From Evidence to Practice: A Narrative Framework for Integrating the Mediterranean Diet into Inflammatory Bowel Disease Management
Abstract
1. Introduction
2. Methods
3. The Mechanisms for Diet in Inflammatory Bowel Disease
4. The Mediterranean Diet in IBD: Transforming Clinical Biomarkers and Patient Outcomes
5. Mediterranean Diet Adherence: A Path to Better Health in IBD
| Author, Year | Population | Primary Objective | Diet Assessment Methods | MED Diet Assessment/Adherence | Results |
|---|---|---|---|---|---|
| Papada et al., 2019 [47] | Outpatient adults with endoscopically proven CD (n = 86) | Characterize the effects of MD adherence on quality of life, disease activity, and inflammatory markers | Assignment of MedDiet scores based on 24 h recall | MedDiet score evaluated by an experienced dietitian | ↑ MedDiet scores in patients with inactive CD versus patients with active CD (p = 0.005) MedDiet score was negatively correlated with Harvey–Bradshaw Index (p < 0.001) and CRP (p = 0.027) |
| Godny et al., 2020 [48] | UC patients who underwent pouch surgery (n = 153) | Assess changes in inflammation markers, and reduced risk of pouchitis development in patients with UC after pouch surgery | Assessment of MD adherence during a 6-month interval between 2015 and 2018 based on a food frequency questionnaire | MED score Adherence defined as MED score ≥ 5 | ↔ MED scores between patients with active and inactive disease (p = 0.10) Patients with <200 mcg/g fecal calprotectin had ↑ MED score versus patients with elevated fecal calprotectin (p < 0.05) ↔ pouchitis development rates in patients with high MED diet adherence versus patients with low adherence (p = 0.17) |
| Naqvi et al., 2021 [49] | Adults (n = 66) with CD and clinical remission (steroid-free, clinical remission with Harvey–Bradshaw Index < 5 for > 3 months) | Assess the relationship between diet and markers of inflammation | A 3-day weighted food/drink intake, reviewed by a dietitian | pMDS score modified to exclude red wine consumption | Increasing daily servings of leafy green vegetables were associated with FCP ≤ 100 μg/mg (p < 0.05) omega-6:omega-3 polyunsaturated fatty acid ratio of 8:1 was associated with CRP ≤ 5 mg/L |
| Fiorindi et al., 2021 [50] | Adults with IBD (n = 62 CD, n = 18 UC) | Assess level of MD adherence in IBD patients with MEDI-LITE questionnaire | MEDI-LITE questionnaire conducted via face-to-face interview | MEDI-LITE questionnaire scores > 11 deemed adherent | ↔ between CD and UC patients in the MEDI-LITE scores (p = 0.543) ↑ MEDI-LITE score in remission CD patients than active CD patients (p < 0.001) No significant differences in MEDI-LITE scores were found in remission UC patients and active UC patients with pouchitis (p = 0.218) |
| Haskey et al., 2022 [52] | Randomized controlled trial Adults (n = 28) with mild-moderate UC in remission (partial Mayo score 0–2) | Examining the proportion of participants achieving high adherence to the MD measured by the MDSSs Changes in diet quality, quality of life, nutritional diet adequacy were also measured as secondary analysis | Two intervention diets were used, the CHD (Canadian Habitual Diet) and the MD The MD group received sessions from dietitians to help adapt to the MD (based on the MD pyramid) with MD specific recipes, 4-week meal plan, food lists The CHD group followed their habitual diet | MDSSs (> 16 points) as measured after 12 weeks deemed adherent | After 12 weeks, there was a significantly higher MDSS in the MD intervention group compared to the CHD group (p = 0.010) and improved diet quality (p = 0.007) as measured by the Healthy Eating Index. No significance in changes in quality-of-life scores in both the groups |
| Celik et al., 2023 [51] | Adults diagnosed with IBD (n = 83; n = 38 UC patients; n = 45 CD patients) | Assess the effect of MD adherence on disease activity (Crohn’s disease activity index; Mayo Score for UC) and quality of life (Short Form-36) in IBD patients | Face-to-face interviews with a dietitian to provide MEDAS scores | MEDAS scores of ≤ 6, 7–9 and ≥ 9 categorized as low, acceptable and high adherence, respectively | Low MD adherence had higher Mayo Clinic scores (p = 0.018) No significant differences in Crohn’s disease activity index scores and BMI with MD adherence (p > 0.05) In UC patients, high MD adherence was associated with better scores in emotional problems (p = 0.03), mental health (p = 0.03), and overall health perception (p < 0.01) UC patients categorized as ’low adherence’ had higher UC Mayo Clinic scores (p = 0.018). In CD patients, MD adherence was not correlated with any sub-dimensions of quality of life measured by the Short Form-36 (p > 0.05). |
6. Current Gaps in Research
7. From Research to Practice: Bridging the Mediterranean Diet and IBD Care
- Use EVOO liberally in cooking (stable to 420 °F) in place of omega-6-rich vegetable oils (e.g., sunflower, corn, soybean, palm, or canola oils).
- Drizzle EVOO on salads, vegetables, grilled fish, chicken, and pasta.
- Use EVOO as a base for salad dressings instead of commercially prepared salad dressings.
- Dip crusty bread in EVOO in place of butter or margarine.
- Add citrus-infused EVOO (e.g., lemon, orange) to breakfast smoothies, oatmeal, and yogurt.
- Add herb-infused EVOO (e.g., garlic, basil, rosemary) to salads, marinades and eggs.
- Dark leafy greens can be used as salads, added to frittatas, eggs, smoothies, and soups.
- Add grated vegetables, such as carrots, zucchini, spinach, and kale to pasta sauces and soups.
- Canned tomato products are rich in lycopene (an antioxidant). A few tomato-centric recipes include shakshuka, stuffed vegetables, stews, curries, baked fish with tomatoes, and marinara sauce.
- Load up sandwiches with vegetables.
- Increase the nutritional value of smoothies by mixing in fruit and leafy greens.
- Top salads with fruit.
- Add fruit to yogurt or cereal.
- Try baked fruit topped with oatmeal, cinnamon, and maple syrup for dessert.
- Roast vegetables to increase flavor, drizzle with olive oil.
- To save preparation time, consider packaged ready-to-eat fresh fruit and vegetables. Frozen and canned fruit and vegetables are budget-friendly options.
- Choose canned vegetables packed in water and look for “no salt added” or “low sodium” options with no added sugar, preservatives. or artificial additives. Even when purchasing “no salt added” options, it is good practice to rinse them under water to remove any additives or preservatives.
- Choose canned fruits packed in water to reduce the sugar content. Whole fruits (e.g., peaches, pears, etc.,) have generally fewer additives than “cocktails”. Check for extra additives, as some products labeled “no sugar” may still contain artificial sweeteners. It is good practice to rinse them under water to remove extra sugar and preservatives.
- Breakfast is one of the easiest ways to increase fiber by consuming whole-grain toast or oatmeal.
- Swap refined grains like white bread, white rice, and pasta for whole grains like brown rice, quinoa, bulgur, barley, and farro.
- Add barley to soups to boost soluble fiber.
- Psyllium can be sprinkled on food.
- Cook, cool, reheat pasta, rice, and potatoes to increase resistant starch.
- Use whole grain flours in baking (e.g., oat flour).
- Choose nuts higher in monounsaturated fats such as almonds, cashews, macadamia, hazelnuts, pistachios, pecans, and walnuts.
- Nuts and seeds can be consumed as nut butter for easier digestion and improved tolerance. This should be favored in those with active disease, recent luminal surgery, and those with known intestinal strictures.
- Opt for raw, unsalted nuts or nut butters without added sugars, salt, or fats.
- A handful of raw nuts makes a healthy, nutrient-rich alternative to processed snacks.
- Tahini (ground sesame seeds) is versatile and can be used in sauces, dressings, or drizzled over roasted vegetables or grain bowls to enhance flavor.
- Add nuts and seeds to enhance dishes like yogurt, smoothies, oatmeal, or fruit.
- Chia seeds expand when moistened, making them ideal for creating jams and puddings.
- Soaking most nuts can improve their digestibility, reduce phytic acid, and enhance nutrient absorption. Soak most nuts for 4 to 12 h, or overnight, to improve their digestibility. Softer nuts, such as cashews, require a shorter soaking time, while harder nuts, like almonds, may benefit from a longer soaking period for optimal results.
- To cook dried beans, use a 1:4 ratio of beans to water. Soak beans overnight to reduce lectins, which can interfere with nutrient absorption and cause discomfort. Discard the soaking water, rinse the beans, and cook in fresh water. Boil for 10–30 min at high heat to deactivate most lectins. Avoid slow cooking or eating raw beans, as they may not reduce lectins effectively.
- Look for canned beans labeled low-sodium or with no salt. Rinse before serving or cooking to remove sodium that is added during processing. Rinsing canned beans can help make them more digestible.
- Lentils may be easier to digest than other starchier legumes like black beans or chickpeas, so start with lentils if other legumes cause too much digestive distress,
- Add legumes to the diet gradually—start with 2 to 4 tablespoons of beans or lentils at a time, then increase intake as the body adjusts.
- Legumes lend themselves to soups, tacos, burritos, and chili, though you can also eat them independently.
- Toss them on top of salads, purée them into a bean dip, or use them as a meat substitute in burgers, stews, and soups.
- Beans can be roasted and used as snacks and salad toppers.
- Replace heavy cream and processed cheese, instead, choose fermented cheeses like feta, Brie, cotija, Swiss, halloumi, ricotta, Manchego, and Parmesan.
- Include fermented dairy (e.g., plain Greek yogurt, kefir) and limit flavored yogurts that tend to be higher in sugar, add flavorings (lemon, maple syrup, berry purees) to sweeten if needed.
- Yogurt, kefir, and aged hard cheeses are lower-lactose options.
- Trial lactose-free options, smaller portions spread throughout the day, if tolerance is an issue.
- Choose white meats (poultry without skin) instead of red meats, pork, or processed meats, sausages, cold meat, or paté.
- Enjoy omega-3-rich fish such as tuna, sardines, and salmon, either fresh or canned.
- Consume red meats (lamb, mutton, beef, pork, veal, goat, horse) less frequently. Opt for lean cuts and prepare in stews, stir-fries, or soups.
- Limit intake of smoked, salted, and processed meats.
- Eggs can be enjoyed daily and are often a well tolerated protein source.
- Aim for moderate portions of 4 ounces per meal.
- Reduce the consumption of packaged, processed foods, and commercial sauces containing maltodextrin, carrageenan, carboxymethylcellulose, polysorbate-80, titanium dioxide, sulfites, and xanthan gum.
- Limit sugary beverages and artificial sweeteners (e.g., aspartame, sucralose, saccharin). Replace soda and juices with water.
- Coffee, tea, and herbal infusions (rich in flavonoids) are allowed, but they should be consumed preferably without any sweetener.
- Avoid high-fat and sugar pastries, industrial bakery products (e.g., cakes, donuts, or cookies), and industrial desserts (e.g., puddings, custard). Save cakes and sweets for special occasions
- Use herbs, spices, garlic, and onions to increase food palatability and reduce the use of salt in cooking.
- Limit to low-risk alcohol consumption. Patients with IBD often report worse gastrointestinal symptoms following alcohol consumption. In the available literature, alcohol use in patients with IBD trends toward harmful effects; however, more research is needed [83].
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benchimol, E.; Bernstein, C.N.; Bitton, A.; Murthy, S.K.; Nguyen, G.C.; Lee, K.; Cooke-Lauder, J.; Siddiq, S.; Windsor, J.W.; Carroll, M.W.; et al. The Impact of Inflammatory Bowel Disease in Canada 2018: A Scientific Report from the Canadian Gastro-Intestinal Epidemiology Consortium to Crohn’s and Colitis Canada. J. Can. Assoc. Gastroenterol. 2019, 2, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.M. Combination therapies: The next major frontier in IBD management. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 761. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; George, J.; Boland, B.S.; Casteele, N.; Sandborn, W.J. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with Inflammatory bowel diseases: A systematic review and meta-analysis. J. Crohns Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, R.; Ben-Horin, S.; Zhuang, X.; Tian, Z.; Li, X.; Ma, R.; Mao, R.; Qiu, Y.; Chen, M. Systematic review with meta-analysis: Environmental and dietary differences of inflammatory bowel disease in Eastern and Western populations. Aliment. Pharmacol. Ther. 2022, 55, 266–276. [Google Scholar] [CrossRef]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in western diet versus mediterranean diet at the gut microbiota-immune system interplay. implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Turpin, W.; Dong, M.; Sasson, G.; Garay, J.A.R.; Espin-Garcia, O.; Lee, S.-H.; Neustaeter, A.; Smith, M.I.; Leibovitzh, H.; Guttman, D.S.; et al. Mediterranean-Like Dietary Pattern Associations With Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; A Dieleman, L.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern improves intestinal inflammation concomitant with reshaping of the bacteriome in ulcerative colitis: A randomized controlled trial. J. Crohns Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults With Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef] [PubMed]
- Khavandegar, A.; Heidarzadeh, A.; Angoorani, P.; Hasani-Ranjbar, S.; Ejtahed, H.-S.; Larijani, B.; Qorbani, M. Adherence to the Mediterranean diet can beneficially affect the gut microbiota composition: A systematic review. BMC Med. Genom. 2024, 17, 91. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Godoy-Brewer, G.; Parian, A.M.; Noorian, S.; Krishna, M.; Shah, N.D.; White, J.; Mullin, G.E. Dietary Interventions for the Treatment of Inflammatory Bowel Diseases: An Updated Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2508–2525. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Suskind, D.L. Current Nutritional Therapies in Inflammatory Bowel Disease: Improving Clinical Remission Rates and Sustainability of Long-Term Dietary Therapies. Nutrients 2023, 15, 668. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, gut microbiome and epigenetics: Emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients 2017, 9, 962. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; E Matarese, L.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; E Mullin, G. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Gordon, M.; Mutlu, E.A.; De Silva, P.S.; Lewis, J.D. Diet Therapy for Inflammatory Bowel Diseases: A Call to the Dining Table. Inflamm. Bowel Dis. 2020, 26, 510–514. [Google Scholar] [CrossRef]
- Aldars-García, L.; Chaparro, M.; Gisbert, J.P. Systematic review: The gut microbiome and its potential clinical application in inflammatory bowel disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Linares, R.; Francés, R.; Gutiérrez, A.; Juanola, O. Bacterial Translocation as Inflammatory Driver in Crohn’s Disease. Front. Cell Dev. Biol. 2021, 9, 703310. [Google Scholar] [CrossRef] [PubMed]
- Ogulur, I.; Yazici, D.; Pat, Y.; Bingöl, E.N.; Babayev, H.; Ardicli, S.; Heider, A.; Rückert, B.; Sampath, V.; Dhir, R.; et al. Mechanisms of gut epithelial barrier impairment caused by food emulsifiers polysorbate 20 and polysorbate 80. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 2441–2455. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The effect of dietary interventions on chronic inflammatory diseases in relation to the microbiome: A systematic review. Nutrients 2021, 13, 3208. [Google Scholar] [CrossRef]
- Khademi, Z.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Dietary Intake of Total Carbohydrates, Sugar and Sugar-Sweetened Beverages, and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2021, 8, 707795. [Google Scholar] [CrossRef]
- Khorshidi, M.; Djafarian, K.; Aghayei, E.; Shab-Bidar, S. A posteriori dietary patterns and risk of inflammatory bowel disease: A meta-analysis of observational studies. Int. J. Vitam. Nutr. Res. 2020, 90, 376–384. [Google Scholar] [CrossRef]
- Peters, V.; Bolte, L.; Schuttert, E.; Andreu-Sánchez, S.; Dijkstra, G.; Weersma, R.; Campmans-Kuijpers, M. Western and Carnivorous Dietary Patterns are Associated with Greater Likelihood of IBD Development in a Large Prospective Population-based Cohort. J. Crohns Colitis 2022, 16, 931–939. [Google Scholar] [CrossRef]
- Milajerdi, A.; Ebrahimi-Daryani, N.; Dieleman, L.A.; Larijani, B.; Esmaillzadeh, A. Association of Dietary Fiber, Fruit, and Vegetable Consumption with Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 735–743. [Google Scholar] [CrossRef]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: Association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2020, 59, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Becherucci, G.; Godny, L.; Mentella, M.C.; Petito, V.; Scaldaferri, F. The Key Nutrients in the Mediterranean Diet and Their Effects in Inflammatory Bowel Disease: A Narrative Review. Nutrients 2024, 16, 4201. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Julliard, W.A.; Myo, Y.P.A.; Perelas, A.; Jackson, P.D.; Thatcher, T.H.; Sime, P.J. Specialized pro-resolving mediators as modulators of immune responses. Semin. Immunol. 2022, 59, 101605. [Google Scholar] [CrossRef]
- Nieva, C.; Pryor, J.; Williams, G.M.; Hoedt, E.C.; Burns, G.L.; Eslick, G.D.; Talley, N.J.; Duncanson, K.; Keely, S. The Impact of Dietary Interventions on the Microbiota in Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2024, 18, 920–942. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Erol Doğan, Ö.; Karaca Çelik, K.E.; Baş, M.; Alan, E.H.; Çağın, Y.F. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients 2024, 16, 1504. [Google Scholar] [CrossRef]
- Strauss, J.C.; Haskey, N.; Ramay, H.R.; Ghosh, T.S.; Taylor, L.M.; Yousuf, M.; Ohland, C.; McCoy, K.D.; Ingram, R.J.M.; Ghosh, S.; et al. Weighted Gene Co-Expression Network Analysis Identifies a Functional Guild and Metabolite Cluster Mediating the Relationship between Mucosal Inflammation and Adherence to the Mediterranean Diet in Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 7323. [Google Scholar] [CrossRef]
- Zhang, Z.; Taylor, L.; Shommu, N.; Ghosh, S.; Reimer, R.; Panaccione, R.; Kaur, S.; Hyun, J.E.; Cai, C.; Deehan, E.C.; et al. A diversified dietary pattern is associated with a balanced gut microbial composition of faecalibacterium and escherichia/Shigella in patients with crohn’s disease in remission. J. Crohns Colitis 2020, 14, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Haigh, L.; Bremner, S.; Houghton, D.; Henderson, E.; Avery, L.; Hardy, T.; Hallsworth, K.; McPherson, S.; Anstee, Q.M. Barriers and Facilitators to Mediterranean Diet Adoption by Patients With Nonalcoholic Fatty Liver Disease in Northern Europe. Clin. Gastroenterol. Hepatol. 2019, 17, 1364–1371.e3. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.E.; McEvoy, C.T.; Prior, L.; Lawton, J.; Patterson, C.C.; Kee, F.; Cupples, M.; Young, I.S.; Appleton, K.; McKinley, M.C.; et al. Barriers to adopting a Mediterranean diet in Northern European adults at high risk of developing cardiovascular disease. J. Hum. Nutr. Diet. 2018, 31, 451–462. [Google Scholar] [CrossRef]
- Damas, O.M.; Maldonado-Contreras, A. Breaking Barriers in Dietary Research: Strategies to Diversify Recruitment in Clinical Studies and Develop Culturally Tailored Diets for Hispanic Communities Living With Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Rojas-Rueda, D.; Basora, J.; Ros, E.; Salas-Salvadó, J. Nut intake and adiposity: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2013, 97, 1346–1355. [Google Scholar] [CrossRef]
- Santos, A.C.N.A.; Duarte de Souza, M.L.; Machado, A.M.; Kümmel Duarte, C. Olive oil and body fat: A systematic review with meta-analysis. Food Funct. 2023, 14, 5516–5536. [Google Scholar] [CrossRef]
- Papada, E.; Amerikanou, C.; Forbes, A.; Kaliora, A.C. Adherence to Mediterranean diet in Crohn’s disease. Eur. J. Nutr. 2020, 59, 1115–1121. [Google Scholar] [CrossRef]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef]
- Naqvi, S.A.; Taylor, L.M.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; Hotte, N.; Shommu, N.; Kaur, S.; Reimer, R.A.; Madsen, K.L.; et al. Dietary patterns, food groups and nutrients in Crohn’s disease: Associations with gut and systemic inflammation. Sci. Rep. 2021, 11, 1674. [Google Scholar] [CrossRef]
- Fiorindi, C.; Dinu, M.; Gavazzi, E.; Scaringi, S.; Ficari, F.; Nannoni, A.; Sofi, F.; Giudici, F. Adherence to mediterranean diet in patients with inflammatory bowel disease. Clin. Nutr. ESPEN 2021, 46, 416–423. [Google Scholar] [CrossRef]
- Çelik, K.; Güveli, H.; Erzin, Y.Z.; Kenger, E.B.; Özlü, T. The Effect of Adherence to Mediterranean Diet on Disease Activity in Patients with Inflammatory Bowel Disease. Turk. J. Gastroenterol. 2023, 34, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Shim, R.C.K.; Davidson-Hunt, A.; Ye, J.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Ghosh, S.; Gibson, D.L. Dietary adherence to the Mediterranean diet pattern in a randomized clinical trial of patients with quiescent ulcerative colitis. Front. Nutr. 2022, 9, 1080156. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.N.; Ingram, R.J.M.; Zhang, Z.; Taylor, L.M.; Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C.; Ghosh, S.; Raman, M. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2021, 6, 754–769. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; Elashry, H.; Salamah, A.; Maher, S.; Abd-Elsalam, S.M.; Hasan, S. Adherence to the Mediterranean Diet Improved Clinical Scores and Inflammatory Markers in Children with Active Inflammatory Bowel Disease: A Randomized Trial. J. Inflamm. Res. 2022, 15, 2075–2086. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Flynn, M.M.; Tierney, A.; Itsiopoulos, C. Is Extra Virgin Olive Oil the Critical Ingredient Driving the Health Benefits of a Mediterranean Diet? A Narrative Review. Nutrients 2023, 15, 2916. [Google Scholar] [CrossRef]
- Alves, E.; Domingues M do, R.; Domingues, P. Chapter 4—Olive oil. In Functional Foods and Their Implications for Health Promotion; Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 97–129. [Google Scholar] [CrossRef]
- Mayr, L.; Grabherr, F.; Schwärzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.-W.; Ruder, B.; Kunz, K.T.R.; et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat. Commun. 2020, 11, 1775. [Google Scholar] [CrossRef]
- Haskey, N.; Ye, J.; Estaki, M.; Meza, A.A.V.; Barnett, J.A.; Yousefi, M.; Birnie, B.W.; Gruenheid, S.; Ghosh, S.; Gibson, D.L. A Mediterranean-like fat blend protects against the development of severe colitis in the mucin-2 deficient murine model. Gut Microbes 2022, 14, 2055441. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative Fingerprint Changes of Toxic Volatiles in Low PUFA Vegetable Oils Under Deep-Frying. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 271–284. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Health Benefits of Dietary Whole Grains: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Haskey, N.; Gold, S.L.; Faith, J.J.; Raman, M. To Fiber or Not to Fiber: The Swinging Pendulum of Fiber Supplementation in Patients with Inflammatory Bowel Disease. Nutrients 2023, 15, 1080. [Google Scholar] [CrossRef]
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of Nuts and Seeds and Health Outcomes Including Cardiovascular Disease, Diabetes and Metabolic Disease, Cancer, and Mortality: An Umbrella Review. Adv. Nutr. 2022, 13, 2136–2148. [Google Scholar] [CrossRef]
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and their effect on gut microbiota, gut function and symptoms in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2020, 12, 2347. [Google Scholar] [CrossRef]
- Preda, C.M.; Istratescu, D.; Nitescu, M.; Manuc, T.; Manuc, M.; Stroie, T.; Catrinoiu, M.; Tieranu, C.; Meianu, C.G.; Tugui, L.; et al. Impact of Dietary Patterns in Inflammatory Bowel Disease Subtypes. Maedica 2023, 18, 174. [Google Scholar] [CrossRef]
- Tor-Roca, A.; Garcia-Aloy, M.; Mattivi, F.; Llorach, R.; Andres-Lacueva, C.; Urpi-Sarda, M. Phytochemicals in Legumes: A Qualitative Reviewed Analysis. J. Agric. Food Chem. 2020, 68, 13486–13496. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Sievenpiper, J.L. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann. N. Y. Acad. Sci. 2017, 1392, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Anderson, B.D.; Cifelli, C.J. The Effects of Dairy Product and Dairy Protein Intake on Inflammation: A Systematic Review of the Literature. J. Am. Coll. Nutr. 2021, 40, 571–582. [Google Scholar] [CrossRef]
- Kempinski, R.; Arabasz, D.; Neubauer, K. Effects of Milk and Dairy on the Risk and Course of Inflammatory Bowel Disease versus Patients’ Dietary Beliefs and Practices: A Systematic Review. Nutrients 2024, 16, 2555. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef]
- Jia, X.; Hu, C.; Wu, X.; Qi, H.; Lin, L.; Xu, M.; Xu, Y.; Wang, T.; Zhao, Z.; Chen, Y.; et al. Evaluating the Effects of Omega-3 Polyunsaturated Fatty Acids on Inflammatory Bowel Disease via Circulating Metabolites: A Mediation Mendelian Randomization Study. Metabolites 2023, 13, 1041. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The un Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Babaei, A.; Pourmotabbed, A.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghoreishy, S.M.; Amirian, P.; Zarpoosh, M.; Mohammadi, H.; Kermani, M.A.H.; et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: A systematic review and dose-response meta-analysis of 4035694 participants. Nutr. Rev. 2024, 82, 861–871. [Google Scholar] [CrossRef]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients with Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Kane, S. Alcohol use in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2021, 17, 211. [Google Scholar]
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392. [Google Scholar] [CrossRef]
| Author, Year | Study Design | Population | Diet Intervention | Outcomes | Results | Limitations |
|---|---|---|---|---|---|---|
| Chicco et al., 2020 [38] | Single arm intervention study | n = 142 adults with active IBD (n = 84 UC; n = 58 CD) Disease activity assessed through the Crohn’s disease activity index for CD and partial Mayo score for UC | Six months of MD adherence Nutritional counseling provided by a nutritionist: ≥2 vegetable servings per meal, 1–2 fruit servings per meal, and 1–2 bread/cereals servings per meal, and olive oil at every meal alongside ≥2 legume servings weekly, ≥2 fish/seafood servings weekly, 2–4 egg servings weekly, and 2 poultry servings weekly and 2 dairy foods servings daily while limiting red meat and sweets to <2 servings per week. Patient adherence was assessed using 24 h recall during nutritional interviews after 6 months | Nutritional status, presence/severity of liver steatosis, therapy response Anthropometric measures: weight, BMI, visceral fat, lean body mass, fat body mass, waist circumference (measured with bioelectrical impedance analysis) Serum lipid profile and lipid function (chemical analysis) Hepatic steatosis (abdominal ultrasound exam) Quality of life (Inflammatory Bowel Disease Questionnaire) | In UC patients: ↓ BMI (p = 0.002) ↓ Waist circumference (p = 0.037) ↓ N of patients with elevated CRP (p = 0.013) ↓ N of patients with FCP > 250 mg/kg (p = 0.049) ↑ Quality of life (p < 0.001) In CD patients: ↓ BMI (p = 0.023) ↓ Waist circumference (p = 0.040) ↓ N of patients with elevated CRP (p = 0.035) ↓ N of patients with FCP > 250 mg/kg (p = 0.035) ↑ Quality of life (p < 0.001) | The lack of a control group, therefore improvements may have occurred independent of the diet. Participants were in clinical remission or affected by mild disease which might lead to overestimated effect of the dietary intervention on disease activity and QoL. Researchers did not use any specific score to quantify adherence to diet, and this was mainly based on patients’ dietary recall. |
| Zhang et al., 2020 [41] | Randomized controlled trial | Adults (n = 40) with luminal CD in remission (Harvey Bradshaw Index < 5) | Two diet groups were studied: Patients that habitually consume a diversified diet pattern [DD] [higher plant-based and lower red and processed meat-based diet] compared to patients following a non-diversified diet pattern [NDD] + MD intervention for 12 weeks Adherence was assessed by 3-day weighted food records | To compare microbiota composition and function between patients in the DD group with patients in the NDD group following a 12-week structured dietary intervention based on principles from the MD | No difference in microbial beta-diversity between the two groups was observed (p = 0.43) The NDD + MD group demonstrated an increase in Faecalibacterium. No association of diet with fecal SCFAs or FCP. | No significant changes in FCP levels observed at week 12, likely due to the clinically and biochemically quiescent baseline disease state. Researchers did not use any specific score to quantify adherence to diet, and this was mainly based on patients’ dietary recall. |
| Lewis et al., 2021 [13] | Randomized control trial | Adults (n = 93, 63% women) with active CD with mild to moderate CD symptoms (short CD Activity Index Score > 175 and < 400) | Participants randomly received either the SCD or the MD for the first 6 weeks (prepared meals consisting of breakfast, lunch, dinner, and 2 snacks) After the first 6 weeks, participants were instructed on food purchase and preparation that aligned with MD Participants completed a 24 h recall at baseline, weeks 6 and 12. These data were used to assign an alternate MD score | Primary outcome: Symptomatic remission at week 6 without increasing CD medication Secondary outcome: changes in FCP and CRP | Symptomatic remission in participants at week 6 (p = 0.77) and week 12 (p = 0.87) was not superior in SCD as compared to MD Among those with an elevated FCP at screening, FCP response was achieved in 8/23 participants (34.8%) with SCD and 4/13 participants (30.8%) with MD (p = 0.83) Among those with elevated CRP at screening, CRP response was achieved in only 2/37 participants (5.4%) with SCD and 1/28 participant (3.6%) with MD (p = 0.68) from screening to week 6 | The study was not designed to assess endoscopic healing. Symptomatic remission was common, few patients achieved combined symptomatic remission and resolution of inflammation. This study included patients with longstanding disease, many of whom had been treated with biologics, which limits generalizability. |
| Haskey et al., 2023 [12] | Randomized controlled trial | Adults (n = 28) with mild-moderate UC in remission (partial Mayo score 0–2) | Two intervention diets were used, the Canadian Habitual Diet and the MD, for 12 weeks The MD group received sessions from dietitians to help adapt to the MD (based on the MD pyramid) The Canadian Habitual Diet group followed their habitual diet MD adherence was assessed using the MD serving score (MDSS) | Assessing whether MD intervention could reduce SCCAI, FCP levels, and microbiome changes | 40% of MD intervention group reported minor improvements in the SCCAI scores, 27% achieved clinical response, whereas 1% reported a decrease in 1-point SCCAI score At week 12, 75% [9/12] of participants in the CHD had an FCP > 100 μg/g vs. 20% [3/15] of participants in the MD group The MD induced alterations in microbial species known to be protective in UC (Alistipes finegoldii and Flavonifractor plautii), as well as the production of short-chain fatty acids (Ruminococcus bromii) | A 12-week follow-up may limit insights into long-term MD effects on disease activity. Results are not generalizable to active IBD patients, as only adults in clinical remission were studied. The small sample size may reduce the study’s statistical power. |
| Strauss and Haskey et al., 2023 [40] | Randomized open-label trial | Adults (n = 40) with active UC (partial Mayo score > 2) | Participants were assigned to MD + low sulfur diet or habitual diet Adherence assessed by the MDS | Improvement in total Mayo score and partial Mayo score | No changes in MDS or FCP were observed within or between groups Marginal improvements in partial Mayo score (median 2.0) were observed from baseline and week 8 in participants following the intervention diet (p = 0.003); however, this also occurred in the habitual diet (p = 0.007) Valerate (SCFA) and glycochenodeoxycholic acid (bile acid) were significantly different between groups at baseline and week 8 (p = 0.05 and p = 0.02, respectively) | Despite a significant decrease in sulfur intake in the MD intervention from baseline to week 8, this did not translate into a reduced FCP. The study sample was heterogeneous in disease activity, reflected by the wide range of partial Mayo scores and total Mayo scores at baseline. Baseline MDS did not differ between intervention groups, nor did it change over time within the intervention group, underscoring the need to assign participants to dietary interventions distinct from their baseline diet. |
| Dogan et al., 2024 [39] | Three-arm intervention study | Adults (n = 46) with mild-to-moderate UC determined by a gastroenterologist | Participants were randomly assigned into three groups: MD, MD + resveratrol (1600 mg/day), MD + curcumin (500 mg/day) for 8 weeks Bi-weekly MD education with dietitian Patient adherence was assessed using the MD adherence scale (MEDAS) with 14 items scored as either 0 or 1 | Truelove–Witts Index of disease activity, serum inflammatory markers, and quality of life (measured by Short Form-36) | Significant improvement post intervention was observed within groups for waist and hip circumference, bowel movements, CRP, erythrocyte sedimentation rate and an increase in quality-of-life scores (p < 0.05) | Absence of clinical biomarkers (e.g., fecal calprotectin, cytokine data, and endoscopic imaging). The study was limited to individuals with mild-to-moderate active disease, which restricts the generalizability of the findings to individuals in remission or with severe active disease. |
| Guiding Principles | |||
|---|---|---|---|
| |||
| Every Meal | |||
| Frequency | Serving Size | Included Foods * | |
| Extra Virgin Olive Oil | 1 serving/main meal | 1 tablespoon | High quality oil (see commentary about choosing quality) |
| Fruit | 1–2 servings/main meal | ½ cup or 1 medium sized piece | A variety of colors in both vegetables and fruits is strongly recommended to ensure intake of a broad range of micronutrients and phytochemicals |
| Vegetables | 2 servings/day plus 1–2 servings/day of leafy greens | ½ cup or 1 medium sized piece plus 1 cup raw | |
| Cereals | 1–2 servings/main meal | 1 cup cooked or 1 slice of bread | Includes bread, pasta, rice, oats Preferably whole grains as tolerated |
| Daily | |||
| Starchy Foods (Resistant Starch) | 1–2 servings/day | 1 cup per day | Includes cooked, cooled reheated rice, pasta, potatoes, winter squash, yams, cassava, and taro |
| Dairy | 2 servings/day | ¾ cup yogurt or 1.5 ounces of hard cheese (cheddar) or 1 cup of milk | Yogurt (Greek yogurt, low sugar), kefir or hard cheese may be better tolerated due to lower lactose content |
| Nuts/Seeds | 1–2 servings/day | 1 ounce or 1/4 of a cup | Without sugar, fat or salt, nut/seed butters may be better tolerated |
| Weekly | |||
| Legumes | 3 servings/week | ¾ cup (150 g) cooked | Includes beans, peas, lentils, edamame, and soy |
| Fatty Fish and Seafood | 2 servings/week | 6 ounces twice per week | Includes salmon, mackerel, tuna, trout, herring, and sardines |
| Eggs | 1 egg/daily | 1 large egg (with yolk and white) | Whole eggs, including those used for cooking and baking |
| White Meat | 2 servings/week | 4 ounces | Includes skinless chicken and turkey Choose lean poultry (e.g., breast, wing, or back portions) |
| Red Meat | 1 serving/week | < 8 ounces per week | Includes pork, beef, and lamb |
| Limit | |||
| Sweets | < 2 servings per week | Includes sugar, candies, pastries, sweetened fruit juice, and soft drinks Fruit should be eaten in place of sweets | |
| Processed Meat | < 1 ounce (30 g) per week | Includes deli meats, ham, sausages, bacon, jerky, and hot dogs | |
| Ultra-Processed Foods | Avoid as much as possible | Includes ice cream, chips/crisps, mass-produced bread and bread products, crackers, biscuits, cookies, instant soups | |
| Additives | Limit | Includes maltodextrin, carrageenan, carboxymethylcellulose, polysorbate-80, titanium dioxide and sulfites, xanthan gum, aspartame, sucralose, saccharin | |
| Alcohol (includes spirits, beer and wine) | Limit | Replace with water or herbal infusions | |
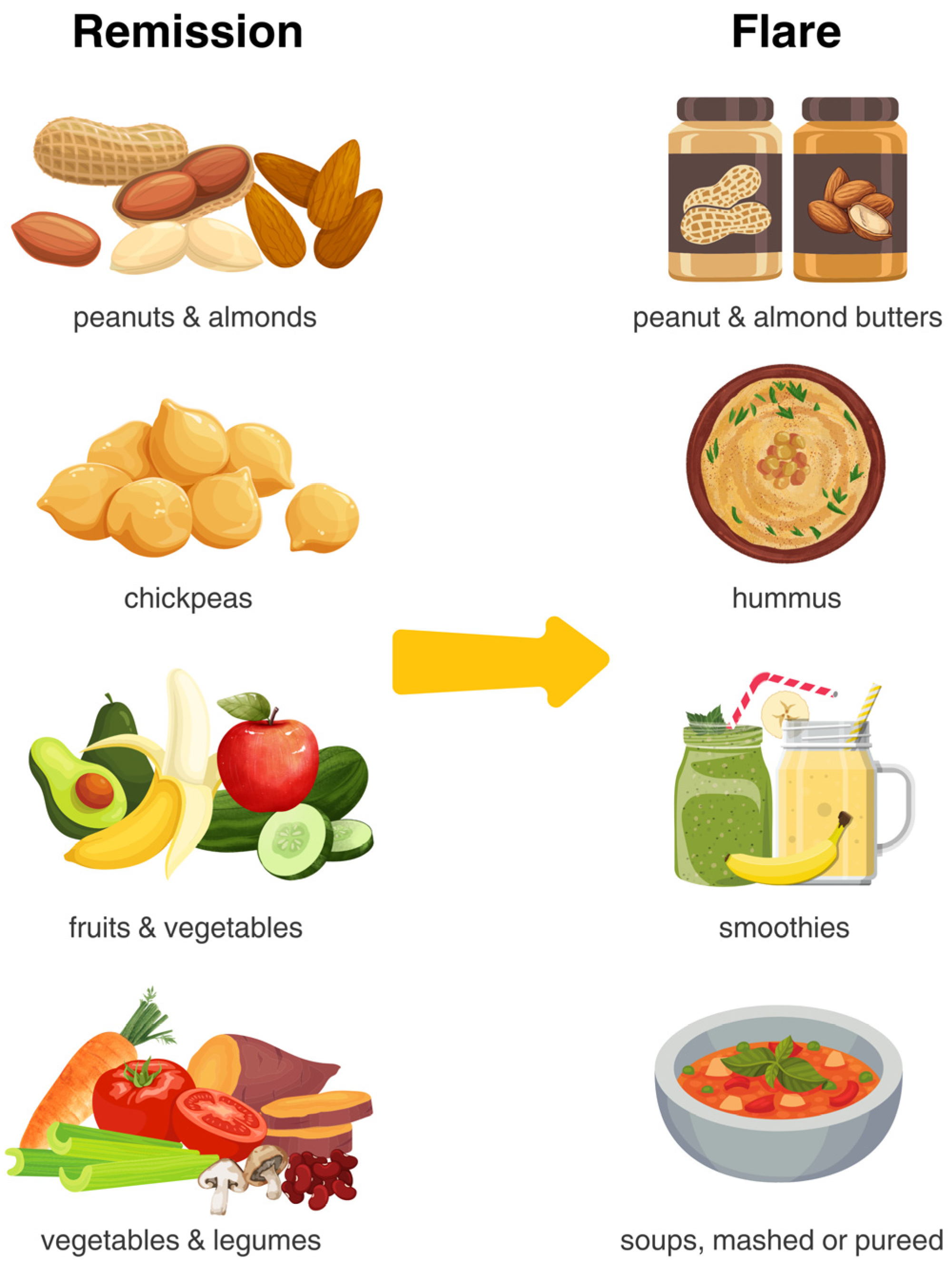
| Active | Strictures/Ileostomy # | Remission | |
|---|---|---|---|
| Fruit | Remove skin/peel Blend into smoothies Apples, bananas and canned/pureed fruit packed in water or juice Pureed fruit (e.g., applesauce, fruit coulis) Cooked/stewed fruit * Limit: dried fruit, coconut, pineapple, prunes | Follow active disease recommendations Smoothies are a great option | No restrictions, based on individual tolerance |
| Vegetables | Cook vegetables until fork tender and remove peels Blend greens into smoothies Consider blended soups * Limit: brussels sprouts, cabbage, cauliflower, kale, asparagus, peas, corn, artichoke | Follow active disease recommendations plus: Avoid skins, tough stalks and seeds as well as raw salads | No restrictions, based on individual tolerance |
| Whole Grains and Starchy Foods (Resistant Starch) | Focus on including soluble fiber: barley, oats, psyllium Green bananas Cook, cool, reheat pasta, rice, sweet potato, and potatoes Limit whole wheat flour, wheat bran | Avoid insoluble fiber, corn hulls, popcorn, wild rice Cook, cool, reheat pasta, rice, and potatoes | Replace refined grains with whole grains, including both insoluble and soluble fiber Cook, cool, reheat pasta, rice, and potatoes No restrictions, based on individual tolerance |
| Nuts and Seeds | Nut and seed butters without added sugar, salt, or fat | Ground nut and seed butters without added sugar, salt, or fat | No restrictions, based on individual tolerance |
| Legumes | Lentils, split pea, tempeh or tofu | Mashed or pureed beans (e.g., hummus) or tofu | No restrictions, based on individual tolerance |
| Dairy Products | Lower lactose, lactose-free or fermented options may be better tolerated | No restrictions, based on individual tolerance | No restrictions, based on individual tolerance |
| Fatty Fish, Eggs, White Meat, and Red Meat | Focus on fish, skinless poultry and eggs while limiting red meat | Stewed, fork tender meat Avoid tougher cuts of meat, unless slow-cooking or stewing (e.g., chuck, brisket, or round, chicken wings), sausages with casing. | No restrictions, based on individual tolerance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, R.G.; Purcell, S.A.; Gold, S.L.; Christiansen, V.; D’Aloisio, L.D.; Raman, M.; Haskey, N. From Evidence to Practice: A Narrative Framework for Integrating the Mediterranean Diet into Inflammatory Bowel Disease Management. Nutrients 2025, 17, 470. https://doi.org/10.3390/nu17030470
Naik RG, Purcell SA, Gold SL, Christiansen V, D’Aloisio LD, Raman M, Haskey N. From Evidence to Practice: A Narrative Framework for Integrating the Mediterranean Diet into Inflammatory Bowel Disease Management. Nutrients. 2025; 17(3):470. https://doi.org/10.3390/nu17030470
Chicago/Turabian StyleNaik, Riya Gautam, Sarah A. Purcell, Stephanie L. Gold, Victoria Christiansen, Leah D. D’Aloisio, Maitreyi Raman, and Natasha Haskey. 2025. "From Evidence to Practice: A Narrative Framework for Integrating the Mediterranean Diet into Inflammatory Bowel Disease Management" Nutrients 17, no. 3: 470. https://doi.org/10.3390/nu17030470
APA StyleNaik, R. G., Purcell, S. A., Gold, S. L., Christiansen, V., D’Aloisio, L. D., Raman, M., & Haskey, N. (2025). From Evidence to Practice: A Narrative Framework for Integrating the Mediterranean Diet into Inflammatory Bowel Disease Management. Nutrients, 17(3), 470. https://doi.org/10.3390/nu17030470







