The Acute Effects of a Fast-Food Meal Versus a Mediterranean Food Meal on the Autonomic Nervous System, Lung Function, and Airway Inflammation: A Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
Randomization, Allocation and Sample Size
2.2. Intervention Protocol
Fast Food Meal and Mediterranean Meal
2.3. Outcome Assessment
2.3.1. Airway Inflammation
2.3.2. Lung Function
2.3.3. Autonomic Nervous System
2.4. Other Procedures
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.; de Castro Mendes, F.; Paciência, I.; Barros, R.; Padrão, P.; Cavaleiro Rufo, J.; Silva, D.; Delgado, L.; Moreira, A.; Moreira, P. Diet quality, asthma and airway inflammation in school-aged children. Eur. Ann. Allergy Clin. Immunol. 2023. epub ahead of print. [Google Scholar]
- Wood, L.G. Diet and lung disease-Are fruits and vegetables the ideal whole-food intervention? Respirology 2021, 26, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Wharton, R.; Wang, J.; Choi, Y.; Eisenberg, E.; Jackson, M.; Hanson, C.; Liu, B.; Washko, G.; Kalhan, R.; Jacobs, D.; et al. Associations of a Plant-centered Diet and Lung Function Decline across Early to Mid-Adulthood: The CARDIA Lung Study. Res. Sq. 2023, 25, 122. [Google Scholar]
- Strüven, A.; Holzapfel, C.; Stremmel, C.; Brunner, S. Obesity, Nutrition and Heart Rate Variability. Int. J. Mol. Sci. 2021, 22, 4215. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Padrão, P.; Teixeira, V.; Carvalho, P.; Delgado, L.; Lopes, C.; Severo, M.; Moreira, P. Dietary patterns and asthma prevalence, incidence and control. Clin. Exp. Allergy 2015, 45, 1673–1680. [Google Scholar] [CrossRef]
- Amazouz, H.; Roda, C.; Beydon, N.; Lezmi, G.; Bourgoin-Heck, M.; Just, J.; Momas, I.; Rancière, F. Mediterranean diet and lung function, sensitization, and asthma at school age: The PARIS cohort. Pediatr. Allergy Immunol. 2021, 32, 1437–1444. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Bioelectronic Medicine: From Preclinical Studies on the Inflammatory Reflex to New Approaches in Disease Diagnosis and Treatment. Cold Spring Harb. Perspect. Med. 2020, 10, a034140. [Google Scholar] [CrossRef]
- Fried, S.; Wemelle, E.; Cani, P.D.; Knauf, C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology 2021, 197, 108721. [Google Scholar] [CrossRef]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romani-Pérez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Carnagarin, R.; Matthews, V.B.; Herat, L.Y.; Ho, J.K.; Schlaich, M.P. Autonomic Regulation of Glucose Homeostasis: A Specific Role for Sympathetic Nervous System Activation. Curr. Diabetes Rep. 2018, 18, 107. [Google Scholar] [CrossRef]
- Costa, J.; Moreira, A.; Moreira, P.; Delgado, L.; Silva, D. Effects of weight changes in the autonomic nervous system: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 110–126. [Google Scholar] [CrossRef]
- George, K.; S., I.J.; Thomas, N.S.; R., B.; K., B. Gender-Based Vegetarian and Nonvegetarian Dietary Impact on Cardiac Autonomic Function of Heart Rate Variability. J. Am. Coll. Nutr. 2021, 40, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Paciência, I.; Rufo, J.C.; Silva, D.; Martins, C.; Mendes, F.; Rama, T.; Rodolfo, A.; Madureira, J.; Delgado, L.; Fernandes, E.d.O.; et al. School environment associates with lung function and autonomic nervous system activity in children: A cross-sectional study. Sci. Rep. 2019, 9, 15156. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.d.C.; Paciência, I.; Rufo, J.C.; Farraia, M.; Silva, D.; Padrão, P.; Delgado, L.; Garcia-Larsen, V.; Moreira, A.; Moreira, P. Increasing Vegetable Diversity Consumption Impacts the Sympathetic Nervous System Activity in School-Aged Children. Nutrients 2021, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Maunder, E.; Dulson, D.K.; Shaw, D.M. Autonomic and Perceptual Responses to Induction of a Ketogenic Diet in Free-Living Endurance Athletes: A Randomized, Crossover Trial. Int. J. Sports Physiol. Perform. 2021, 16, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Laugero, K.D.; Keim, N.L. A Diet Pattern Characterized by Sugar-Sweetened Beverages Is Associated with Lower Decision-Making Performance in the Iowa Gambling Task, Elevated Stress Exposure, and Altered Autonomic Nervous System Reactivity in Men and Women. Nutrients 2023, 15, 3930. [Google Scholar] [CrossRef]
- Connett, G.J. Asthma, classical conditioning, and the autonomic nervous system—A hypothesis for why children wheeze. Arch. Dis. Child. 2024, 109, 462–467. [Google Scholar] [CrossRef]
- Mendes, F.d.C.; Paciência, I.; Rufo, J.C.; Farraia, M.; Silva, D.; Padrão, P.; Delgado, L.; Garcia-Larsen, V.; Moreira, A.; Moreira, P. Higher diversity of vegetable consumption is associated with less airway inflammation and prevalence of asthma in school-aged children. Pediatr. Allergy Immunol. 2021, 32, 925–936. [Google Scholar] [CrossRef]
- Christensen, J.H. Omega-3 Polyunsaturated Fatty Acids and Heart Rate Variability. Front. Physiol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Kagawa, D.; Fujii, A.; Ohtsuka, M.; Murase, T. Ingestion of coffee polyphenols suppresses deterioration of skin barrier function after barrier disruption, concomitant with the modulation of autonomic nervous system activity in healthy subjects. Biosci. Biotechnol. Biochem. 2018, 82, 879–884. [Google Scholar] [CrossRef]
- Ennequin, G.; Thivel, D.; Mourot, L.; Isacco, L. Physically active men present a healthier cardiometabolic profile in response to a balanced meal compared to inactive men. Eur. J. Appl. Physiol. 2023, 123, 283–297. [Google Scholar] [CrossRef]
- Cozzolino, D.; Esposito, K.; Palmiero, G.; De Bellis, A.; Furlan, R.; Perrotta, S.; Perrone, L.; Torella, D.; del Giudice, E.M. Cardiac Autonomic Regulation in Response to a Mixed Meal Is Impaired in Obese Children and Adolescents: The Role Played by Insulin Resistance. J. Clin. Endocrinol. Metab. 2014, 99, 3199–3207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Lorenzo, A.; Bernardini, S.; Gualtieri, P.; Cabibbo, A.; Perrone, M.A.; Giambini, I.; Di Renzo, L. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: A randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2017, 54, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Schönknecht, Y.B.; Crommen, S.; Stoffel-Wagner, B.; Coenen, M.; Fimmers, R.; Stehle, P.; Ramirez, A.; Egert, S. APOE ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits. Nutrients 2021, 13, 3924. [Google Scholar] [CrossRef] [PubMed]
- Ade, C.J.; Rosenkranz, S.K.; Harms, C.A. The effects of short-term fish oil supplementation on pulmonary function and airway inflammation following a high-fat meal. Eur. J. Appl. Physiol. 2014, 114, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, S.R.; Bradley, M.M.; Siegle, G.J.; Roecklein, K.A.; Dix, A. Publication guidelines and recommendations for pupillary measurement in psychophysiological studies. Psychophysiology 2022, 59, e14035. [Google Scholar] [CrossRef]
- Ferencova, N.; Visnovcova, Z.; Olexova, L.B.; Tonhajzerova, I. Eye pupil—A window into central autonomic regulation via emotional/cognitive processing. Physiol. Res. 2021, 70 (Suppl. S4), S669–S682. [Google Scholar] [CrossRef]
- Jenkins, Z.M.; Eikelis, N.; Phillipou, A.; Castle, D.J.; Wilding, H.E.; Lambert, E.A. Autonomic Nervous System Function in Anorexia Nervosa: A Systematic Review. Front. Neurosci. 2021, 15, 682208. [Google Scholar] [CrossRef]
- Stang, J.; Stensrud, T.; Mowinckel, P.; Carlsen, K.-H. Parasympathetic Activity and Bronchial Hyperresponsiveness in Athletes. Med. Sci. Sports Exerc. 2016, 48, 2100–2107. [Google Scholar] [CrossRef]
- Silva, D.; Moreira, R.; Sokhatska, O.; Beltrão, M.; Montanha, T.; Garcia-Larsen, V.; Villegas, R.; Severo, M.; Pizarro, A.; Pinto, M.; et al. Meal-exercise challenge and physical activity reduction impact on immunity and inflammation (MERIIT trial). Contemp. Clin. Trials Commun. 2018, 10, 177–189. [Google Scholar] [CrossRef]
- Blüher, S.; Petroff, D.; Keller, A.; Wagner, A.; Classen, J.; Baum, P. Effect of a 1-Year Obesity Intervention (KLAKS Program) on Preexisting Autonomic Nervous Dysfunction in Childhood Obesity. J. Child Neurol. 2015, 30, 1174–1181. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Gangat, A.; Millar, P.J. A single high-fat Western meal modulates vascular responsiveness to sympathetic activation at rest and during exercise in humans: A randomized controlled trial. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H529–H538. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Thoracic, S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. J. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar]
- National Institute for Health and Care Excellence. Measuring Fractional Exhaled Nitric Oxide Concentration in Asthma: NIOX MINO, NIOX VERO and NObreath. 2014. Available online: https://www.nice.org.uk/guidance/dg12 (accessed on 1 July 2024).
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Stanojevic, S. GLI-2012 Excel Sheet Calculator. Global Lung Function Initiative. 2013. Available online: https://gli-calculator.ersnet.org/index.html (accessed on 1 June 2015).
- Couto, M.; Silva, D.; Santos, P.; Queirós, S.; Delgado, L.; Moreira, A. Exploratory study comparing dysautonomia between asthmatic and non-asthmatic elite swimmers. Rev. Port. Pneumol. 2015, 21, 22–29. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2015. Available online: https://ginasthma.org/ (accessed on 1 June 2015).
- World Health Organization. Global Database on Body Mass Index: BMI Classification; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Lu, C.-L.; Zou, X.; Orr, W.C.; Chen, J.D.Z. Postprandial Changes of Sympathovagal Balance Measured by Heart Rate Variability. Dig. Dis. Sci. 1999, 44, 857–861. [Google Scholar] [CrossRef]
- Nagai, N.; Sakane, N.; Hamada, T.; Kimura, T.; Moritani, T. The effect of a high-carbohydrate meal on postprandial thermogenesis and sympathetic nervous system activity in boys with a recent onset of obesity. Metabolism 2005, 54, 430–438. [Google Scholar] [CrossRef]
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9, 57. [Google Scholar] [CrossRef]
- Kurti, S.P.; Rosenkranz, S.K.; Levitt, M.; Cull, B.J.; Teeman, C.S.; Emerson, S.R.; Harms, C.A. Does moderate intensity exercise attenuate the postprandial lipemic and airway inflammatory response to a high-fat meal? BioMed Res. Int. 2015, 2015, 647952. [Google Scholar] [CrossRef]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Mendes, F.d.C.; Delgado, L.; Padrão, P.; Paciência, I.; Barros, R.; Rufo, J.C.; Silva, D.; Moreira, A.; Moreira, P. Diet and Asthma: A Narrative Review. Appl. Sci. 2023, 13, 6398. [Google Scholar] [CrossRef]
- Sorli-Aguilar, M.; Martin-Lujan, F.; Flores-Mateo, G.; Arija-Val, V.; Basora-Gallisa, J.; Sola-Alberich, R.; for the RESET Study Group investigators. Dietary patterns are associated with lung function among Spanish smokers without respiratory disease. BMC Pulm. Med. 2016, 16, 162. [Google Scholar] [CrossRef]
- Serra, H.C.O.A.; Rudakoff, L.C.S.; Muniz, A.K.O.A.; Magalhães, E.I.d.S.; Bragança, M.L.B.M.; Silva, A.A.M.d.; Vianna, E.d.S.O.; Bettiol, H.; Barbieri, M.A. Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil. Nutrients 2023, 15, 3165. [Google Scholar] [CrossRef] [PubMed]
- Mattos, S.; da Cunha, M.R.; Silva, M.I.B.; Serfaty, F.; Tarvainen, M.P.; Klein, M.R.S.T.; Neves, M.F. Effects of weight loss through lifestyle changes on heart rate variability in overweight and obese patients: A systematic review. Clin. Nutr. 2022, 41, 2577–2586. [Google Scholar] [CrossRef]
- Ziegler, D.; Strom, A.; Nowotny, B.; Zahiragic, L.; Nowotny, P.J.; Carstensen-Kirberg, M.; Herder, C.; Roden, M. Effect of Low-Energy Diets Differing in Fiber, Red Meat, and Coffee Intake on Cardiac Autonomic Function in Obese Individuals with Type 2 Diabetes. Diabetes Care 2015, 38, 1750–1757. [Google Scholar] [CrossRef]
- Tada, Y.; Yoshizaki, T.; Tanaka, I.; Kanehara, R.; Kato, M.; Hatta, N.; Hida, A.; Kawano, Y. Higher energy intake at dinner decreases parasympathetic activity during nighttime sleep in menstruating women: A randomized controlled trial. Physiol. Behav. 2018, 194, 252–259. [Google Scholar] [CrossRef]
- Loper, H.; Leinen, M.; Bassoff, L.; Sample, J.; Romero-Ortega, M.; Gustafson, K.J.; Taylor, D.M.; Schiefer, M.A. Both high fat and high carbohydrate diets impair vagus nerve signaling of satiety. Sci. Rep. 2021, 11, 10394. [Google Scholar] [CrossRef]
- Yan, Y.; Ramanan, D.; Rozenberg, M.; McGovern, K.; Rastelli, D.; Vijaykumar, B.; Yaghi, O.; Voisin, T.; Mosaheb, M.; Chiu, I.; et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity 2021, 54, 499–513.e5. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Glime, G.N.E.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.M.; Ghiadoni, L. Polyphenols, Antioxidants and the Sympathetic Nervous System. Curr. Pharm. Des. 2018, 24, 130–139. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of Marine-Derived n-3 Polyunsaturated Fatty Acids on C-Reactive Protein, Interleukin 6 and Tumor Necrosis Factor α: A Meta-Analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef] [PubMed]
- Thesing, C.S.; Bot, M.; Milaneschi, Y.; Giltay, E.J.; Penninx, B.W. Omega-3 polyunsaturated fatty acid levels and dysregulations in biological stress systems. Psychoneuroendocrinology 2018, 97, 206–215. [Google Scholar] [CrossRef]
- Dai, J.; Lampert, R.; Wilson, P.W.; Goldberg, J.; Ziegler, T.R.; Vaccarino, V. Mediterranean dietary pattern is associated with improved cardiac autonomic function among middle-aged men: A twin study. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 366–373. [Google Scholar] [CrossRef]
- Tentolouris, N.; Tsigos, C.; Perea, D.; Koukou, E.; Kyriaki, D.; Kitsou, E.; Daskas, S.; Daifotis, Z.; Makrilakis, K.; Raptis, S.; et al. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism 2003, 52, 1426–1432. [Google Scholar] [CrossRef]
- Palmer, A.C.; Healy, K.; Barffour, M.A.; Siamusantu, W.; Chileshe, J.; Schulze, K.J.; West, K.P., Jr.; Labrique, A.B. Provitamin A Carotenoid–Biofortified Maize Consumption Increases Pupillary Responsiveness among Zambian Children in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2551–2558. [Google Scholar] [CrossRef]
- Healy, K.; Palmer, A.C.; A Barffour, M.; Schulze, K.J.; Siamusantu, W.; Chileshe, J.; West, K.P.; Labrique, A.B. Nutritional Status Measures Are Correlated with Pupillary Responsiveness in Zambian Children. J. Nutr. 2018, 148, 1160–1166. [Google Scholar] [CrossRef]
- Yalcin, H.; Çapar, T.D. Bioactive Compounds of Fruits and Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 723–745. [Google Scholar]
- Papadopoulou, R.T.; Theodorou, M.R.; Ieong, C.S.; Ballantyne, K.; Marshall, D.; Verney, A.; Roig, M.; Nichols, B.; Gerasimidis, K. The Acute Effect of Meal Timing on the Gut Microbiome and the Cardiometabolic Health of the Host: A Crossover Randomized Control Trial. Ann. Nutr. Metab. 2020, 76, 322–333. [Google Scholar] [CrossRef]
- Catalin, R.-E.; Martin-Lujan, F.; Salamanca-Gonzalez, P.; Palleja-Millan, M.; Villalobos, F.; Santigosa-Ayala, A.; Pedret, A.; Valls-Zamora, R.M.; Sola, R.; on behalf of the MEDISTAR Research Group Investigators. Mediterranean Diet and Lung Function in Adults Current Smokers: A Cross-Sectional Analysis in the MEDISTAR Project. Nutrients 2023, 15, 1272. [Google Scholar] [CrossRef] [PubMed]
- Bakolis, I.; Hooper, R.; Bachert, C.; Lange, B.; Haahtela, T.; Keil, T.; Hofmaier, S.; Fokkens, W.; Rymarczyk, B.; Janson, C.; et al. Dietary patterns and respiratory health in adults from nine European countries—Evidence from the GA2LEN study. Clin. Exp. Allergy 2018, 48, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Potts, J.F.; Omenaas, E.; Heinrich, J.; Svanes, C.; Garcia-Aymerich, J.; Burney, P.G.; Jarvis, D.L. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur. Respir. J. 2017, 50, 1602286. [Google Scholar] [CrossRef]
- Rosenkranz, S.K.; Townsend, D.K.; Steffens, S.E.; Harms, C.A. Effects of a high-fat meal on pulmonary function in healthy subjects. Eur. J. Appl. Physiol. 2010, 109, 499–506. [Google Scholar] [CrossRef]
- McDiarmid, K.P.; Wood, L.G.; Upham, J.W.; MacDonald-Wicks, L.K.; Shivappa, N.; Hebert, J.R.; Scott, H.A. The Impact of Meal Dietary Inflammatory Index on Exercise-Induced Changes in Airway Inflammation in Adults with Asthma. Nutrients 2022, 14, 4392. [Google Scholar] [CrossRef]
- Damoun, N.; Amekran, Y.; Taiek, N.; El Hangouche, A.J. Heart rate variability measurement and influencing factors: Towards the standardization of methodology. Glob. Cardiol. Sci. Pract. 2024, 2024, e202435. [Google Scholar] [CrossRef]
- Farrell, M.C.; Giza, R.J.; Shibao, C.A. Race and sex differences in cardiovascular autonomic regulation. Clin. Auton. Res. 2020, 30, 371–379. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, L.; Jin, D.; Lin, Z.; Zhang, Q.; Zhang, M. Assessment of Combination of Automated Pupillometry and Heart Rate Variability to Detect Driving Fatigue. Front. Public Health 2022, 10, 828428. [Google Scholar] [CrossRef]
| Total | |
|---|---|
| Female | 26 (57) |
| Age, years (median, 25th–75th) | 25, 22–30 |
| Caucasian | 43 (94) |
| Height, cm (mean ± SD) | 169 ± 10.4 |
| BMI | |
| Average weight (18.5–24.9 kg/m2) | 25 (54) |
| Overweight (25–29.9 kg/m2) | 17 (37) |
| Obese (≥30 kg/m2) | 4 (9) |
| Active smokers | 7 (15) |
| Asthma diagnosis | 13 (28.3) |
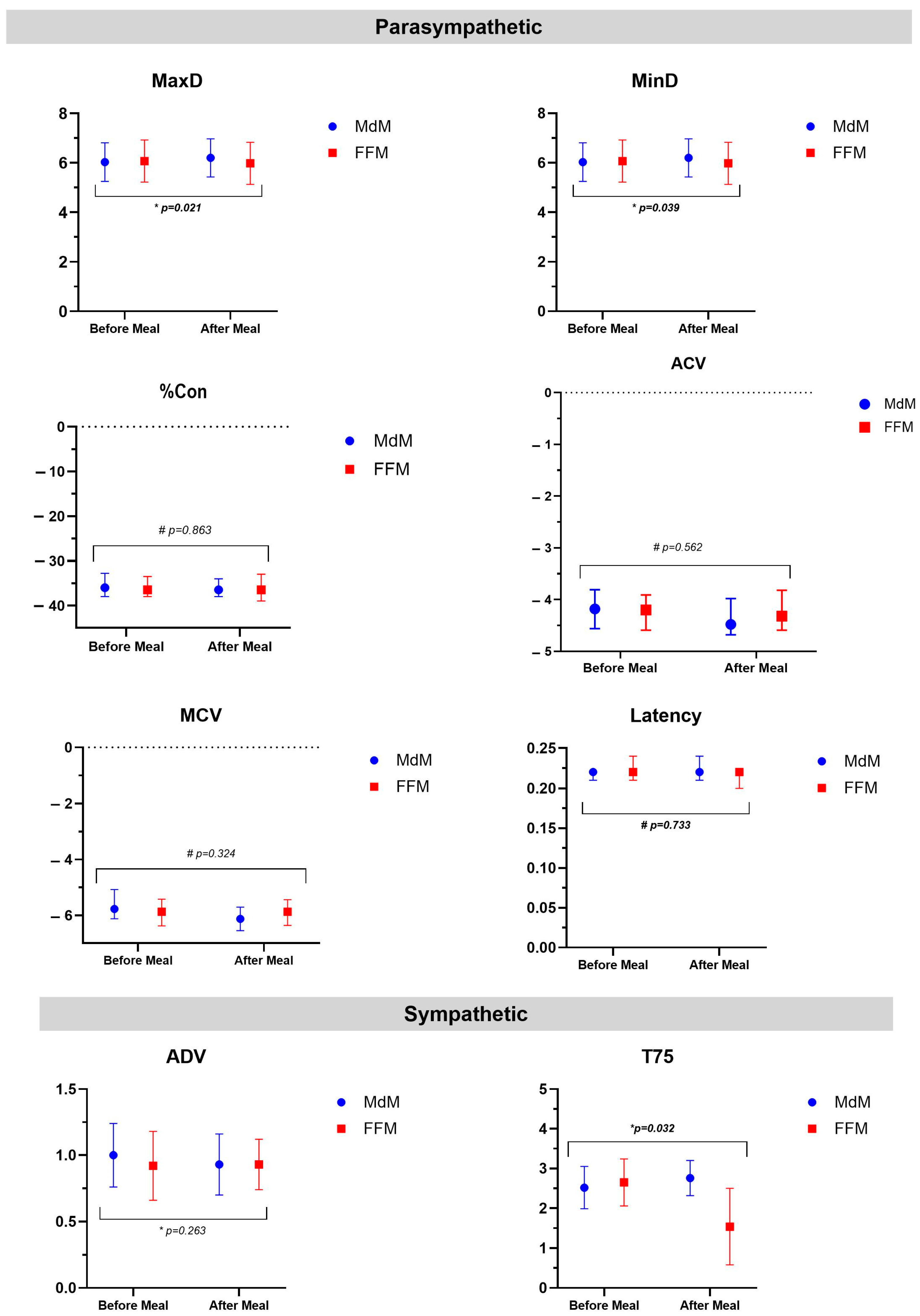
| MdM | FFM | ∆ Between Meals | |||||
|---|---|---|---|---|---|---|---|
| Measurements | Before | After | p | Before | After | p | |
| Parasympathetic | |||||||
| MaxD, mm (mean ± SD) | 6.03 ± 0.78 | 6.20 ± 0.77 | 0.043 a | 6.07 ± 0.85 | 5.98 ± 0.85 | 0.249 a | 0.26 ± 0.71 |
| MinD, mm (mean ± SD) | 3.93 ± 0.62 | 3.97 ± 0.60 | 0.128 a | 3.92 ± 0.66 | 3.87 ± 0.72 | 0.352 a | 0.17 ± 0.51 |
| %Con (%) | −36.0 (−38.0; −32.8) | −36.5 (−38.0; −34.0) | 0.072 b | −36.5 (−38.0; −33.5) | −36.5 (−39.0; −33.0) | 0.467 b | 0.50 (−2.00; 2.50) |
| Latency, s | 0.22 (0.21; 0.22) | 0.22 (0.21; 0.24) | 0.461 b | 0.22 (0.21; 0.24) | 0.22 (0.20; 0.22) | 0.876 b | 0.0 (−0.015; 0.015) |
| ACV, mm/s | −4.18 (−4.56; −3.81) | −4.48 (−4.68; −3.98) | 0.035 b | −4.20 (−4.59; −3.91) | −4.32 (−4.59; −3.82) | 0.483 b | −0.060 (−0.29; 0.26) |
| MCV, mm/s | −5.77 (−6.12; −5.08) | −6.13 (−6.55; −5.71) | 0.003 b | −5.87 (−6.38; −5.42) | −5.87 (−6.36; −5.44) | 0.324 b | −0.03 (−0.85; 0.37) |
| Sympathetic | |||||||
| ADV, mm/s (mean ± SD) | 1.00 ± 0.24 | 0.93 ± 0.23 | 0.030 a | 0.92 ± 0.26 | 0.93 ± 0.19 | 0.412 a | 1.62 ± 9.53 |
| T75, s (mean ± SD) | 2.52 ± 0.53 | 2.76 ± 0.44 | 0.454 a | 2.65 ± 0.59 | 1.54 ± 0.96 | 0.012 a | 1.84 ± 0.67 |
| MdM | FFM | |||||
|---|---|---|---|---|---|---|
| Measurements | Before | After | p | Before | After | p |
| Lung function | ||||||
| FVC, median (25th; 75th) | 4.08 (3.55; 5.40) | 4.08 (3.44; 4.98) | 0.023 b | 4.13 (3.45; 5.52) | 4.19 (3.52; 5.44) | 0.029 b |
| FEV1, median (25th; 75th) | 3.67 (2.93; 4.44) | 3.58 (2.94; 4.41) | 0.055 b | 3.65 (3.03; 4.48) | 3.73 (3.00; 4.45) | 0.245 b |
| FEV1/FVC | 85.7 ± 5.43 | 86.2 ± 5.20 | 0.311 a | 85.6 ± 5.70 | 86.3 ± 5.29 | 0.118 a |
| FEF25/75 | 4.0 ± 1.11 | 4.09 ± 1.21 | 0.110 a | 4.11 ± 1.10 | 4.09 ± 1.13 | 0.817 a |
| Airway inflammation | ||||||
| FeNO, ppb, median (25th; 75th) | 25.7 (16.3; 49.3) | 30.8 (25.6; 62.3) | <0.001 b | 25.0 (14.3; 47.0) | 29.3 (17.0; 51.7) | 0.040 b |
| Fixed Effects | Random Effects | Model Fit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Meal FFM vs. MdM | Time After vs. Before | Intercept | Residual | Log-Likelihood | AIC | BIC | ||
| MaxD | |||||||||
| Model 1 | |||||||||
| Estimate | 6.09 | −0.08 | 0.04 | -- | -- | −138.56 | 287.13 | 302.50 | |
| SE | 0.12 | 0.07 | 0.06 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.47 | 0.17 | -- | -- | -- | |
| SD | -- | -- | -- | 0.68 | 0.41 | -- | -- | -- | |
| DF | 53.81 | 117.99 | 117.19 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.215 | 0.508 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 8.83 | −0.08 | 0.04 | -- | -- | −140.15 | 300.30 | 331.05 | |
| SE | 0.83 | 0.07 | 0.06 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.40 | 0.17 | -- | -- | -- | |
| SD | -- | -- | -- | 0.63 | 0.41 | -- | -- | -- | |
| DF | 35.27 | 117.89 | 117.22 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.222 | 0.508 | -- | -- | -- | -- | -- | |
| MinD | |||||||||
| Model 1 | |||||||||
| Estimate | 3.96 | −0.06 | 0.01 | -- | -- | −98.90 | 207.79 | 223.04 | |
| SE | 0.09 | 0.05 | 0.05 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.33 | 0.10 | -- | -- | -- | |
| SD | -- | -- | -- | 0.58 | 0.32 | -- | -- | -- | |
| DF | 51.20 | 113.80 | 113.05 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.231 | 0.792 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 6.03 | −0.06 | 0.01 | -- | -- | −102.11 | 224.22 | 254.72 | |
| SE | 0.71 | 0.05 | 0.05 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.30 | 0.10 | -- | -- | -- | |
| SD | -- | -- | -- | 0.55 | 0.32 | -- | -- | -- | |
| DF | 35.03 | 113.76 | 113.19 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.233 | 0.788 | -- | -- | -- | -- | -- | |
| %Con | |||||||||
| Model 1 | |||||||||
| Estimate | −35.20 | −0.13 | −0.21 | -- | -- | −376.14 | 762.28 | 777.60 | |
| SE | 0.64 | 0.29 | 0.29 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 14.53 | 3.81 | -- | -- | -- | |
| SD | -- | -- | -- | 3.29 | 1.82 | -- | -- | -- | |
| DF | 48.58 | 115.53 | 114.96 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.651 | 0.470 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | −30.36 | −0.13 | −0.21 | -- | -- | −372.72 | 765.43 | 796.06 | |
| SE | 5.03 | 0.29 | 0.29 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 15.39 | 3.29 | -- | -- | -- | |
| SD | -- | -- | -- | 3.92 | 1.82 | -- | -- | -- | |
| DF | 34.83 | 115.30 | 114.91 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.652 | 0.474 | -- | -- | -- | -- | -- | |
| ACV | |||||||||
| Model 1 | |||||||||
| Estimate | −4.21 | 0.13 | −0.04 | -- | -- | −144.81 | 299.62 | 314.62 | |
| SE | 0.09 | 0.08 | 0.08 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.17 | 0.25 | -- | -- | -- | |
| SD | -- | -- | -- | 0.41 | 0.50 | -- | -- | -- | |
| DF | 86.37 | 118.36 | 116.42 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.109 | 0.629 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | −5.03 | 0.12 | −0.04 | -- | -- | −150.01 | 320.01 | 350.01 | |
| SE | 0.58 | 0.08 | 0.08 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.15 | 0.25 | -- | -- | -- | |
| SD | -- | -- | -- | 0.39 | 0.50 | -- | -- | -- | |
| DF | 34.84 | 117.40 | 115.76 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.127 | 0.624 | -- | -- | -- | -- | -- | |
| MCV | |||||||||
| Model 1 | |||||||||
| Estimate | −5.78 | −0.003 | −0.28 | -- | -- | −245.37 | 500.73 | 516.08 | |
| SE | 0.16 | 0.16 | 0.16 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.35 | 1.01 | -- | -- | -- | |
| SD | -- | -- | -- | 0.59 | 1.01 | -- | -- | -- | |
| DF | 107.93 | 119.07 | 116.67 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.984 | 0.079 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | −6.34 | −2.61 × 10−3 | −0.28 | -- | -- | −250.77 | 521.54 | 552.23 | |
| SE | 1.03 | 0.16 | 0.16 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.41 | 0.64 | -- | -- | -- | |
| SD | -- | -- | -- | 1.01 | 1.01 | -- | -- | -- | |
| DF | 35.55 | 118.34 | 116.43 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.987 | 0.079 | -- | -- | -- | -- | -- | |
| ADV | |||||||||
| Model 1 | |||||||||
| Estimate | 0.97 | −0.02 | −0.03 | -- | -- | 167.48 | −22.95 | −7.93 | |
| SE | 0.03 | 0.03 | 0.03 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.03 | 0.03 | -- | -- | -- | |
| SD | -- | -- | -- | 0.16 | 0.17 | -- | -- | -- | |
| DF | 74.79 | 108.15 | 107.24 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.440 | 0.229 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 1.08 | −0.02 | −0.03 | -- | -- | 5.35 | 9.29 | 39.33 | |
| SE | 0.23 | 0.03 | 0.03 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.03 | 0.03 | -- | -- | -- | |
| SD | -- | -- | -- | 0.16 | 0.17 | -- | -- | -- | |
| DF | 33.54 | 107.01 | 106.35 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.482 | 0.233 | -- | -- | -- | -- | -- | |
| T75 | |||||||||
| Model 1 | |||||||||
| Estimate | 2.98 | −0.67 | −0.54 | -- | -- | −97.76 | 205.52 | 217.56 | |
| SE | 0.17 | 0.18 | 0.18 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.16 | 0.61 | -- | -- | -- | |
| SD | -- | -- | -- | 0.17 | 0.78 | -- | -- | -- | |
| DF | 79.0 | 79.0 | 79.0 | -- | -- | -- | -- | -- | |
| p | <0.001 | <0.001 | <0.001 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 3.46 | −0.68 | −0.52 | -- | -- | −97.63 | 215.27 | 239.34 | |
| SE | 0.63 | 0.16 | 0.17 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.10 | 0.53 | -- | -- | -- | |
| SD | -- | -- | -- | 0.07 | 0.73 | -- | -- | -- | |
| DF | 74.0 | 74.0 | 74.0 | -- | -- | -- | -- | -- | |
| p | <0.001 | <0.001 | 0.002 | -- | -- | -- | -- | -- | |
| Fixed Effects | Random Effects | Model Fit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Meal FFM vs. MdM | Time After vs. Before | Intercept | Residual | Log-Likelihood | AIC | BIC | ||
| FVC | |||||||||
| Model 1 | |||||||||
| Estimate | 4.33 | 0.039 | −0.079 | -- | -- | −629.08 | 1302.15 | 1369.67 | |
| SE | 0.16 | 0.023 | 0.023 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 1.027 | 0.021 | -- | -- | -- | |
| SD | -- | -- | -- | 1.013 | 0.146 | -- | -- | -- | |
| DF | 40.82 | 117.07 | 117.01 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.097 | <0.001 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 1.63 | 0.039 | −0.079 | -- | -- | −14.72 | 75.44 | 146.17 | |
| SE | 1.35 | 0.023 | 0.023 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.963 | 0.021 | -- | -- | -- | |
| SD | -- | -- | -- | 0.981 | 0.146 | -- | -- | -- | |
| DF | 21.98 | 117.01 | 116.98 | -- | -- | -- | -- | -- | |
| p | 0.238 | 0.096 | <0.001 | -- | -- | -- | -- | -- | |
| FEV1 | |||||||||
| Model 1 | |||||||||
| Estimate | 3.70 | 0.02 | −0.04 | -- | -- | −75.21 | 160.42 | 170.53 | |
| SE | 0.13 | 0.02 | 0.02 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.66 | 0.01 | -- | -- | -- | |
| SD | -- | -- | -- | 0.81 | 0.11 | -- | -- | -- | |
| DF | 40.76 | 117.06 | 117.0 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.186 | 0.017 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 2.18 | 0.02 | −0.04 | -- | -- | −14.72 | 75.44 | 146.17 | |
| SE | 1.15 | 0.017 | 0.018 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 0.71 | 0.84 | -- | -- | -- | |
| SD | -- | -- | -- | 0.013 | 0.11 | -- | -- | -- | |
| DF | 21.99 | 117.02 | 116.99 | -- | -- | -- | -- | -- | |
| p | 0.072 | 0.186 | 0.017 | -- | -- | -- | -- | -- | |
| FEV1/FVC | |||||||||
| Model 1 | |||||||||
| Estimate | 102.52 | 0.32 | 0.83 | -- | -- | −430.14 | 870.28 | 885.63 | |
| SE | 1.0 | 0.39 | 0.39 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 36.38 | 5.90 | -- | -- | -- | |
| SD | -- | -- | -- | 6.03 | 2.43 | -- | -- | -- | |
| DF | 18.76 | 113.45 | 114.32 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.411 | 0.033 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 123.91 | 0.31 | −2.31 | -- | -- | −379.58 | 805.17 | 875.75 | |
| SE | 7.59 | 0.39 | 1.21 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 29.13 | 5.90 | -- | -- | -- | |
| SD | -- | -- | -- | 5.40 | 2.43 | -- | -- | -- | |
| DF | 22.12 | 116.39 | 22.0 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.433 | 0.06 | -- | -- | -- | -- | -- | |
| FEF25/75 | |||||||||
| Model 1 | |||||||||
| Estimate | 4.03 | 0.005 | 0.04 | -- | -- | −204.30 | 418.59 | 434.38 | |
| SE | 0.18 | 0.04 | 0.04 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 1.22 | 0.07 | -- | -- | -- | |
| SD | -- | -- | -- | 1.11 | 0.26 | -- | -- | -- | |
| DF | 42.27 | 117.17 | 117.01 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.905 | 0.349 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | 4.61 | 0.004 | 0.830 | -- | -- | −104.90 | 219.80 | 235.18 | |
| SE | 1.35 | 0.04 | 0.39 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 1.26 | 1.12 | -- | -- | -- | |
| SD | -- | -- | -- | 0.07 | 0.26 | -- | -- | -- | |
| DF | 22.03 | 117.13 | 116.1 | -- | -- | -- | -- | -- | |
| p | 0.002 | 0.922 | 0.349 | -- | -- | -- | -- | -- | |
| FeNO | |||||||||
| Model 1 | |||||||||
| Estimate | 44.08 | −4,15 | 3.79 | -- | -- | −705.05 | 1422.09 | 1440.51 | |
| SE | 6.81 | 2.19 | 2.16 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 1758.83 | 185.82 | -- | -- | -- | |
| SD | -- | -- | -- | 41.94 | 13.63 | -- | -- | -- | |
| DF | 44.19 | 116.37 | 116.08 | -- | -- | -- | -- | -- | |
| p | <0.001 | 0.058 | 0.083 | -- | -- | -- | -- | -- | |
| Model 2 | |||||||||
| Estimate | −7.01 | −4.22 | 3.80 | -- | -- | −629.08 | 1302.15 | 1369.67 | |
| SE | 56.95 | 2.19 | 2.16 | -- | -- | -- | -- | -- | |
| Variance | -- | -- | -- | 1776.84 | 42.15 | -- | -- | -- | |
| SD | -- | -- | -- | 185.82 | 13.63 | -- | -- | -- | |
| DF | 23.08 | 116.07 | 116.07 | -- | -- | -- | -- | -- | |
| p | 0.903 | 0.057 | 0.082 | -- | -- | -- | -- | -- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.; Mendes, F.C.; Stanzani, V.; Moreira, R.; Pinto, M.; Beltrão, M.; Sokhatska, O.; Severo, M.; Padrão, P.; Garcia-Larsen, V.; et al. The Acute Effects of a Fast-Food Meal Versus a Mediterranean Food Meal on the Autonomic Nervous System, Lung Function, and Airway Inflammation: A Randomized Crossover Trial. Nutrients 2025, 17, 614. https://doi.org/10.3390/nu17040614
Silva D, Mendes FC, Stanzani V, Moreira R, Pinto M, Beltrão M, Sokhatska O, Severo M, Padrão P, Garcia-Larsen V, et al. The Acute Effects of a Fast-Food Meal Versus a Mediterranean Food Meal on the Autonomic Nervous System, Lung Function, and Airway Inflammation: A Randomized Crossover Trial. Nutrients. 2025; 17(4):614. https://doi.org/10.3390/nu17040614
Chicago/Turabian StyleSilva, Diana, Francisca Castro Mendes, Vânia Stanzani, Rita Moreira, Mariana Pinto, Marília Beltrão, Oksana Sokhatska, Milton Severo, Patrícia Padrão, Vanessa Garcia-Larsen, and et al. 2025. "The Acute Effects of a Fast-Food Meal Versus a Mediterranean Food Meal on the Autonomic Nervous System, Lung Function, and Airway Inflammation: A Randomized Crossover Trial" Nutrients 17, no. 4: 614. https://doi.org/10.3390/nu17040614
APA StyleSilva, D., Mendes, F. C., Stanzani, V., Moreira, R., Pinto, M., Beltrão, M., Sokhatska, O., Severo, M., Padrão, P., Garcia-Larsen, V., Delgado, L., Moreira, A., & Moreira, P. (2025). The Acute Effects of a Fast-Food Meal Versus a Mediterranean Food Meal on the Autonomic Nervous System, Lung Function, and Airway Inflammation: A Randomized Crossover Trial. Nutrients, 17(4), 614. https://doi.org/10.3390/nu17040614









