Impact of Sexual Dimorphism on Therapy Response in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: From Conventional and Nutritional Approaches to Emerging Therapies
Abstract
1. Introduction
2. The Importance of Sexual Dimorphism in MASLD: The Relevance of Steroids Metabolism
3. Impact of Sexual Dimorphisms on Conventional and Emerging Therapeutic Strategies
3.1. Diet-Based Treatment
3.2. Antihyperglycemic Treatment
3.3. Lipid Lowering Treatment
3.4. Nucleotide-Based Therapies
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lekakis, V.; Papatheodoridis, G.V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- O’Hara, J.; Finnegan, A.; Dhillon, H.; Ruiz-Casas, L.; Pedra, G.; Franks, B.; Morgan, G.; Hebditch, V.; Jonsson, B.; Mabhala, M.; et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: The GAIN study. JHEP Rep. 2020, 2, 100142. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.T.; Taylor, R.J.; Bayliss, S.; Hagstrom, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, A.W.; Peleg, N.; et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 2020, 158, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Lassen, P.B.; Nori, N.; Bedossa, P.; Genser, L.; Aron-Wisnewsky, J.; Poitou, C.; Surabattula, R.; Nielsen, M.J.; Karsdal, M.A.; Leeming, D.J.; et al. Fibrogenesis marker PRO-C3 is higher in advanced liver fibrosis and improves in patients undergoing bariatric surgery. J. Clin. Endocrinol. Metab. 2022, 107, e1356–e1366. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Staels, B. Hepatic sexual dimorphism—Implications for non-alcoholic fatty liver disease. Nat. Rev. Endocrinol. 2021, 17, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Vandel, J.; Dubois-Chevalier, J.; Gheeraert, C.; Derudas, B.; Raverdy, V.; Thuillier, D.; Gaal, L.; Francque, S.; Pattou, F.; Staels, B.; et al. Hepatic Molecular Signatures Highlight the Sexual Dimorphism of Nonalcoholic Steatohepatitis (NASH). Hepatology 2021, 73, 920–936. [Google Scholar] [CrossRef]
- Eslam, M.; George, J. Genetic contributions to NAFLD: Leveraging shared genetics to uncover systems biology. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 40–52. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D.; Gudnason, V.; Eiriksdottir, G.; Garcia, M.E.; Launer, L.J.; et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Kozlitina, J.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Hobbs, H.H.; Cohen, J.C. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 2017, 49, 842–847. [Google Scholar] [CrossRef]
- Schulze, K.; Nault, J.; Villanueva, A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J. Hepatol. 2016, 65, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Moeller, G.; Adamski, J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2009, 301, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.; Gane, E.; Schwabe, C.; Yoon, K.T.; Heo, J.; Scott, R.; Lee, J.; Lee, J.I.; Kweon, Y.O.; Weltman, M.; et al. A phase I/II study of ARO-HSD, an RNA interference therapeutic, for the treatment of non-alcoholic steatohepatitis. J. Hepatol. 2023, 78, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, F.; Zhang, S.; Liu, J.; Gao, G.; Xie, J.; Wang, Y. Oligonucleotide therapies for nonalcoholic steatohepatitis. Mol. Ther. Nucleic Acids 2022, 35, 102184. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A.; et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Falzarano, C.; Lofton, T.; Osei-Ntansah, A.; Oliver, T.; Southward, T.; Stewart, S.; Andrisse, S. Nonalcoholic fatty liver disease in women and girls with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2022, 107, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Hutison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine aspects of metabolic dysfunction associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction associated steatotic liver disease. Trends Mol. Med. 2024, 30, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Paschou, S.A.; Armeni, E.; Polyzos, S.A.; Goulis, D.G.; Lambrinoudaki, I. Non-alcoholic fatty liver disease through the female lifespan: The role of sex hormones. J. Endocrinol. Investig. 2022, 45, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Della Torre, S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD. Pathophysiol. Cells 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Turola, E.; Petta, S.; Vanni, E.; Milosa, F.; Valenti, L.; Critelli, R.; Miele, L.; Maccio, L.; Calvaruso, V.; Fracanzani, A.L.; et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis. Models Mech. 2015, 8, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Barone, M.; Mitro, N.; Lolli, F.; Pedretti, S.; Caruso, D.; Maggi, A.; Della Torre, S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol. Metab. 2020, 32, 97–108. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int. J. Mol. Sci. 2019, 20, 2841. [Google Scholar] [CrossRef]
- Charlton, M.; Angulo, P.; Chalasani, N.; Merriman, R.; Viker, K.; Charatcharoenwitthaya, P.; Sanderson, S.; Gawrieh, S.; Krishnan, A.; Lindor, K.; et al. Low circulating levels of dehydroepiandrosterone in histologically advanced nonalcoholic fatty liver disease. Hepatology 2008, 47, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, S.; Zhang, X.; Fan, Y.; Liu, M. Low serum dehydroepiandrosterone is associated with diabetic dyslipidemia risk in males with type 2 diabetes. Front. Endocrinol. 2023, 14, 1272797. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Jornayvaz, F.R. Endocrine causes of nonalcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 11053–11076. [Google Scholar] [CrossRef] [PubMed]
- Marginean, C.M.; Pirscoveanu, D.; Cazacu, S.M.; Popescu, M.S.; Marginean, I.C.; Iacob, G.A.; Popescu, M. Non-Alcoholic Fatty Liver Disease, Awareness of a Diagnostic Challenge-A Clinician’s Perspective. Gastroenterol. Insights 2024, 15, 1028–1053. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. 2013, 217, 25–45. [Google Scholar] [CrossRef]
- Fernandez, C.J.; Chacko, E.C.; Pappachan, J.M. Male Obesity-related Secondary Hypogonadism—Pathophysiology, Clinical Implications and Management. Eur. Endocrinol. 2019, 15, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Namatame, L.A.; Kang, M.; Buchthal, S.D.; Ernst, T.; Monroe, K.R.; Shepherd, J.A.; Wilkens, L.R.; Boushey, C.J.; Marchand, L.L.; et al. Differences in the association of diet quality with body fat distribution between men and women. Eur. J. Clin. Nutr. 2020, 74, 1434–1441. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Moore, J.B. Practical Lifestyle Management of Nonalcoholic Fatty Liver Disease for Busy Clinicians. Diabetes Spectr. 2024, 37, 39–47. [Google Scholar] [CrossRef]
- Wirfalt, E.; Hedblad, B.; Gullberg, B.; Mattisson, I.; Andrén, C.; Rosander, U.; Janzon, L.; Berglund, G. Food patterns and components of the metabolic syndrome in men and women: A cross-sectional study within the Malmo diet and cancer cohort. Am. J. Epidemiol. 2001, 154, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, M.; Paye, S.; Benayoun, B.A. Sex as a biological variable in nutrition research: From human studies to animal models. Annu. Rev. Nutr. 2022, 42, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Su, J.; Andres, J.; Henning, A.; Ren, J. Sex Differences in Fat Distribution and Muscle Fat Infiltration in the Lower Extremity: A Retrospective Diverse-Ethnicity 7T MRI Study in a Research Institute Setting in the USA. Diagnostics 2024, 14, 2260. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Elbers, J.M.; Asscheman, H.; Seidell, J.C.; Megens, J.A.; Gooren, L.J. Long-term testosterone administration increases visceral fat in female to male transsexuals. J. Clin. Endocrinol. Metab. 1997, 82, 2044–2047. [Google Scholar] [CrossRef] [PubMed]
- Elbers, J.M.; Giltay, E.J.; Teerlink, T.; Scheffer, P.G.; Asscheman, H.; Seidell, J.C.; Gooren, L.J. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin. Endocrinol. 2003, 58, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. Metabolic implications of body fat distribution. Diabetes Care 1991, 14, 1132–1143. [Google Scholar] [CrossRef]
- Della Torre, S.; Mitro, N.; Meda, C.; Lolli, F.; Pedretti, S.; Barcella, M.; Ottobrini, L.; Metzger, D.; Caruso, D.; Maggi, A. Short-Term Fasting Reveals Amino Acid Metabolism as a Major Sex-Discriminating Factor in the Liver. Cell Metab. 2018, 28, 256–267. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015, 3, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Smeets, A.J.; Luscombe-Marsh, N.D. Sex differences in energy homeostatis following a diet relatively high in protein exchanged with carbohydrate, assessed in a respiration chamber in humans. Physiol. Behav. 2009, 97, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Goossens, G.H.; Astrup, A.; Saris, W.H.M.; Blaak, E.E. Sexual Dimorphism in Body Weight Loss, Improvements in Cardiometabolic Risk Factors and Maintenance of Beneficial Effects 6 Months after a Low-Calorie Diet: Results from the Randomized Controlled DiOGenes Trial. Nutrients 2021, 13, 1588. [Google Scholar] [CrossRef]
- Bédard, A.; Corneau, L.; Lamarche, B.; Dodin, S.; Lemieux, S. Sex-related differences in the effects of the mediterranean diet on glucose and insulin homeostasis. J. Nutr. Metab. 2014, 2014, 424130. [Google Scholar] [CrossRef]
- Leblanc, V.; Bégin, C.; Hudon, A.M.; Royer, M.M.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in the long-term effects of a nutritional intervention program promoting the Mediterranean diet: Changes in dietary intakes, eating behaviors, anthropometric and metabolic variables. Nutr. J. 2014, 13, 107. [Google Scholar] [CrossRef]
- Carruba, G.; Granata, O.M.; Pala, V.; Campisi, I.; Agostara, B.; Cusimano, R.; Ravazzolo, B.; Traina, A. A Traditional Mediterranean Diet Decreases Endogenous Estrogens in Healthy Postmenopausal Women. Nutr. Cancer 2006, 56, 253–259. [Google Scholar] [CrossRef]
- D’Abbondanza, M.; Ministrini, S.; Pucci, G.; Nulli Migliola, E.; Martorelli, E.E.; Gandolfo, V.; Siepi, D.; Lupattelli, G.; Vaudo, G. Very Low-Carbohydrate Ketogenic Diet for the Treatment of Severe Obesity and Associated Non-Alcoholic Fatty Liver Disease: The Role of Sex Differences. Nutrients 2020, 12, 2748. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Frias-Toral, E.; Reytor-González, C.; Annunziata, G.; Proganò, M.; Savastano, S.; Simancas-Racines, D.; Colao, A.; Barrea, L. Weight loss, changes in body composition and inflammatory status after a very low-energy ketogenic therapy (VLEKT): Does gender matter? J. Transl. Med. 2024, 22, 949. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Costabile, G.; Bergia, R.E.; Hjorth, T.; Campbell, W.W.; Landberg, R.; Riccardi, G.; Giacco, R. The effects of Mediterranean diets with low or high glycemic index on plasma glucose and insulin profiles are different in adult men and women: Data from MEDGI-Carb randomized clinical trial. Clin. Nutr. 2023, 42, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B.; Gallwitz, B.; Dagogo-Jack, S.; Thieu, V.; Garcia-Perez, L.E.; Pavo, I.; Yu, M.; Robertson, K.E.; Zhang, N.; Giorgino, F. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes. Metab. 2018, 20, 409–418. [Google Scholar] [CrossRef]
- Onishi, Y.; Oura, T.; Nishiyama, H.; Ohyama, S.; Takeuchi, M.; Iwamoto, N. Subgroup analysis of phase 3 studies of dulaglutide in Japanese patients with type 2 diabetes. Endocr. J. 2016, 63, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Caroleo, P.; Arcidiacono, B.; Corigliano, D.M.; Giuliano, S.; Brunetti, F.S.; Tanyolac, S.; Foti, D.P.; Puccio, L.; et al. Long-Term Effectiveness of Liraglutide for Weight Management and Glycemic Control in Type 2 Diabetes. Int. J. Environ. Res. Public Health 2019, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Buysschaert, M.; Preumont, V.; Oriot, P.; Paris, I.; Ponchon, M.; Scarniere, D.; Selvais, P. One-year metabolic outcomes in patients with type 2 diabetes treated with exenatide in routine practice. Diabetes Metab. 2010, 36, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, H.; Wei, W.; Fang, T. Gender-related different effects of a combined therapy of Exenatide and Metformin on overweight or obesity patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2016, 30, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, G.; Bosisio, R.; Calabresi, L.; Magni, P.; Pavanello, C.; Pazzucconi, F.; Pazzucconi, F.; Sirtori, C.R. Gender-related lipid and/or lipoprotein responses to statins in subjects in primary and secondary prevention. J. Clin. Lipidol. 2015, 9, 226–233. [Google Scholar] [CrossRef]
- Smiderle, L.; Lima, L.O.; Hutz, M.H.; Van der Sand, C.R.; Van der Sand, L.C.; Wagner Ferreira, M.E.; Canibal Pires, R.; Almeida, S.; Fiegenbaum, M. Evaluation of sexual dimorphism in the efficacy and safety of simvastatin/atorvastatin therapy in a southern Brazilian cohort. Arq. Bras. Cardiol. 2014, 103, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sever, P.; Gouni-Berthold, I.; Keech, A.; Giugliano, R.; Pedersen, T.R.; Im, K.; Wang, H.; Knusel, B.; Sabatine, M.S.; O’Donoghue, M.L. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the FOURIER Trial. Eur. J. Prev. Cardiol. 2021, 28, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bittner, V.; McGinniss, J.; Schwartz, G.; Bhatt, D.; Chua, T.; Asita de Silva, H.; Diaz, R.; Dorobantu, M.; Goodman, S.; Robert Harrington, A.; et al. Alirocumab and cardiovascular outcomes in women after an acute coronary syndrome: An ODYSSEY outcomes trial analysis. J. Am. Coll. Cardiol. 2020, 75, 1854. [Google Scholar] [CrossRef]
- Fabbrini, E.; Rady, B.; Koshkina, A.; Ayyar, V.S.; DiProspero, N.; Hegge, J.; Hamilton, H.; Ding, Z.M.; Afrazi, M.; Nicholas, A.; et al. Phase 1 trials of PNPLA3 siRNA in I148M homozygous patients with MAFLD. N. Engl. J. Med. 2024, 391, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Kamzolas, I.; Harder, L.M.; Oakley, F.; Trautwein, C.; Hatting, M.; Ross, T.; Bernardo, B.; Oldenburger, A.; Hjuler, S.T.; et al. An unbiased ranking of murine dietary models based on their proximity to human metabolic dysfunction-associated steatotic liver disease (MASLD). Nat. Metab. 2024, 6, 1178–1196. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Maggi, A. Sex Differences: A Resultant of an Evolutionary Pressure? Cell Metab. 2017, 25, 499–505. [Google Scholar] [CrossRef]
- Sprankle, K.W.; Knappenberger, M.A.; Locke, E.J.; Thompson, J.H.; Vinovrski, M.F.; Knapsack, K.; Kolwicz, S.C., Jr. Sex- and Age-Specific Differences in Mice Fed a Ketogenic Diet. Nutrients 2024, 16, 2731. [Google Scholar] [CrossRef]
- De Souza, G.O.; Wasinski, F.; Donato, J., Jr. Characterization of the metabolic differences between male and female C57BL/6 mice. Life Sci. 2022, 301, 20636. [Google Scholar] [CrossRef] [PubMed]
- Maric, I.; Krieger, J.P.; van der Velden, P.; Börchers, S.; Asker, M.; Vujicic, M.; Wernstedt Asterholm, I.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 828522. [Google Scholar] [CrossRef]
- Oraha, J.; Enriquez, R.F.; Herzog, H.; Lee, N.J. Sex-specific changes in metabolism during the transition from chow to high-fat diet feeding are abolished in response to dieting in C57BL/6J mice. Int. J. Obes. 2022, 46, 1749–1758. [Google Scholar] [CrossRef]
- Guerra-Cantera, S.; Frago, L.M.; Collado-Perez, R.; Canelles, S.; Ros, P.; Freire-Regatillo, A.; Jiménez-Hernaiz, M.; Barrios, V.; Argente, J.; Chowen, J.A. Sex differences in metabolic recuperation after weight loss in high fat diet-induces obese mice. Front. Endocrinol. 2021, 12, 796661. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.J.; Robinson, L.S.; Salinero, A.E.; Abi-Ghanem, C.; Mansour, F.M.; Kelly, R.D.; Tyagi, A.; Brawley, R.R.; Ogg, J.D.; Zuloaga, K.L. High-fat diet exacerbates cognitive decline in mouse model of Alzheimer’s disease and mixed dementia in a sex-dependent manner. J. Neuroinflamm. 2022, 19, 110. [Google Scholar] [CrossRef]
- Braga-Tibaes, J.R.; Azarcoya-Barrera, J.; Wollin, B.; Veida-Silva, H.; Makarowski, A.; Vine, D.; Tsai, S.; Jacobs, R.; Richard, C. Sex differences distinctly impact high-fat diet-induced dysfunction in Wistar rats. J. Nutr. 2022, 152, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Salinero, A.E.; Anderson, B.M.; Zuloaga, K.L. Sex differences in the metabolic effects of diet-induced obesity in mice. Int. J. Obes. 2018, 42, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef]
- Arnetz, L.; Ekberg, N.R.; Alvarsson, M. Sex differences in type 2 diabetes: Focus on disease course and outcomes. Diabetes Metab. Syndr. Obes. 2014, 7, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Vazquez, J.T.; Boulger, E.; Liu, H.; Xue, P.; Hussain, M.A.; Wolfe, A. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci Rep. 2017, 7, 1661. [Google Scholar] [CrossRef] [PubMed]
- Van Sinderen, M.; Steinberg, G.; Jorgensen, S.B.; Honeyman, J.; Chow, J.D.Y.; Simpson, E.R.; Jones, M.E.E.; Boon, W.C. Sexual dimorphism in the glucose homeostasis phenotype of the Aromatase Knockout (ArKO) mice. J. Steroid Biochem. Mol. Biol. 2017, 170, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Bai, B.; Gao, Q.; Li, S.; Zhao, Y. 17β-estradiol regulates glucose metabolism and insulin secretion in rat islet β cells through GPER and Akt/mTOR/GLUT2 pathway. Front. Endocrinol. 2019, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Kempegowda, P.; Arlt, W.; O’Reilly, M.W. Mechanisms in endocrinology: The sexually dimorphic role of androgens in human metabolic disease. Eur. J. Endocrinol. 2017, 177, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Allard, C.; Morford, J.J.; Xu, W.; Liu, S.; Molinas, A.J.; Butcher, S.M.; Fine, N.H.F.; Blandino-Rosano, M.; Sure, V.N.; et al. Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 2018, 3, e98607. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Xu, W.; Jacobson, D.A.; Wicksteed, B.; Allard, C.; Zhang, G.; De Gendt, K.; Kim, S.H.; Wu, H.; Zhang, H.; et al. Extranuclear actions of the androgen receptor enhance glucose-stimulated secretion in male. Cell Metab. 2016, 23, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2020, 46, 101102. [Google Scholar] [CrossRef]
- Sachinidis, A.; Nikolic, D.; Stoian, A.P.; Papanas, N.; Tarar, O.; Rizvi, A.A.; Rizzo, M. Cardiovascular outcomes trials with incretin-based medications: A critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism 2020, 111, 154343. [Google Scholar] [CrossRef]
- Kaiafa, G.; Veneti, S.; Polychronopoulos, G.; Pilalas, D.; Daios, S.; Kanellos, I.; Didangelos, T.; Pagoni, S.; Savopoulos, C. Is HbA1c an ideal biomarker of well-controlled diabetes? Postgrad. Med. J. 2021, 97, 380–383. [Google Scholar] [CrossRef]
- Pencek, R.; Blickensderfer, A.; Li, Y.; Brunell, S.C.; Anderson, P.W. Exenatide twice daily: Analysis of effectiveness and safety data stratified by age, sex, race, duration of diabetes, and body mass index. Postgrad. Med. 2012, 124, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef]
- Shah, M.; Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 2014, 15, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, C. The effects of the new therapeutic treatments for diabetes mellitus on the male reproductive Axis. Front. Endocrinol. 2022, 13, 821113. [Google Scholar] [CrossRef]
- Overgaard, R.V.; Petri, K.C.C.; Jacobsen, L.V.; Jensen, C.B. Liraglutide 3.0 mg for weight management: A population pharmacokinetic analysis. Clin. Pharmacokinet. 2016, 55, 1413–1422. [Google Scholar] [CrossRef]
- Martin, A.; Lang, S.; Goeser, T.; Demir, M.; Steffen, H.; Kasper, P. Management of dyslipidemia in patients with non-alcoholic fatty liver disease. Curr. Atheroscler. Rep. 2022, 24, 533–546. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Palmisano, B.; Zhu, L.; Eckel, R.; Stafford, J. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E.; Luceri, C.; Mannucci, E.; Rotella, C.M.; Raimondi, L. Gender-related drug effect on several markers of oxidation stress in diabetes patients with and without complications. Eur. J. Pharmacol. 2015, 766, 86–90. [Google Scholar] [CrossRef]
- Coban, N.; Onat, A.; Guclu-Geyik, F.; Can, G.; Erginel-Unaltuna, N. Sex- and obesity-specific association of aromatase (CYP19A1) gene variant with apolipoprotein B and hypertension. Arch. Med. Res. 2015, 46, 564–571. [Google Scholar] [CrossRef]
- Karjalainen, A.; Heikkinen, J.; Savolainen, M.J.; Backstrom, A.C.; Kesaniemi, Y.A. Mechanisms regulating LDL metabolism in subjects on peroral and transdermal estrogen replacement therapy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1101–1106. [Google Scholar] [CrossRef]
- Zhang, Y.; Klein, K.; Sugathan, A.; Nassery, N.; Dombkowski, A.; Zanger, U.M.; Waxman, D.J. Transcriptional profiling of human liver identifies sex-biased genes associated with polygenic dyslipidemia and coronary artery disease. PLoS ONE 2011, 6, e23506. [Google Scholar] [CrossRef]
- Athyros, V.G.; Tziomalos, K.; Gossios, T.D. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet 2010, 376, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Romiti, G.F.; Campolongo, G.; Ruscio, E.; Sciomer, S.; Gianfrilli, D.; Raparelli, V. Gender related differences in treatment and response to statins in primary and secondary cardiovascular prevention: The never-ending debate. Pharmacol. Res. 2017, 117, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Fei, S.F.; Tong, D.B.; Xue, C.; Li, J.J. Sex difference in circulating PCSK9 and its clinical implications. Front. Pharmacol. 2022, 13, 953845. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri, N.; Macchi, C.; Meroni, M.; Lanti, C.; Ricci, C.; Maggioni, M.; Fracanzani, A.L.; Badiali, S.; Fargion, S.; et al. Liver fat accumulation is associated with circulating PCSK9. Ann. Med. 2016, 48, 384–391. [Google Scholar] [CrossRef]
- Trépo, E.; Valenti, L. Update on NAFLD genetics: From new variants to the clinic. J. Hepatol. 2020, 72, 1196–1209. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.L.; et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Donati, B.; Fares, R.; Lombardi, R.; Mancina, R.M.; Romeo, S.; Valenti, L. PNPLA3I148M polymorphism and progressive liver disease. World J. Gastroenterol. 2013, 19, 6969–6978. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef]
- Li, Q.; Qu, H.Q.; Rentfro, A.R.; Grove, M.L.; Mirza, S.; Lu, Y.; Hanis, C.L.; Fallon, M.B.; Boerwinkle, E.; Fisher-Hoch, S.P.; et al. PNPLA3 polymorphisms and liver aminotransferase levels in a Mexican American population. Clin. Investig. Med. 2012, 35, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Caviglia, G.P.; Birolo, G.; Armandi, A.; Pennisi, G.; Pelusi, S.; Younes, R.; Liguori, A.; Perez-Diaz-Del-Campo, N.; Nicolosi, A.; et al. Impact of PNPLA3 rs738409 Polymorphism on the Development of Liver-Related Events in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Rosso, C.; Della Torre, S. Sex-specific effects of PNPLA3 I148M. Liver Int. 2024, in press. [CrossRef] [PubMed]
- Linden, D.; Ahnmark, A.; Pingitore, P.; Ciociola, E.; Ahlstedt, I.; Andreasson, A.C.; Sasidharan, K.; Madeyski-Bengtson, K.; Zurek, M.; Mancina, R.M.; et al. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Mol. Metab. 2019, 22, 49–61. [Google Scholar] [CrossRef] [PubMed]
- BasuRay, S.; Wang, Y.; Smagris, E.; Cohen, J.C.; Hobbs, H.H. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Banini, B.A.; Kumar, D.P.; Cazanave, S.; Seneshaw, M.; Mirshahi, F.; Santhekadur, P.K.; Wang, L.; Guan, H.P.; Oseini, A.M.; Alonso, C.; et al. Identification of a metabolic, transcriptomic, and molecular signature of patatin-like phospholipase domain containing 3- mediated acceleration of steatohepatitis. Hepatology 2021, 73, 1290–1306. [Google Scholar] [CrossRef] [PubMed]
- Arrowhead Pharmaceuticals. Available online: https://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-pharmaceuticals-gains-full-rights-nash-candidate-aro (accessed on 10 January 2025).
- Huang, Y.; He, S.; Li, J.Z.; Li, J.Z.; Seo, Y.K.; Osborne, T.F.; Cohen, J.C.; Hobbs, H.H. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl. Acad. Sci. USA 2010, 107, 7892–7897. [Google Scholar] [CrossRef]
- Fernández-Suárez, M.E.; Daimiel, L.; Villa-Turégano, G.; Pavón, M.V.; Busto, R.; Escolà-Gil, J.C.; Platt, F.M.; Lasunción, M.A.; Martínez-Botas, J.; Gómez-Coronado, D. Selective estrogen receptor modulators (SERMs) affect cholesterol homeostasis through the master regulators SREBP and LXR. Biomed. Pharmacother. 2021, 141, 111871. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Ostadreza, M.; Jamialahmadi, O.; Pelusi, S.; Rrapaj, E.; Casirati, E.; Passignani, G.; Norouziesfahani, M.; Sinopoli, E.; Baselli, G.; et al. Interaction between estrogen receptor-α and PNPLA3 I148M variant drives fatty liver disease susceptibility in women. Nat. Med. 2023, 29, 2643–2655. [Google Scholar] [CrossRef] [PubMed]
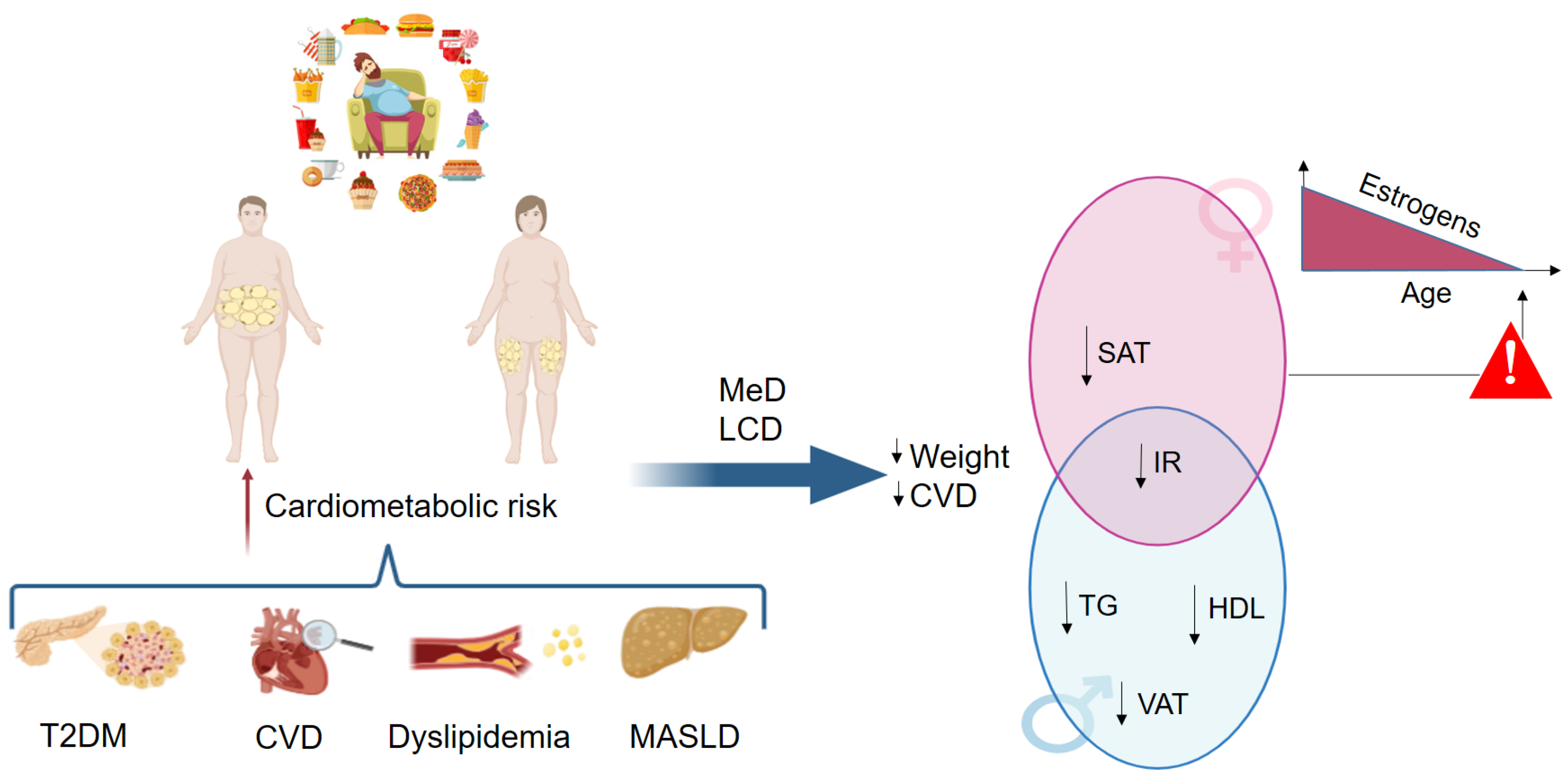


| Ref. | n, Sex: Age | Intervention and Overall Dosage | Conclusions |
|---|---|---|---|
| Nutritional intervention | |||
| Trouwborst et al. [50] | n = 782 M: 276 (42.5 ± 6.0) F: 506 (41 ± 6.3) | 6-month low-calorie diet | Women were less responsive to the diet compared with men in terms of cardiometabolic risk parameters |
| Bédard et al. [51] | n = 69 M: 37 (42.6 ± 7.3) F: 32 (41.2 ± 7.3) | 4-week MeD | Men improved cardiovascular health through the reduction in postprandial insulin concentration |
| Leblanc et al. [52] | n = 123 M: 64 (41 ± 7.9) F: 59 (41.8 ± 6.7) | 12-week MeD | Men showed a significant improvement of metabolic profile |
| Carruba et al. [53] | n = 115 Post-menopausal women | 6-month MeD | Traditional Mediterranean diet significantly reduces endogenous estrogen in healthy postmenopausal women. |
| Vitale et al. [56] | n = 156 M: 74 (55.7 ± 10.7) F: 82 (55.1 ± 10.7) | 12-week high- versus low-GI diet | Women, compared to men, showed a better metabolic improvement |
| D’Abbondanza et al. [54] | n = 70 M: 28 (20–62) F: 42 (17–67) | 25-day very low-carbohydrate ketogenic diet | Men compared to pre-menopausal women, showed a better response in terms of body weight loss and improvement in MASLD |
| Muscogiuri et al. [55] | n = 42 M: 21 (37.7 ± 10.7) F: 21 (32.7 ± 8.7) | 45-day very low-energy ketogenic diet | Men compared to women showed a better response in terms of weight loss and reduction in inflammation |
| Antihyperglycemic treatment | |||
| Gallwitz et al. [57] | n = 3375 M: 1673 (56.8 ± 9.8) F: 1702 (55.9 ± 10.1) | Dulaglutide 1.5 mg and 0.75 mg for 12 months | Significant improvement in glycaemic control irrespective of gender |
| Onishi et al. [58] | n = 855 M: 649 (56.9 ± 10.1) F: 206 (58.7 ± 11.3) | Dulaglutide 0.75 mg for 26 weeks | Body weight reduction during treatment is more pronounced in women compared to men |
| Mirabelli et al. [59] | n = 40 (57.5 ± 6.6) M: 18 (na) F: 22 (na) | Liraglutide 1.2 mg or 1.8 mg for a minimum follow-up of 5 years | Prolonging treatment exerted a lasting benefit in women |
| Buysschaert et al. [60] | n = 184 (59 ± 11) M: 110 (na) F: 74 (na) | Exenatide 5 μg or 10 μg for 9 and 12 months | Body weight reduction during treatment with exenatide is more pronounced in women compared to men |
| Quan et al. [61] | n = 105 M: 51 (48.4 ± 9.8) F: 54 (49.1 ± 10.4) | Exenatide 5 μg + metformin 0.5 g for 4 weeks Exenatide 10 μg + metformin 0.5 g for 24 weeks | Combination therapy showed better results in women compared with men |
| Lipid lowering therapy | |||
| Mombelli et al. [62] | n = 337 M: 171 (57.5 ± 9.2) F: 166 (59.4 ± 8.8) | Atorvastatin or rosuvastatin 10 mg/day Pravastatin and simvastatin 20 mg/day Fluvastatin 80 mg/day | Women compared with men showed a greater reduction in LDL-C |
| Smiderle et al. [63] | n = 495 M: 164 (59.9 ± 11.1) F: 331 (62.3 ± 10.7) | Simvastatin or atorvastatin for approximately 6 months | Adverse drug reactions were more frequent in women than in men |
| Sever et al. [64] | n = 27,564 M: 20,795 (62 ± 9) F: 6769 (64.1 ± 8.8) | Evolocumab or placebo for 2.2 years | Similar efficacy regardless of age in both men and women |
| Bittner et al. [65] | n = 18,924 M: 14,162 (mean age 57) F: 4762 (mean age 62) | Alirocumab 75 mg or placebo for a median follow-up of 2.8 years | Improvement of cardiovascular outcomes regardless of sex |
| Nucleotide-based therapy | |||
| Fabbrini et al. [66] | Study 1: n = 55 (24–65) M: 24 (na) F: 31 (na) Study 2: n = 9 (38–61) M: 8 (na) F: 1 (na) | Study 1: Placebo or JNJ-0795 10/25/75/200/400 mg Study 2: Placebo or 75 mg JNJ-0795 | A single dose of GalNac-conjugated PNPLA3 siRNA reduced liver fat content, but no sexual dimorphism has been currently described concerning treatment response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dileo, E.; Saba, F.; Parasiliti-Caprino, M.; Rosso, C.; Bugianesi, E. Impact of Sexual Dimorphism on Therapy Response in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: From Conventional and Nutritional Approaches to Emerging Therapies. Nutrients 2025, 17, 477. https://doi.org/10.3390/nu17030477
Dileo E, Saba F, Parasiliti-Caprino M, Rosso C, Bugianesi E. Impact of Sexual Dimorphism on Therapy Response in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: From Conventional and Nutritional Approaches to Emerging Therapies. Nutrients. 2025; 17(3):477. https://doi.org/10.3390/nu17030477
Chicago/Turabian StyleDileo, Eleonora, Francesca Saba, Mirko Parasiliti-Caprino, Chiara Rosso, and Elisabetta Bugianesi. 2025. "Impact of Sexual Dimorphism on Therapy Response in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: From Conventional and Nutritional Approaches to Emerging Therapies" Nutrients 17, no. 3: 477. https://doi.org/10.3390/nu17030477
APA StyleDileo, E., Saba, F., Parasiliti-Caprino, M., Rosso, C., & Bugianesi, E. (2025). Impact of Sexual Dimorphism on Therapy Response in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: From Conventional and Nutritional Approaches to Emerging Therapies. Nutrients, 17(3), 477. https://doi.org/10.3390/nu17030477








