Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
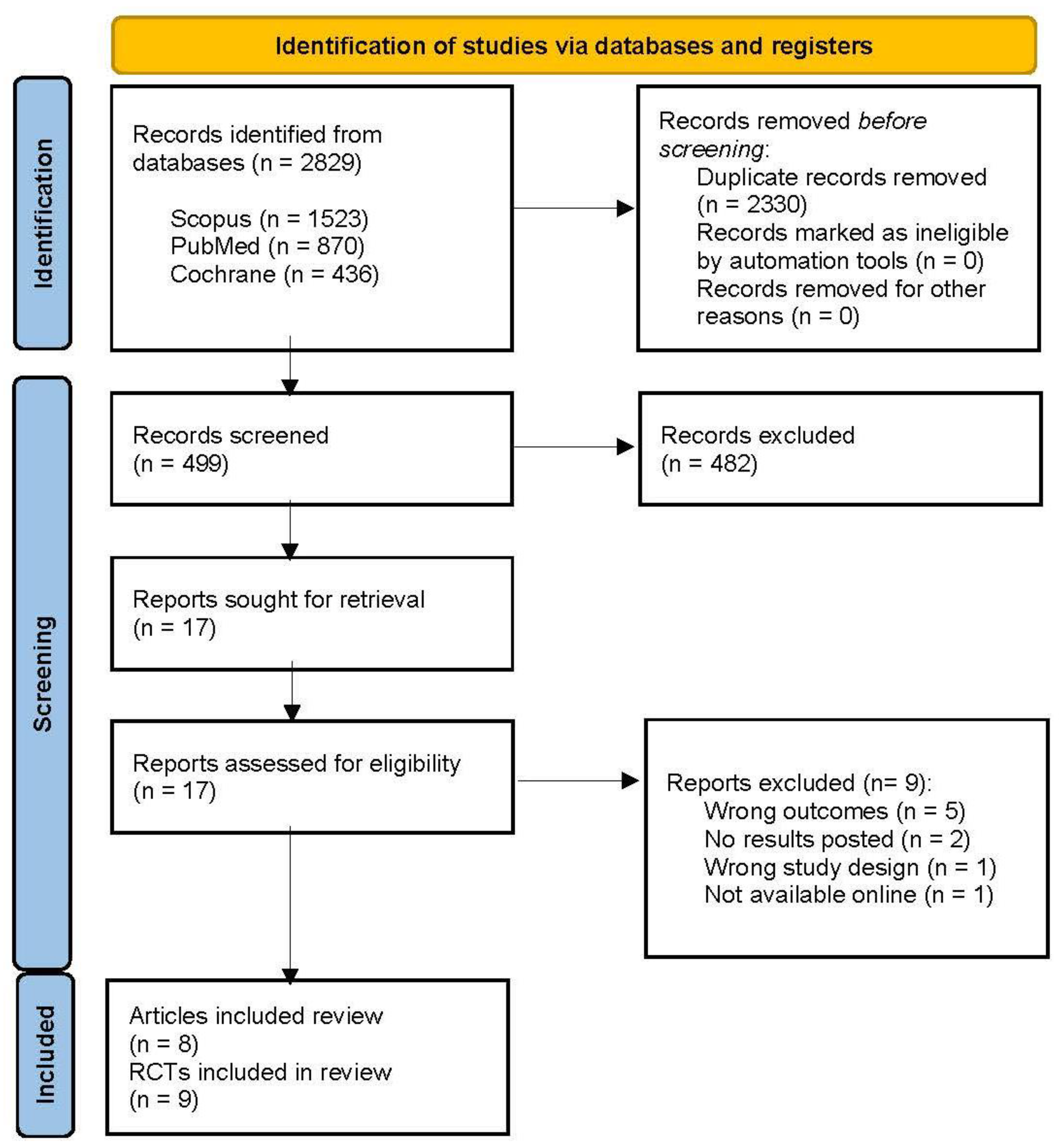
2.1. Literature Research
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
2.4. Outcomes
2.5. Quality Assessment
3. Results
3.1. The Characteristics of the Included Studies
3.2. Chewing Gum and Appetite Regulation
3.2.1. Hunger
3.2.2. Desire to Eat
3.2.3. Preoccupation with Food
3.3. Chewing Gum and Satiety
3.4. Chewing Gum and Energy Intake
3.5. Chewing Gum and Weight Loss
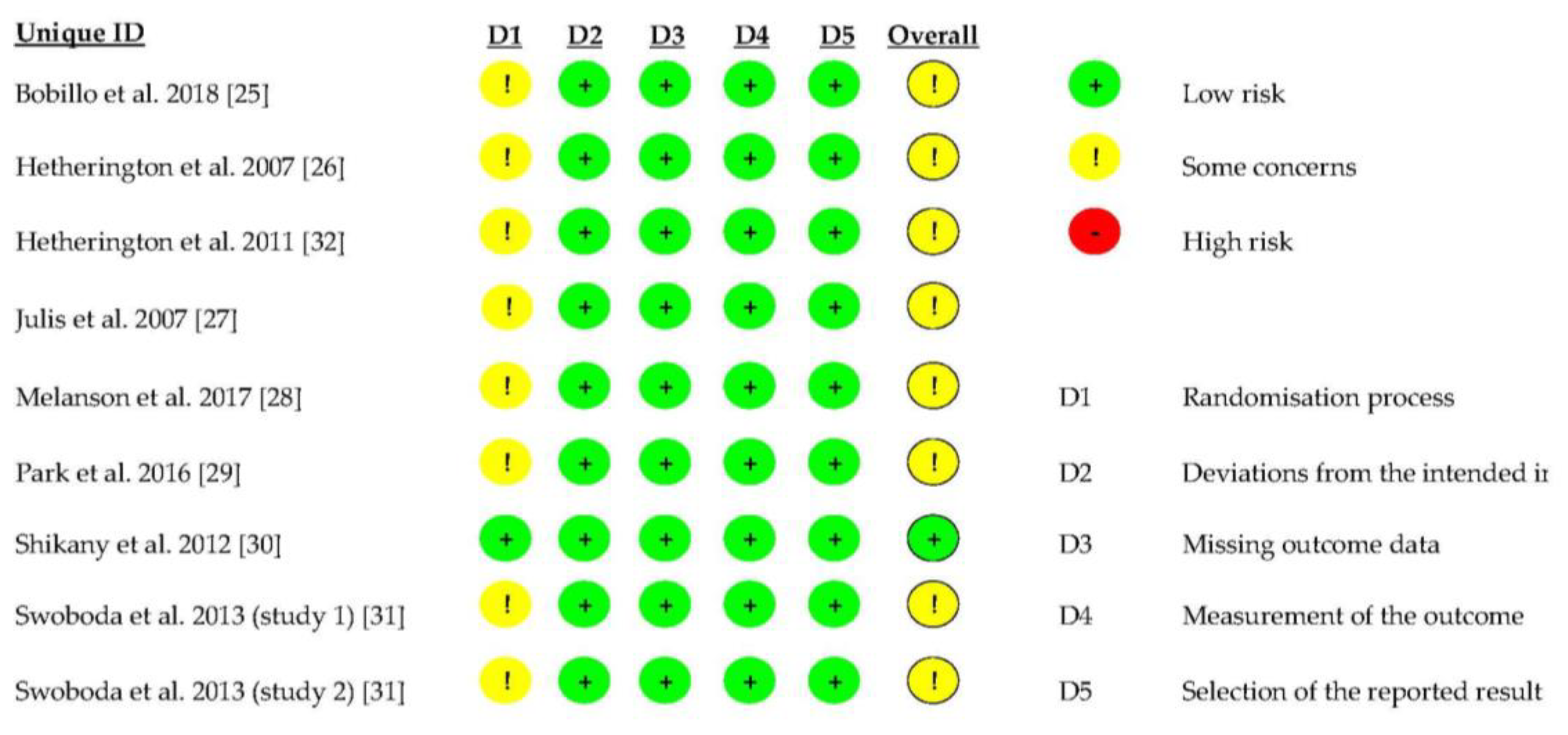
3.6. Quality of the RCTs Included in This Systematic Review
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Status Report on Noncommunicable Diseases. 2014. Available online: https://www.who.int/publications/i/item/9789241564854 (accessed on 29 May 2024).
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 29 May 2024).
- Gibbons, C.; Hopkins, M.; Beaulieu, K.; Oustric, P.; Blundell, J.E. Issues in Measuring and Interpreting Human Appetite (Satiety/Satiation) and Its Contribution to Obesity. Curr. Obes. Rep. 2019, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite Control: Methodological Aspects of the Evaluation of Foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Considine, R.V. Oral Processing Effort, Appetite and Acute Energy Intake in Lean and Obese Adults. Physiol. Behav. 2013, 120, 173–181. [Google Scholar] [CrossRef]
- Lasschuijt, M.P.; de Graaf, K.; Mars, M. Effects of Oro-Sensory Exposure on Satiation and Underlying Neurophysiological Mechanisms-What Do We Know So Far? Nutrients 2021, 13, 1391. [Google Scholar] [CrossRef]
- Slyper, A. Oral Processing, Satiation and Obesity: Overview and Hypotheses. Diabetes Metab. Syndr. Obes. 2021, 14, 3399–3415. [Google Scholar] [CrossRef]
- Blundell, J.; De Graaf, K.; Finlayson, G.; Halford, J.; Hetherington, M.; King, N.; Stubbs, R. Measuring Food Intake, Hunger, Satiety and Satiation in the Laboratory. In Handbook of Assessment Methods for Eating Behaviours and Weight-Related Problems: Measures, Theory and Research, 2nd ed.; Sage Publications: London, UK, 2009. [Google Scholar]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The Use of Visual Analogue Scales to Assess Motivation to Eat in Human Subjects: A Review of Their Reliability and Validity with an Evaluation of New Hand-Held Computerized Systems for Temporal Tracking of Appetite Ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef]
- Maruyama, K.; Sato, S.; Ohira, T.; Maeda, K.; Noda, H.; Kubota, Y.; Nishimura, S.; Kitamura, A.; Kiyama, M.; Okada, T.; et al. The Joint Impact on Being Overweight of Self Reported Behaviours of Eating Quickly and Eating until Full: Cross Sectional Survey. BMJ 2008, 337, 1091–1093. [Google Scholar] [CrossRef]
- Leong, S.L.; Madden, C.; Gray, A.; Waters, D.; Horwath, C. Faster Self-Reported Speed of Eating Is Related to Higher Body Mass Index in a Nationwide Survey of Middle-Aged Women. J. Am. Diet. Assoc. 2011, 111, 1192–1197. [Google Scholar] [CrossRef]
- Katagiri, S.; Nitta, H.; Nagasawa, T.; Izumi, Y.; Kanazawa, M.; Matsuo, A.; Chiba, H.; Miyazaki, S.; Miyauchi, T.; Nakamura, N.; et al. Reduced Masticatory Function in Non-Elderly Obese Japanese Adults. Obes. Res. Clin. Pract. 2011, 5, e279–e286. [Google Scholar] [CrossRef]
- Sánchez-Ayala, A.; Campanha, N.H.; Garcia, R.C.M.R. Relationship between Body Fat and Masticatory Function. J. Prosthodont. 2013, 22, 120–125. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Hu, L.; Li, Z.; Li, R.; Li, C.; Wang, S. Improvement in Chewing Activity Reduces Energy Intake in One Meal and Modulates Plasma Gut Hormone Concentrations in Obese and Lean Young Chinese Men. Am. J. Clin. Nutr. 2011, 94, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hollis, J.H. Increasing the Number of Chews before Swallowing Reduces Meal Size in Normal-Weight, Overweight, and Obese Adults. J. Acad. Nutr. Diet. 2014, 114, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Miquel-Kergoat, S.; Azais-Braesco, V.; Burton-Freeman, B.; Hetherington, M.M. Effects of Chewing on Appetite, Food Intake and Gut Hormones: A Systematic Review and Meta-Analysis. Physiol. Behav. 2015, 151, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Krop, E.M.; Hetherington, M.M.; Nekitsing, C.; Miquel, S.; Postelnicu, L.; Sarkar, A. Influence of Oral Processing on Appetite and Food Intake—A Systematic Review and Meta-Analysis. Appetite 2018, 125, 253–269. [Google Scholar] [CrossRef]
- Robinson, E.; Almiron-Roig, E.; Rutters, F.; De Graaf, C.; Forde, C.G.; Smith, C.T.; Nolan, S.J.; Jebb, S.A. A Systematic Review and Meta-Analysis Examining the Effect of Eating Rate on Energy Intake and Hunger. Am. J. Clin. Nutr. 2014, 100, 123–151. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Szu, S.Y. Effects of Gum Chewing on Recovery From Postoperative Ileus: A Randomized Clinical Trail. J. Nurs. Res. 2022, 30, E233. [Google Scholar] [CrossRef]
- Ikeda, A.; Miyamoto, J.J.; Usui, N.; Taira, M.; Moriyama, K. Chewing Stimulation Reduces Appetite Ratings and Attentional Bias toward Visual Food Stimuli in Healthy-Weight Individuals. Front. Psychol. 2018, 9, 99. [Google Scholar] [CrossRef]
- Aslani, A.; Rostami, F. Medicated Chewing Gum, a Novel Drug Delivery System. J. Res. Med. Sci. 2015, 20, 403. [Google Scholar] [CrossRef]
- Redruello-Requejo, M.; González-Rodríguez, M.; Samaniego-Vaesken, M.d.L.; Montero-Bravo, A.; Partearroyo, T.; Varela-Moreiras, G. Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population. Nutrients 2021, 13, 1845. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, L4898. [Google Scholar] [CrossRef] [PubMed]
- Bobillo, C.; Finlayson, G.; Martínez, A.; Fischman, D.; Beneitez, A.; Ferrero, A.J.; Fernández, B.E.; Mayer, M.A. Short-Term Effects of a Green Coffee Extract-, Garcinia c Ambogia- and L-Carnitine-Containing Chewing Gum on Snack Intake and Appetite Regulation. Eur. J. Nutr. 2018, 57, 607–615. [Google Scholar] [CrossRef]
- Hetherington, M.M.; Boyland, E. Short-Term Effects of Chewing Gum on Snack Intake and Appetite. Appetite 2007, 48, 397–401. [Google Scholar] [CrossRef]
- Julis, R.A.; Mattes, R.D. Influence of Sweetened Chewing Gum on Appetite, Meal Patterning and Energy Intake. Appetite 2007, 48, 167–175. [Google Scholar] [CrossRef]
- Melanson, K.J.; Kresge, D.L. Chewing Gum Decreases Energy Intake at Lunch Following a Controlled Breakfast. Appetite 2017, 118, 1–7. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Inui, T.; Kergoat, S.; Kelley, M.; Burton-Freeman, B. Short-Term Effects of Chewing Gum on Satiety and Afternoon Snack Intake in Healthy Weight and Obese Women. Physiol. Behav. 2016, 159, 64–71. [Google Scholar] [CrossRef]
- Shikany, J.M.; Thomas, A.S.; McCubrey, R.O.; Mark Beasley, T.; Allison, D.B. Randomized Controlled Trial of Chewing Gum for Weight Loss. Obesity 2012, 20, 547–552. [Google Scholar] [CrossRef]
- Swoboda, C.; Temple, J.L. Acute and Chronic Effects of Gum Chewing on Food Reinforcement and Energy Intake. Eat. Behav. 2013, 14, 149–156. [Google Scholar] [CrossRef]
- Hetherington, M.M.; Regan, M.F. Effects of Chewing Gum on Short-Term Appetite Regulation in Moderately Restrained Eaters. Appetite 2011, 57, 475–482. [Google Scholar] [CrossRef]
- Njike, V.Y.; Smith, T.M.; Shuval, O.; Shuval, K.; Edshteyn, I.; Kalantari, V.; Yaroch, A.L. Snack Food, Satiety, and Weight. Adv. Nutr. 2016, 7, 866. [Google Scholar] [CrossRef]
- Skoczek-Rubińska, A.; Bajerska, J. The Consumption of Energy Dense Snacks and Some Contextual Factors of Snacking May Contribute to Higher Energy Intake and Body Weight in Adults. Nutr. Res. 2021, 96, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.B.; Greatwood, H.C.; McCullough, D.; Kirwan, R.; Duckworth, L.C.; Sutton, L.; Gately, P.J. The Effect of Discretionary Snack Consumption on Overall Energy Intake, Weight Status, and Diet Quality: A Systematic Review. Obes. Rev. 2024, 25, e13693. [Google Scholar] [CrossRef] [PubMed]
- Komai, N.; Motokubota, N.; Suzuki, M.; Hayashi, I.; Moritani, T.; Nagai, N. Thorough Mastication Prior to Swallowing Increases Postprandial Satiety and the Thermic Effect of a Meal in Young Women. J. Nutr. Sci. Vitaminol. 2016, 62, 288–294. [Google Scholar] [CrossRef]
- Rakha, A.; Mehak, F.; Shabbir, M.A.; Arslan, M.; Ranjha, M.M.A.N.; Ahmed, W.; Socol, C.T.; Rusu, A.V.; Hassoun, A.; Aadil, R.M. Insights into the Constellating Drivers of Satiety Impacting Dietary Patterns and Lifestyle. Front. Nutr. 2022, 9, 1002619. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiao, X.; Li, Y.; Zheng, J.; Li, W.; Zhang, Q.; Wang, Z. The Effect of Gum Chewing on Blood GLP-1 Concentration in Fasted, Healthy, Non-Obese Men. Endocrine 2015, 50, 93–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. Increasing the Number of Masticatory Cycles Is Associated with Reduced Appetite and Altered Postprandial Plasma Concentrations of Gut Hormones, Insulin and Glucose. Br. J. Nutr. 2013, 110, 384–390. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Nehaoua, A.; Shanaida, M.; Semenova, Y.; Piscopo, S.; Menzel, A.; Voloshyn, V.; Voloshyn, O.; Shanaida, V.; et al. Pharmacological Treatments and Natural Biocompounds in Weight Management. Pharmaceuticals 2023, 16, 212. [Google Scholar] [CrossRef]
- Peters, B.; Vahlhaus, J.; Pivovarova-Ramich, O. Meal Timing and Its Role in Obesity and Associated Diseases. Front. Endocrinol. 2024, 15, 1359772. [Google Scholar] [CrossRef]
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Comparison |
|
|
| Outcomes |
|
|
| Study type |
|
|
| Author | Participants | Intervention | Results | ||||
|---|---|---|---|---|---|---|---|
| N | BMI | Design | Effect on Appetite Regulation | Effect on Satiety | Effect on Energy Intake | Effect on Weight Loss | |
| Hetherington and Boyland(2007) [26] | 60 | NOB or OW | Fixed lunch on arrival. Gum chewed 1 h, 2 h, and 3 h after lunch. Access to ad libitum snacks 3 h after lunch. Intake recorded at home by diet records; within subjects. | Hunger * and desire to eat sweet snacks * ↓ in the chewing gum condition. No effect on desire to eat salty snacks. | Fullness * ↑ in the chewing gum condition. | Energy intake * ↓ in the chewing gum condition. | NA |
| Julis and Mattes(2007) [27] | 47 | OW | Standard breakfast and lunch. Gum chewed at a fixed time or before meal for 20 min before food consumption. Intake recorded at home by diet records; within subjects. | Hunger α ↑ after gum chewing, but desire to eat α and desire to eat sweet snacks ↓ in the chewing gum condition. No effect on salty snacks, fatty snacks, or preoccupation with food. | Fullness α ↓ after gum chewing. | Energy intake ↓ in the chewing gum condition. | NA |
| Hetherington and Regan(2011) [32] | 60 | NOB or OB | Fixed lunch on arrival. Gum chewed 1 h, 2 h, and 3 h after lunch. Access to ad libitum snacks 3 h after lunch. Intake recorded at home by diet records; within subjects. | Hunger **, desire to eat **, desire to eat sweet snacks **, desire to eat salty snacks ** and desire to eat a snack ** ↓ in the chewing gum condition. | Fullness * ↑ in the chewing gum condition. | NA | NA |
| Shikany J et al. (2012) [30] | 201 | OB | Eight-week intervention with chewing gum and printed nutrition information. Participants chewed gum six times a day for a total of 90 min/day (20 min after breakfast, lunch, and dinner and chewed an additional 10 min mid-morning, mid-afternoon, and 1–2 h after dinner). | NA | NA | NA | No effect on weight loss. |
| Swoboda and Temple (study 1) (2013) [31] | 44 | OW | Gum chewed for 10 min upon arrival at the laboratory. Reinforcement game to earn points in order to earn food (lower or high energy density). Intake recorded at home with diet records; within subjects. | Hunger β was ↓ in the chewing gum condition. | NA | Energy intake of healthy food * ↓ in the chewing gum condition but no effect on total daily energy intake. | NA |
| Swoboda and Temple(study 2) (2013) [31] | 54 | OW | Gum chewed every single occasion before food for a week. Intake recorded at home using diet records; within subjects. | NA | NA | Energy intake per meal * ↓ in the chewing gum condition but no effect on total daily energy intake. | NA |
| Park et al.(2016) [29] | 50 | NOB and OB | Fixed lunch on arrival. Gum chewed 1 h, 2 h, and 3 h after lunch. Access to ad libitum snacks 3 h after lunch. Intake recorded at home using diet records; within subjects. | Hunger * and desire to eat * ↓ in the chewing gum condition. | Fullness ↑ in the chewing gum condition. | Energy intake from snacks ↓ in the chewing gum condition. Carbohydrate intake * was ↓ in the chewing gum condition. | NA |
| Melanson and Kresge(2017) [28] | 33 | NOB or OW | Fixed breakfast on arrival. Gum chewed for 20 min, 10 min into ventilated hood indirect calorimetry, and 3 h after breakfast. Access to ad libitum snacks 3 h after breakfast. Intake recorded at home using diet records; within subjects. | Hunger * ↓ in the chewing gum condition only after first chewing period. No effect on desire to eat, desire to eat something sweet, or desire to eat something salty. | Fullness ↑ in the chewing gum condition. | Energy intake * ↓ in the chewing gum condition. | NA |
| Bobillo et al.(2018) [25] | 57 | NOB or OW | Fixed lunch on arrival. Gum chewed 1 h, 2 h, 3 h, and 4 h after lunch. Access to ad libitum snacks 4 h after lunch. Intake recorded at home in diet records; within subjects. | Hunger * ↓ in the chewing gum condition. | Fullness * ↑ in the chewing gum condition. | Total energy intake from snacks * ↓ for active gum when compared to no gum. No effect of placebo gum. | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-ten Hoevel, C.; Llauradó, E.; Valls, R.M.; Besora-Moreno, M.; Queral, J.; Solà, R.; Pedret, A. Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review. Nutrients 2025, 17, 435. https://doi.org/10.3390/nu17030435
Jiménez-ten Hoevel C, Llauradó E, Valls RM, Besora-Moreno M, Queral J, Solà R, Pedret A. Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review. Nutrients. 2025; 17(3):435. https://doi.org/10.3390/nu17030435
Chicago/Turabian StyleJiménez-ten Hoevel, Claudia, Elisabet Llauradó, Rosa M. Valls, Maria Besora-Moreno, Judit Queral, Rosa Solà, and Anna Pedret. 2025. "Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review" Nutrients 17, no. 3: 435. https://doi.org/10.3390/nu17030435
APA StyleJiménez-ten Hoevel, C., Llauradó, E., Valls, R. M., Besora-Moreno, M., Queral, J., Solà, R., & Pedret, A. (2025). Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review. Nutrients, 17(3), 435. https://doi.org/10.3390/nu17030435






