Inflammatory Bowel Disease, Gastrointestinal Graft-Versus-Host Disease and Immune Checkpoint Inhibitors Induced Colitis: Similar Diseases to Treat with Fecal Microbiota Transplantation
Abstract
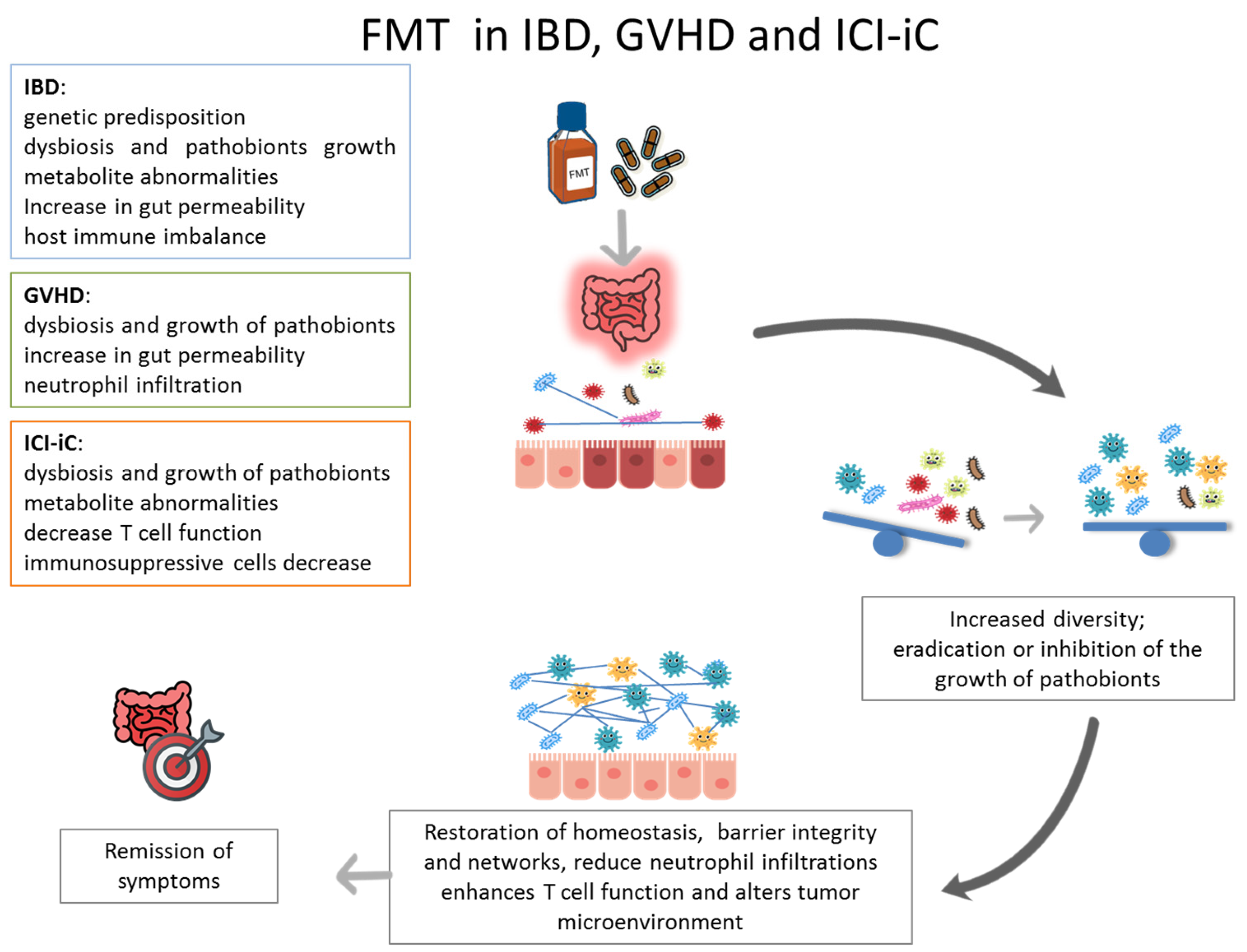
1. Introduction
2. Methods
3. FMT in Inflammatory Bowel Disease
3.1. FMT in Ulcerative Colitis
3.2. FMT in Crohn’s Disease
4. FMT in Immune Checkpoint Inhibitors Induced Colitis
5. FMT in Gastrointestinal Graft-Versus-Host Disease
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| FMT | Fecal Microbiota Transplantation |
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| ICI-iC | Immune Checkpoint Inhibitor-Induced Colitis |
| GI-GVHD | Gastrointestinal Graft-Versus-Host Disease |
| RCT | Randomized Controlled Trial |
References
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. MODIFY I and MODIFY II Investigators. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hunt, A.; Danziger, L.; Drwiega, E.N. A Comparison of Currently Available and Investigational Fecal Microbiota Transplant Products for Recurrent Clostridioides difficile Infection. Antibiotics 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [CrossRef]
- Hamamah, S.; Gheorghita, R.; Lobiuc, A.; Sirbu, I.O.; Covasa, M. Fecal microbiota transplantation in non-communicable diseases: Recent advances and protocols. Front. Med. 2022, 9, 1060581. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Löwenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Sood, A.; Mahajan, R.; Singh, A.; Midha, V.; Mehta, V.; Narang, V.; Singh, T.; Singh Pannu, A. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J. Crohns Colitis 2019, 13, 1311–1317. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: Results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef] [PubMed]
- van Lingen, E.; Nooij, S.; Terveer, E.M.; Crossette, E.; Prince, A.L.; Bhattarai, S.K.; Watson, A.; Galazzo, G.; Menon, R.; Szabady, R.L.; et al. Faecal Microbiota Transplantation Engraftment After Budesonide or Placebo in Patients With Active Ulcerative Colitis Using Pre-selected Donors: A Randomized Pilot Study. J. Crohns Colitis 2024, 18, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Grunert, P.; Stallhofer, J.; Löffler, B.; Baier, M.; Rödel, J.; Kiehntopf, M.; Neugebauer, S.; Pieper, D.H.; Junca, H.; et al. Transfer of FRozen Encapsulated multi-donor Stool filtrate for active ulcerative Colitis (FRESCO): Study protocol for a prospective, multicenter, double-blind, randomized, controlled trial. Trials 2022, 23, 173. [Google Scholar] [CrossRef]
- Raja, S.S.; Costello, S.P.; Rayner, C.K.; Day, A.; Portmann, L.; Uylaki, W.; Wheeler, R.; Saxon, S.; Tucker, E.C.; Fon, J.; et al. Examining the role of faecal microbiota transplantation for inducing remission in resistant ulcerative proctitis and distal ulcerative colitis (up-FMT). J. Crohns Colitis 2025, 19, jjaf169. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Kao, D.; Wong, K.; Jijon, H.; Moayyedi, P.; Franz, R.; McDougall, C.; Hotte, N.; Panaccione, R.; Semlacher, E.; Kroeker, K.I.; et al. Preliminary Results From a Multicenter, Randomized Trial Using Fecal Microbiota Transplantation to Induce Remission in Patients With Mild-to-Moderate Crohn’s Disease. Am. J. Gastroenterol. 2024, 120, 1334–1344. [Google Scholar] [CrossRef]
- Chukhlovin, A.B.; Goloshchapov, O.V.; Shchukina, O.B.; Kharitidis, A.M.; Zhloba, A.A.; Subbotina, T.F.; Kusakin, A.V.; Kosarev, O.V.; Tsai, V.V.; Kalinin, R.S.; et al. Changes in Gut Phageome and Bacteriome Following Fecal Microbiota Transfer in Patients with Intestinal Graft-Versus-Host Disease and Crohn’s Disease. Microorganisms 2025, 13, 2337. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Halsey, T.M.; Thomas, A.S.; Hayase, T.; Ma, W.; Abu-Sbeih, H.; Sun, B.; Parra, E.R.; Jiang, Z.D.; DuPont, H.L.; Sanchez, C.; et al. Microbiome alteration via fecal microbiota transplantation is effective for refractory immune checkpoint inhibitor-induced colitis. Sci. Transl. Med. 2023, 15, eabq4006. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, A.; Waters, N.R.; Smith, N.; Dai, A.; Slingerland, J.; Aleynick, N.; Febles, B.; Gogia, P.; Socci, N.D.; Lumish, M.; et al. Immune-Related Colitis Is Associated with Fecal Microbial Dysbiosis and Can Be Mitigated by Fecal Microbiota Transplantation. Cancer Immunol. Res. 2024, 12, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.Y.; Do, E.J.; Bae, D.J.; Kim, S.; Kweon, M.N.; Song, J.S.; et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Halwachs, B.; Bayer, N.; Huber-Krassnitzer, B.; Schulz, E.; Uhl, B.; Gaksch, L.; Hatzl, S.; Bachmayr, V.; Kleissl, L.; et al. Antibiotic use and ileocolonic immune cells in patients receiving fecal microbiota transplantation for refractory intestinal GvHD: A prospective cohort study. Ther. Adv. Hematol. 2021, 12, 20406207211058333. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Qi, J.; Ma, X.; Qi, X.; Wu, D.; Xu, Y. Fecal microbiota transplantation combined with ruxolitinib as a salvage treatment for intestinal steroid-refractory acute GVHD. Exp. Hematol. Oncol. 2022, 11, 96. [Google Scholar] [CrossRef]
- Malard, F.; Loschi, M.; Huynh, A.; Cluzeau, T.; Guenounou, S.; Legrand, F.; Magro, L.; Orvain, C.; Charbonnier, A.; Panz-Klapuch, M.; et al. Pooled allogeneic faecal microbiota MaaT013 for steroid-resistant gastrointestinal acute graft-versus-host disease: A single-arm, multicentre phase 2 trial. EClinicalMedicine 2023, 62, 102111. [Google Scholar] [CrossRef]
- Yang, K.; Du, J.; Huang, F.; Si, Y.; Gu, Y.; Xu, N.; Fan, Z.; Xue, R.; Wang, P.; Yao, X.; et al. Fecal microbiota transplantation for refractory chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation: A pilot open-label, non-placebo-controlled study. BMC Med. 2025, 23, 498. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Villanacci, V.; Reggiani-Bonetti, L.; Salviato, T.; Leoncini, G.; Cadei, M.; Albarello, L.; Caputo, A.; Aquilano, M.C.; Battista, S.; Parente, P. Histopathology of IBD Colitis. A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD). Pathologica 2021, 113, 39–53. [Google Scholar] [CrossRef]
- Parente, P.; Ascani, S.; Maiorano, B.A.; Zanelli, M.; Ciardiello, D. Letter: Be careful of gastrointestinal CMV infection in adverse events from ICIs therapy in solid tumours. Aliment. Pharmacol. Ther. 2023, 57, 916–917. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Kozuch, P.L.; Hanauer, S.B. Treatment of inflammatory bowel disease: A review of medical therapy. World J. Gastroenterol. 2008, 14, 354–377. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Jauregui-Amezaga, A.; Smet, A. The Microbiome in Inflammatory Bowel Disease. J. Clin. Med. 2024, 13, 4622. [Google Scholar] [CrossRef]
- Imdad, A.; Pandit, N.G.; Zaman, M.; Minkoff, N.Z.; Tanner-Smith, E.E.; Gomez-Duarte, O.G.; Acra, S.; Nicholson, M.R. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2023, 4, CD012774. [Google Scholar]
- Igbo, C.A.; Ezeano, C.; Adeniran, O.; Taha, M.; Annan, A.A.; Nriagu, V.C.; Boateng, S.; Williams, M.C.; Onyali, C. The impact of fecal microbiota transplantation on refractory ulcerative colitis: A systematic review and Meta-Analysis of randomised controlled trials. BMC Gastroenterol. 2025, 25, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Y.; Zhang, Y.; Zhao, T.; Cong, J. Fecal microbiota transplantation for induction of remission in Crohn’s disease: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2023, 38, 62. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Arafat Hossain, M. A comprehensive review of immune checkpoint inhibitors for cancer treatment. Int. Immunopharmacol. 2024, 143, 113365. [Google Scholar] [CrossRef] [PubMed]
- Parente, P.; Maiorano, B.A.; Ciardiello, D.; Cocomazzi, F.; Carparelli, S.; Guerra, M.; Ingravallo, G.; Cazzato, G.; Carosi, I.; Maiello, E.; et al. Clinic, Endoscopic and Histological Features in Patients Treated with ICI Developing GI Toxicity: Some News and Reappraisal from a Mono-Institutional Experience. Diagnostics 2022, 12, 685. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaïfaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef]
- Zhou, Y.; Medik, Y.B.; Patel, B.; Zamler, D.B.; Chen, S.; Chapman, T.; Schneider, S.; Park, E.M.; Babcock, R.L.; Chrisikos, T.T.; et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 2023, 220, e20221333. [Google Scholar] [CrossRef]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e22. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Herrera, L.N.; Tang, T.; Altan, M.; Chaftari, A.P.; Okhuysen, P.C.; Jenq, R.R.; Wang, Y. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J. Immunother. Cancer 2019, 7, 242. [Google Scholar] [CrossRef]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, N.; Luo, Q.; Jiang, L.; He, B.; Yuan, X.; Shen, L. Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef]
- Hamilton, B.K. Updates in chronic graft-versus-host disease. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 648–654. [Google Scholar] [CrossRef]
- Wong, N.A. Gastrointestinal pathology in transplant patients. Histopathology 2015, 66, 467–479. [Google Scholar] [CrossRef]
- Patil, P.A.; Zhang, X. Pathologic Manifestations of Gastrointestinal and Hepatobiliary Injury in Immune Checkpoint Inhibitor Therapy. Arch. Pathol. Lab. Med. 2021, 145, 571–582. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Biliński, J.; Jasiński, M.; Basak, G.W. The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines 2022, 10, 837. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, Y.; Wu, D. Fecal microbiota transplantation for graft-versus-host disease: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 587. [Google Scholar]
- Wekking, D.; Ende, T.V.D.; Bijlsma, M.F.; Vidal-Itriago, A.; Nieuwdorp, M.; van Laarhoven, H.W.M. Fecal microbiota transplantation to enhance cancer treatment outcomes across different cancer types: A systematic literature review. Cancer Treat. Rev. 2025, 140, 103025. [Google Scholar] [CrossRef] [PubMed]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Bryant, R.V.; Costello, S.P.; Day, A.S.; Forbes, B.; Haifer, C.; Hold, G.; Kelly, C.R.; Li, A.; Pakuwal, E.; et al. Translational strategies for oral delivery of faecal microbiota transplantation. Gut 2025, 74, 2096–2117. [Google Scholar] [CrossRef]
| Authors | Patient Population | Study Design | Number of Recipients | FMT Adminitration/Protocol | Donor Type | Primary Outcome | Major Findings |
|---|---|---|---|---|---|---|---|
| Rossen et al. [6] | Mild to moderate active UC | Double-blind, randomised, placebo-controlled | 50 | Nasoduodenal tube | Own FMT VS healthy donors FMT | Clinical remission | No statistical difference |
| Moayyedi et al. [7] | Active UC without infectious diarrhea | Double-blind, randomised, placebo-controlled | 75 | Via enema | Plalcebo VS healthy donors FMT | Clinical remission | Clinical remission in the FMT group |
| Paramsothy et al. [8] | Active UC | Double-blind, randomised, placebo-controlled | 85 | Colonoscopy followed by enema | Plalcebo VS healthy multidonors FMT | Clinical remission | Clinical remission in the FMT group |
| Costello et al. [9] | Mild to moderate active UC | Double-blind, randomised, placebo-controlled | 73 | Colonoscopy followed by enema | Own FMT VS healthy donors FMT | Clinical remission | Clinical remission in the FMT group |
| Sood et al. [10] | Clinical remission UC | Double-blind, randomised, placebo-controlled | 61 | Colonoscopy | Plalcebo VS healthy donors FMT | Maintain clinical remission | Clinical remission in the FMT group |
| Crothers et al. [11] | Active UC | Double-blind, randomised, placebo-controlled | 12 | Oral administration of frozen encapsulated FMT | Plalcebo VS healthy donors cFMT | Clinical remission | Safety |
| Haifer et al. [12] | Active UC | Double-blind, randomised, placebo-controlled | 35 | Oral administration of lyophilised encapsulated FMT | Plalcebo VS healthy donors lyophilised FMT | Clinical remission with endoscopic remission or response | Clinical remission in the FMT group |
| van Lingen et al. [13] | Active mild to moderate disease | Double-blind, randomised, placebo-controlled | 24 | Colonoscopy | Plalcebo VS healthy donors FMT | Engraftment of donor microbiota after FMT | Pretreatment with budesonide did not significantly influence engraftment or clinical response |
| Stallmach et al. [14] | Mild to moderate active UC | Double-blind, randomised, placebo-controlled | 174 | Oral administration of frozen encapsulated FMT | Placebo VS sterile FMT VS healthy donors FMT | Clinical remission | Microbial and immunologic changes after FMT |
| Raja et al. [15] | Resistant ulcerative proctitis (UP) or distal UC | Double-blind, randomised, placebo-controlled | 30 | Via enema | Single-donor FMT | Safety and tolerability of FMT therapy | FMT enema was well tolerated and efficacy in inducing clinical remission |
| Sokol et al. [16] | CD in clinical remission | Single-blind, randomized, placebo-controlled | 17 | Colonoscopy | Plalcebo VS healthy donors FMT | Clinical remission | No statistical difference |
| Kao et al. [17] | Mild-to-moderate CD | Double-blind, randomised, placebo-controlled | 44 | Colonoscopy followed by capsules | Plalcebo VS healthy donors FMT | Clinical and endoscopic remission | No statistical difference |
| Chukhlovin et al. [18] | Remission-to-high activity CD | Case series | 15 | Oral administration of frozen encapsulated FMT | Healthy donors FMT | Clinical response | Ten patients with complete clinical response |
| Wang et al. [19] | * Urothelial carcinoma refractory to standard chemotherapy threated with CTLA-4 and PD-1; ** Prostate cancer refractory to chemotherapy and hormonal therapy who received two doses of ipilimumab | Case series | 2 | * Colonoscopy single dose; ** two doses of FMT | Single healthy donor FMT | Complete resolution of clinical symptoms following treatment with FMT | *Complete resolution; **Complete resolution after the second FMT |
| Davar et al. [20] | Melanoma patients refractory to anti–PD-1 therapy | Case series | 15 | Colonoscopy | FMT from anti-PD-1 responder and anti–PD-1 therapy | FMT salvage therapy | Six of 15 patients with clinical benefit |
| Baruch et al. [21] | Melanoma patients refractory to anti–PD-1 therapy | Case series | 10 | Colonoscopy | FMT from anti-PD-1 responder and anti–PD-1 therapy | FMT salvage therapy | Three of 10 patients with clinical benefit |
| Halsey et al. [22] | Different cancers treated with CTLA-4 and PD-1 or combined drugs | Case series | 12 | Colonoscopy | FMT from healthy donors | FMT salvage therapy | Ten patients achieved symptom improvement after FMT, including seven patients who had a complete clinical response. Three patients required repeat FMT, one of which had response. At the end of the study period, 92% achieved immune-mediated colitis clinical remission |
| Elkrief et al. [23] | Different cancers treated with CTLA-4 and PD-1 or combined drugs | Case series | 5 | Colonoscopy | FMT from healthy donors | FMT salvage therapy | Four of 5 patients exhibited improvement in immune checkpoint inhibitors Colitis symptoms following FMT |
| Kim et al. [24] | Different cancers treated with PD-1 | Case series | 13 | Colonoscopy | FMT from anti-PD-1 responder | FMT salvage therapy | Six of 13 patients acghieved clinical benefits |
| Kakihana et al. [25] | GI-GVHD | Case series | 4 | Nasoduodenal tube | FMT from healthy donors | FMT salvage therapy | Three achieved complete responses; one partial responses |
| Spindelboeck et al. [26] | GI-GVHD | Case series | 9 | Colonoscopy | FMT from healthy donors | FMT salvage therapy | Four patients with clinical response and was observed a change in immune cell patterns |
| Zhao et al. [27] | GI-GVHD | Open-label, non-randomized | 41 | Nasoduodenal tube | None FMT VS healthy donors FMT | Clinical remission | Clinical remission in the FMT group |
| Liu et al. [28] | GI-GVHD threated with Ruxolitinib | Case series | 21 | Oral administration of frozen encapsulated FMT | FMT from healthy donors | FMT salvage therapy | Fifteen patients with clinical response, 10 of them with complete response |
| Malard et al. [29] | GI-GVHD | Case series | 76 | Via rectal catheter | Pooled allogeneic faecal microbiota MaaT013 at leat one dose | FMT salvage therapy | Thirtynine patients with clinical response, 22 of them with complete response |
| Yang et al. [30] | GI-GVHD | Case series | 12 | Colonoscopy | FMT from healthy donors | FMT salvage therapy | Six patients with clinical response, 1 of them with complete response |
| Chukhlovin et al. [18] | GI-GVHD | Case series | 12 | Oral administration of frozen encapsulated FMT | Healthy donors FMT | Clinical response | Five patients with complete clinical response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscaglia, G.; Gentile, A.; Parente, P.; Calvo, A.; Fontana, R.; Continisio, A.; Di Brina, A.L.P.; Ciardiello, D.; McIlwain, G.; Latiano, A.; et al. Inflammatory Bowel Disease, Gastrointestinal Graft-Versus-Host Disease and Immune Checkpoint Inhibitors Induced Colitis: Similar Diseases to Treat with Fecal Microbiota Transplantation. Nutrients 2025, 17, 3788. https://doi.org/10.3390/nu17233788
Biscaglia G, Gentile A, Parente P, Calvo A, Fontana R, Continisio A, Di Brina ALP, Ciardiello D, McIlwain G, Latiano A, et al. Inflammatory Bowel Disease, Gastrointestinal Graft-Versus-Host Disease and Immune Checkpoint Inhibitors Induced Colitis: Similar Diseases to Treat with Fecal Microbiota Transplantation. Nutrients. 2025; 17(23):3788. https://doi.org/10.3390/nu17233788
Chicago/Turabian StyleBiscaglia, Giuseppe, Annamaria Gentile, Paola Parente, Annamaria Calvo, Rosanna Fontana, Antonio Continisio, Anna Laura Pia Di Brina, Davide Ciardiello, Gillian McIlwain, Anna Latiano, and et al. 2025. "Inflammatory Bowel Disease, Gastrointestinal Graft-Versus-Host Disease and Immune Checkpoint Inhibitors Induced Colitis: Similar Diseases to Treat with Fecal Microbiota Transplantation" Nutrients 17, no. 23: 3788. https://doi.org/10.3390/nu17233788
APA StyleBiscaglia, G., Gentile, A., Parente, P., Calvo, A., Fontana, R., Continisio, A., Di Brina, A. L. P., Ciardiello, D., McIlwain, G., Latiano, A., Perri, F., & Palmieri, O. (2025). Inflammatory Bowel Disease, Gastrointestinal Graft-Versus-Host Disease and Immune Checkpoint Inhibitors Induced Colitis: Similar Diseases to Treat with Fecal Microbiota Transplantation. Nutrients, 17(23), 3788. https://doi.org/10.3390/nu17233788






