, P.; Urroz Elizalde, M.; Vega-Gil, N.; Sánchez Santiago, B.; Zorrilla Martínez, I.; Jiménez-Mercado, M.; Jódar, E.; Landeta Manzano, A.; Campo Hoyos, C.; Frías Iniesta, J. Efficacy and Safety of Calcifediol in Young Adults with Vitamin D Deficiency: A Phase I, Multicentre, Clinical Trial—POSCAL Study. Nutrients 2024, 16, 306. [Google Scholar] [CrossRef]
- Lee, J.P.; Tansey, M.; Jetton, J.G.; Krasowski, M.D. Vitamin D Toxicity: A 16-Year Retrospective Study at an Academic Medical Center. Lab. Med. 2018, 49, 123–129. [Google Scholar] [CrossRef]
- Ross, A.; Taylor, C.; Yaktine, A. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011; Available online: http://www.nap.edu/catalog/13050 (accessed on 3 May 2024).


| Parameter | Placebo | Calcifediol 100 µg | Calcifediol 125 µg | Total |
|---|---|---|---|---|
| (n = 55) | (n = 104) | (n = 110) | (n = 269) | |
| Age, mean (SD) years | 53.8 (12.9) | 55.4 (16.0) | 55.7 (16.1) | 55.2 (15.4) |
| Sex: female, n (%) | 43 (78.2) | 79 (76.0) | 76 (69.1) | 198 (73.6) |
| BMI, mean (SD) kg/m2 | 30.5 (8.3) | 29.3 (7.2) | 29.5 (6.6) | 29.7 (7.2) |
| BMI, n (%): | ||||
| <18.5 kg/m2 | 0 | 2 (1.9) | 3 (2.7) | 5 (1.9) |
| ≥18.5 <25 kg/m2 | 19 (34.5) | 29 (27.9) | 24 (21.8) | 72 (26.8) |
| ≥25 <30 kg/m2 | 12 (21.8) | 30 (28.8) | 37 (33.6) | 79 (29.4) |
| ≥30 kg/m2 | 24 (43.6) | 42 (40.4) | 46 (41.8) | 112 (41.6) |
| Main comorbidities: | ||||
| Hypertension, n (%) | 28 (50.9) | 52 (50.0) | 53 (48.2) | 133 (49.4) |
| Menopause, n (%) | 21 (38.2) | 49 (47.1) | 46 (41.8) | 116 (43.1) |
| Placebo | Calcifediol | Calcifediol | Total | |||||
|---|---|---|---|---|---|---|---|---|
| (N = 55) | 100 µg (N = 104) | 125 µg (N = 113) | (N = 272) | |||||
| n (%) | E | n (%) | E | n (%) | E | n (%) | E | |
| TEAE | 21 (38.2) | 47 | 33 (31.7) | 62 | 41 (36.3) | 81 | 95 (34.9) | 190 |
| Non-serious TEAE | 21 (38.2) | 44 | 32 (30.8) | 59 | 40 (35.4) | 76 | 93 (34.2) | 179 |
| Serious TEAE | 3 (5.5) | 3 | 3 (2.9) | 3 | 4 (3.5) | 5 | 10 (3.7) | 11 |
| Related TEAE | 7 (12.7) | 7 | 0 | 0 | 2 (1.8) | 2 | 9 (3.3) | 9 |
| Related serious TEAE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe TEAE | 3 (5.5) | 3 | 3 (2.9) | 3 | 2 (1.8) | 3 | 8 (2.9) | 9 |
| TEAE leading to discontinuation | 1 (1.8) | 1 | 2 (1.9) | 2 | 6 (5.3) | 6 | 9 (3.3) | 9 |
| Parameter Visit | Placebo (N = 55) | Calcifediol 100 µg (N = 104) | Calcifediol 125 µg (N = 113) | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Alkaline phosphatase (U/L) | ||||||
| Week 16 | 54 | 2.3 (13.03) | 102 | −3.8 (17.22) | 105 | −9.1 (26.29) |
| Week 52 | 41 | −5.7 (27.67) | 91 | −4.4 (13.93) | 93 | −7.8 (21.16) |
| Total serum Ca (mg/dL) | ||||||
| Week 16 | 53 | 0.13 (0.336) | 102 | 0.09 (0.351) | 105 | 0.12 (0.407) |
| Week 52 | 40 | 0.15 (0.399) | 91 | 0.11 (0.384) | 93 | 0.16 (0.352) |
| Phosphorous (nmol/L) | ||||||
| Week 16 | 54 | 0.034 (0.178) | 103 | 0.025 (0.180) | 105 | 0.022 (0.239) |
| Week 52 | 41 | 0.054 (0.189) | 92 | 0.049 (0.191) | 93 | 0.015 (0.226) |
| Parathyroid hormone (pg/mL) | ||||||
| Week 16 | 50 | −8.3 (20.32) | 100 | −14.3 (20.19) | 101 | −16.1 (22.73) |
| Week 52 | 37 | −8.6 (19.29) | 90 | −15.6 (19.06) | 89 | −15.8 (21.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Castrillón, J.L.; Jódar-Gimeno, E.; Nociar, J.; Lojka, M.; Nikolov, D.; Cereto-Castro, F.; Novković, S.; Tarantino, U.; Mehsen-Cetre, N.; Arranz, P.; et al. A Randomized Phase II/III Trial Evaluating the Efficacy and Safety of 100 and 125 µg of Calcifediol Weekly Treatment of Severe Vitamin D Deficiency. Nutrients 2025, 17, 672. https://doi.org/10.3390/nu17040672
Pérez-Castrillón JL, Jódar-Gimeno E, Nociar J, Lojka M, Nikolov D, Cereto-Castro F, Novković S, Tarantino U, Mehsen-Cetre N, Arranz P, et al. A Randomized Phase II/III Trial Evaluating the Efficacy and Safety of 100 and 125 µg of Calcifediol Weekly Treatment of Severe Vitamin D Deficiency. Nutrients. 2025; 17(4):672. https://doi.org/10.3390/nu17040672
Chicago/Turabian StylePérez-Castrillón, Jose Luis, Esteban Jódar-Gimeno, Ján Nociar, Michal Lojka, Dimitar Nikolov, Fernando Cereto-Castro, Snežana Novković, Umberto Tarantino, Nadia Mehsen-Cetre, Paula Arranz, and et al. 2025. "A Randomized Phase II/III Trial Evaluating the Efficacy and Safety of 100 and 125 µg of Calcifediol Weekly Treatment of Severe Vitamin D Deficiency" Nutrients 17, no. 4: 672. https://doi.org/10.3390/nu17040672
APA StylePérez-Castrillón, J. L., Jódar-Gimeno, E., Nociar, J., Lojka, M., Nikolov, D., Cereto-Castro, F., Novković, S., Tarantino, U., Mehsen-Cetre, N., Arranz, P., Martínez Ostalé, C., García-Bea, A., & Gilaberte, I. (2025). A Randomized Phase II/III Trial Evaluating the Efficacy and Safety of 100 and 125 µg of Calcifediol Weekly Treatment of Severe Vitamin D Deficiency. Nutrients, 17(4), 672. https://doi.org/10.3390/nu17040672








