Folate Supplementation Awareness Among Women of Reproductive Age in Poland: Focus on Active Forms and Updated National Recommendations
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Sociodemographic Characteristics
3.2. Baseline Knowledge and Practices Related to Folate Supplementation Among Women with and Without Infertility
3.3. Multivariable Analysis of Predictors of 5-MTHF Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAP | American Academy of Pediatrics |
| ACOG | American College of Obstetricians and Gynecologists |
| ART | Assisted Reproductive Technologies |
| CDC | Centers for Disease Control and Prevention |
| CI | Confidence Interval |
| DHFR | Dihydrofolate Reductase |
| EFSA | European Food Safety Authority |
| MTHFR | Methylenetetrahydrofolate Reductase |
| NTD | Neural Tube Defect |
| OR | Odds Ratio |
| USPSTF | U.S. Preventive Services Task Force |
| UMFA | Unmetabolized Folic Acid |
| WHO | World Health Organization |
| 5-MTHF | 5-methyltetrahydrofolate |
References
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.M.; Boers, R.G.; Boers, J.B.; Schäffers, O.J.M.; van der Meeren, L.E.; Steegers-Theunissen, R.P.M.; Gribnau, J.; Schoenmakers, S. Genome-Wide Methylation Profiling of Maternal Cell-Free DNA Using Methylated DNA Sequencing (MeD-Seq) Indicates a Placental and Immune-Cell Signature. Eur. J. Clin. Investig. 2025, 55, e14363. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Zauche, L.H.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Kaldygulova, L.; Ukybassova, T.; Aimagambetova, G.; Gaiday, A.; Tussupkaliyev, A. Biological Role of Folic Acid in Pregnancy and Possible Therapeutic Application for the Prevention of Preeclampsia. Biomedicines 2023, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Laurence, K.M.; James, N.; Miller, M.H.; Tennant, G.B.; Campbell, H. Double-Blind Randomised Controlled Trial of Folate Treatment before Conception to Prevent Recurrence of Neural-Tube Defects. Br. Med. J. Clin. Res. Ed 1981, 282, 1509–1511. [Google Scholar] [CrossRef]
- Abate, B.B.; Kumsa, H.; Kibret, G.A.; Wodaynew, T.; Habtie, T.E.; Kassa, M.; Munie, M.A.; Temesgen, D.; Tilahun, B.D.; Merchaw, A.; et al. Preconception Folic Acid and Multivitamin Supplementation for the Prevention of Neural Tube Defect: An Umbrella Review of Systematic Review and Meta-Analysis. Neuroepidemiology 2025, 59, 412–425. [Google Scholar] [CrossRef]
- Viswanathan, M.; Urrutia, R.P.; Hudson, K.N.; Middleton, J.C.; Kahwati, L.C. Folic Acid Supplementation to Prevent Neural Tube Defects: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 330, 460–466. [Google Scholar] [CrossRef]
- Samaniego-Vaesken, M.d.L.; Morais-Moreno, C.; Carretero-Krug, A.; Puga, A.M.; Montero-Bravo, A.M.; Partearroyo, T.; Gregorio, V.-M. Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review. Nutrients 2024, 16, 3154. [Google Scholar] [CrossRef]
- Steenweg-de Graaff, J.; Roza, S.J.; Steegers, E.A.; Hofman, A.; Verhulst, F.C.; Jaddoe, V.W.; Tiemeier, H. Maternal Folate Status in Early Pregnancy and Child Emotional and Behavioral Problems: The Generation R Study. Am. J. Clin. Nutr. 2012, 95, 1413–1421. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The Pharmacokinetic Advantage of 5-Methyltetrahydrofolate for Minimization of the Risk for Birth Defects. Sci. Rep. 2018, 8, 4096. [Google Scholar] [CrossRef]
- Venn, B.J.; Green, T.J.; Moser, R.; Mann, J.I. Comparison of the Effect of Low-Dose Supplementation with L-5-Methyltetrahydrofolate or Folic Acid on Plasma Homocysteine: A Randomized Placebo-Controlled Study. Am. J. Clin. Nutr. 2003, 77, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.M.; Elango, R.; Devlin, A.M.; Mayer, C.; Hutcheon, J.A.; Karakochuk, C.D. Supplementation with (6S)-5-Methyltetrahydrofolic Acid Appears as Effective as Folic Acid in Maintaining Maternal Folate Status While Reducing Unmetabolised Folic Acid in Maternal Plasma: A Randomised Trial of Pregnant Women in Canada. Br. J. Nutr. 2024, 131, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.M.; Aleliunas, R.E.; Loh, S.P.; Khor, G.L.; Harvey-Leeson, S.; Glier, M.B.; Kitts, D.D.; Green, T.J.; Devlin, A.M. L-5-Methyltetrahydrofolate Supplementation Increases Blood Folate Concentrations to a Greater Extent than Folic Acid Supplementation in Malaysian Women. J. Nutr. 2018, 148, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Servy, E.J.; Jacquesson-Fournols, L.; Cohen, M.; Menezo, Y.J.R. MTHFR Isoform Carriers. 5-MTHF (5-Methyl Tetrahydrofolate) vs Folic Acid: A Key to Pregnancy Outcome: A Case Series. J. Assist. Reprod. Genet. 2018, 35, 1431–1435. [Google Scholar] [CrossRef]
- Kubo, Y.; Fukuoka, H.; Kawabata, T.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Ohkubo, T.; Oshida, K.; Yanagisawa, N.; et al. Distribution of 5-Methyltetrahydrofolate and Folic Acid Levels in Maternal and Cord Blood Serum: Longitudinal Evaluation of Japanese Pregnant Women. Nutrients 2020, 12, 1633. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic Acid versus 5- Methyl Tetrahydrofolate Supplementation in Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Cirillo, M.; Fucci, R.; Rubini, S.; Coccia, M.E.; Fatini, C. 5-Methyltetrahydrofolate and Vitamin B12 Supplementation Is Associated with Clinical Pregnancy and Live Birth in Women Undergoing Assisted Reproductive Technology. Int. J. Environ. Res. Public. Health 2021, 18, 12280. [Google Scholar] [CrossRef]
- Periconceptional Folic Acid Supplementation to Prevent Neural Tube Defects. Available online: https://www.who.int/tools/elena/interventions/folate-periconceptional (accessed on 10 November 2025).
- CDC. Folic Acid: Sources and Recommended Intake. Available online: https://www.cdc.gov/folic-acid/about/intake-and-sources.html (accessed on 10 November 2025).
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to Increasing Maternal Folate Status by Supplemental Folate Intake and Reduced Risk of Neural Tube Defects Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3328. [Google Scholar] [CrossRef]
- ACOG. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet. Gynecol. 2019, 133, e78–e89. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Genetics. Folic Acid for the Prevention of Neural Tube Defects. Pediatrics 1999, 104, 325–327. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Folic Acid Supplementation to Prevent Neural Tube Defects: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2023, 330, 454–459. [Google Scholar] [CrossRef]
- Seremak-Mrozikiewicz, A.; Bomba-Opoń, D.; Drews, K.; Kaczmarek, P.; Wielgoś, M.; Sieroszewski, P. Stanowisko Ekspertów Polskiego Towarzystwa Ginekologów i Położników w zakresie suplementacji folianów oraz warunków stosowania dodatkowej suplementacji choliny oraz witamin B6 i B12 w okresie przedkoncepcyjnym, ciąży i połogu. Ginekol. Perinatol. Prakt. 2024, 9, 154–156. [Google Scholar]
- Guideline for the Prevention, Diagnosis and Treatment of Infertility: Summary of Recommendations. Available online: https://www.who.int/publications/i/item/B09599 (accessed on 1 December 2025).
- Kwapien, P.; Mrowiec, M.; Janus, D.; Chmura, K.; Surmiak, P.; Mrowiec, E. Wiedza Kobiet Na Temat Suplementacji Diety Kwasem Foliowym Przez Caly Okres Rozrodczy. Ginekol. Poloznictwo 2021, 16, 1–5. [Google Scholar]
- Sobek, G.; Socha, P.; Pawelec, S.; Łuszczki, E.; Dereń, K.; Mazur, A. Ocena wiedzy kobiet na temat potrzeby stosowania suplementów diety w okresie ciąży. Stand. Medyczne/Pediatria 2017, 14, 126–135. [Google Scholar]
- Oliver, E.M.; Grimshaw, K.E.C.; Schoemaker, A.A.; Keil, T.; McBride, D.; Sprikkelman, A.B.; Ragnarsdottir, H.S.; Trendelenburg, V.; Emmanouil, E.; Reche, M.; et al. Dietary Habits and Supplement Use in Relation to National Pregnancy Recommendations: Data from the EuroPrevall Birth Cohort. Matern. Child Health J. 2014, 18, 2408–2425. [Google Scholar] [CrossRef]
- Taye, M.; Mohammed, T.; Taye, W.; Glagn, M.; Yihune, M. Compliance with Iron-Folate Supplementation among Pregnant Women in Southern Ethiopia: A Multi-Center Cross-Sectional Study. Reprod. Health 2025, 22, 22. [Google Scholar] [CrossRef]
- Felipe-Dimog, E.B.; Yu, C.-H.; Ho, C.-H.; Liang, F.-W. Factors Influencing the Compliance of Pregnant Women with Iron and Folic Acid Supplementation in the Philippines: 2017 Philippine Demographic and Health Survey Analysis. Nutrients 2021, 13, 3060. [Google Scholar] [CrossRef]
- Ignaszak-Kaus, N.; Ozegowska, K.; Piekarski, P.; Pawelczyk, L.; Jędrzejczak, P. Planning and Preparation for Pregnancy among Women with and without a History of Infertility. Ginekol. Pol. 2018, 89, 74–79. [Google Scholar] [CrossRef]
- Sharma, A.; Kamboj, N.; Saraswathy, K.N.; Puri, M.; Babu, N.; Mahajan, C. Knowledge, Attitude, and Practice of Infertility: A Comparative Study in Infertile and Fertile Indian Women. J. Biosoc. Sci. 2023, 55, 947–959. [Google Scholar] [CrossRef]
- Kadir, M.; Hood, R.B.; Mínguez-Alarcón, L.; Maldonado-Cárceles, A.B.; Ford, J.B.; Souter, I.; Chavarro, J.E.; Gaskins, A.J.; EARTH Study Team. Folate Intake and Ovarian Reserve among Women Attending a Fertility Center. Fertil. Steril. 2022, 117, 171–180. [Google Scholar] [CrossRef]
- Ren, M.; Zhong, B.; Ding, R.; Fan, W. The Influence of Self-Control Fatigue and Information Source Credibility on the Gap Between Attitude and Behavior. Adv. Cogn. Psychol. 2023, 19, 201–210. [Google Scholar] [CrossRef]
- Fohr, I.P.; Prinz-Langenohl, R.; Brönstrup, A.; Bohlmann, A.M.; Nau, H.; Berthold, H.K.; Pietrzik, K. 5,10-Methylenetetrahydrofolate Reductase Genotype Determines the Plasma Homocysteine-Lowering Effect of Supplementation with 5-Methyltetrahydrofolate or Folic Acid in Healthy Young Women. Am. J. Clin. Nutr. 2002, 75, 275–282. [Google Scholar] [CrossRef]
- van der Put, N.M.; Gabreëls, F.; Stevens, E.M.; Smeitink, J.A.; Trijbels, F.J.; Eskes, T.K.; van den Heuvel, L.P.; Blom, H.J. A Second Common Mutation in the Methylenetetrahydrofolate Reductase Gene: An Additional Risk Factor for Neural-Tube Defects? Am. J. Hum. Genet. 1998, 62, 1044–1051. [Google Scholar] [CrossRef]
| Variable | Total (n = 188) | Without Infertility (n = 94) | With Infertility (n = 94) | p-Value | χ2 |
|---|---|---|---|---|---|
| Knowledge of nutrients recommended during pregnancy | 185 (98.4%) | 91 (96.8%) | 94 (100.0%) | 0.244 | 1.35 |
| Familiarity with folate/folic acid supplementation recommendations for pregnancy | 165 (87.8%) | 80 (85.1%) | 85 (90.4%) | 0.373 | 0.79 |
| Awareness of different folate forms available in supplements | 150 (79.8%) | 68 (72.3%) | 82 (87.2%) | 0.018 | 5.57 |
| Recognition of methylated folate (5-MTHF) | 125 (66.5%) | 52 (55.3%) | 73 (77.7%) | 0.002 * | 9.55 |
| Understanding of differences between folate forms | 92 (48.9%) | 36 (38.3%) | 56 (59.6%) | 0.005 * | 7.68 |
| Knowledge of the MTHFR gene and its role in folate metabolism | 110 (58.5%) | 37 (39.4%) | 73 (77.7%) | <=0.001 * | 26.84 |
| Exposure to information about potential adverse effects of synthetic folic acid | 80 (42.6%) | 37 (39.4%) | 43 (45.7%) | 0.460 | 0.54 |
| Reported use of folate/folic acid supplements during pregnancy | 186 (98.9%) | 92 (97.9%) | 94 (100.0%) | 0.477 | 0.51 |
| Initiation of supplementation ≥3 months before conception | 128 (68.1%) | 45 (47.9%) | 83 (88.3%) | <0.001 * | 33.51 |
| Use of the active folate form (5-MTHF) | 104 (55.3%) | 42 (44.7%) | 62 (66.0%) | 0.005 * | 7.77 |
| Choosing supplements based on specialist recommendations | 136 (72.3%) | 69 (73.4%) | 67 (71.3%) | 0.870 | 0.03 |
| Self-rated knowledge: good/very good | 124 (65.9%) | 56 (59.6%) | 68 (72.3%) | 0.090 | 2.87 |
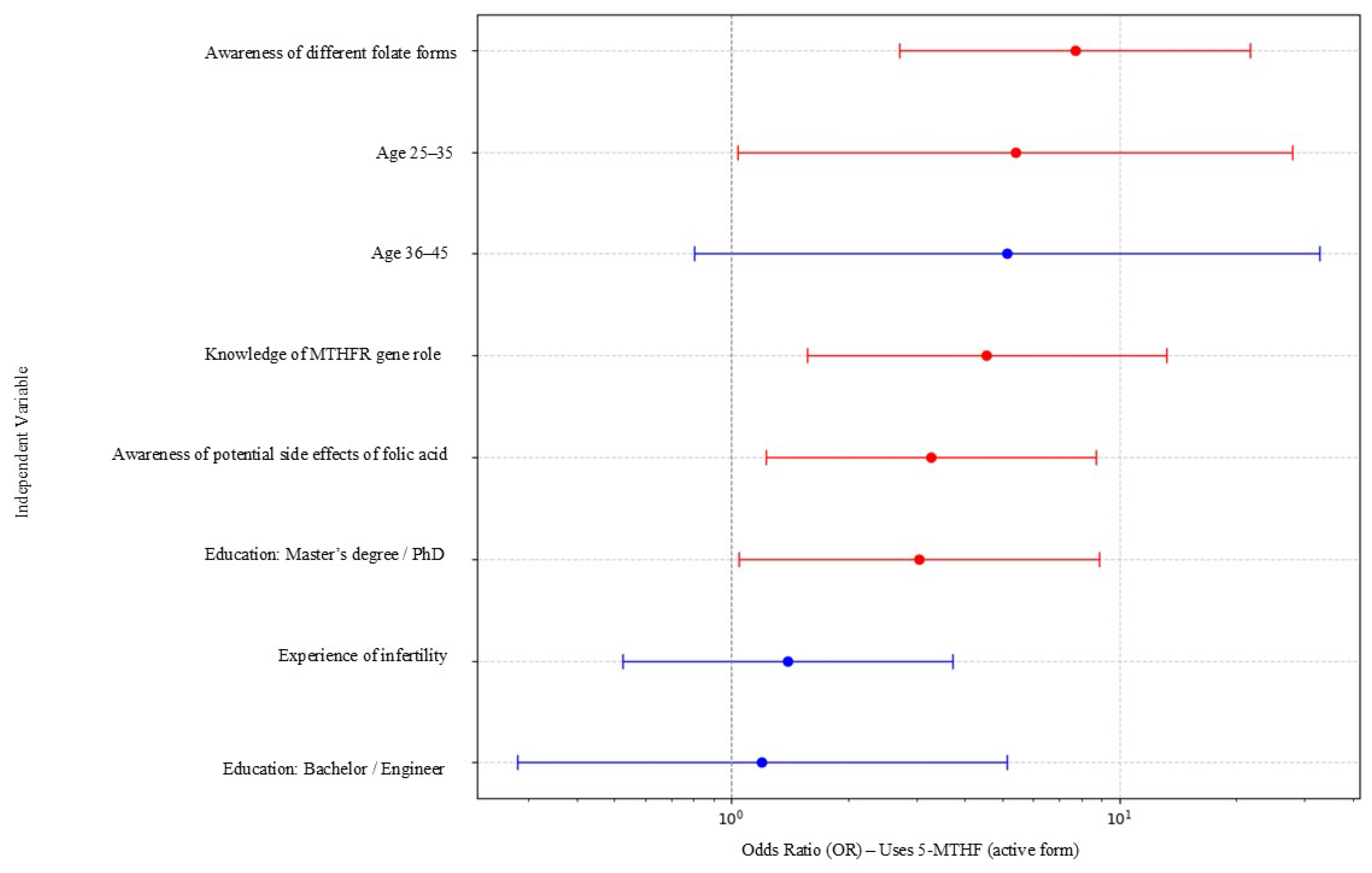
| Variable | Odds Ratio (OR) | 95% CI Lower | 95% CI Upper |
|---|---|---|---|
| Awareness of different folate forms | 6.79 | 1.40 | 32.82 |
| Knowledge of MTHFR gene role | 3.22 | 1.28 | 8.10 |
| Awareness of potential side effects of folic acid | 2.72 | 1.19 | 6.24 |
| Education: Master’s degree/PhD | 3.02 | 1.05 | 8.65 |
| Age 25–35 | 4.59 | 1.18 | 17.79 |
| Age 36–45 | 3.27 | 0.74 | 14.42 |
| Education: Bachelor/Engineer | 1.43 | 0.36 | 5.64 |
| Experience of infertility | 1.21 | 0.52 | 2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarska, O.; Zaryczny-Trojan, L.; Minkiewicz-Zochniak, A. Folate Supplementation Awareness Among Women of Reproductive Age in Poland: Focus on Active Forms and Updated National Recommendations. Nutrients 2025, 17, 3881. https://doi.org/10.3390/nu17243881
Barbarska O, Zaryczny-Trojan L, Minkiewicz-Zochniak A. Folate Supplementation Awareness Among Women of Reproductive Age in Poland: Focus on Active Forms and Updated National Recommendations. Nutrients. 2025; 17(24):3881. https://doi.org/10.3390/nu17243881
Chicago/Turabian StyleBarbarska, Olga, Lidia Zaryczny-Trojan, and Anna Minkiewicz-Zochniak. 2025. "Folate Supplementation Awareness Among Women of Reproductive Age in Poland: Focus on Active Forms and Updated National Recommendations" Nutrients 17, no. 24: 3881. https://doi.org/10.3390/nu17243881
APA StyleBarbarska, O., Zaryczny-Trojan, L., & Minkiewicz-Zochniak, A. (2025). Folate Supplementation Awareness Among Women of Reproductive Age in Poland: Focus on Active Forms and Updated National Recommendations. Nutrients, 17(24), 3881. https://doi.org/10.3390/nu17243881






