Exploring Dietary Supplement Utilization Patterns Among African American Survivors of Prostate and Breast Cancer: A Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Eligibility, and Recruitment
2.2. Measures
2.3. Statistical Analyses
3. Results
3.1. Study Population Characteristics
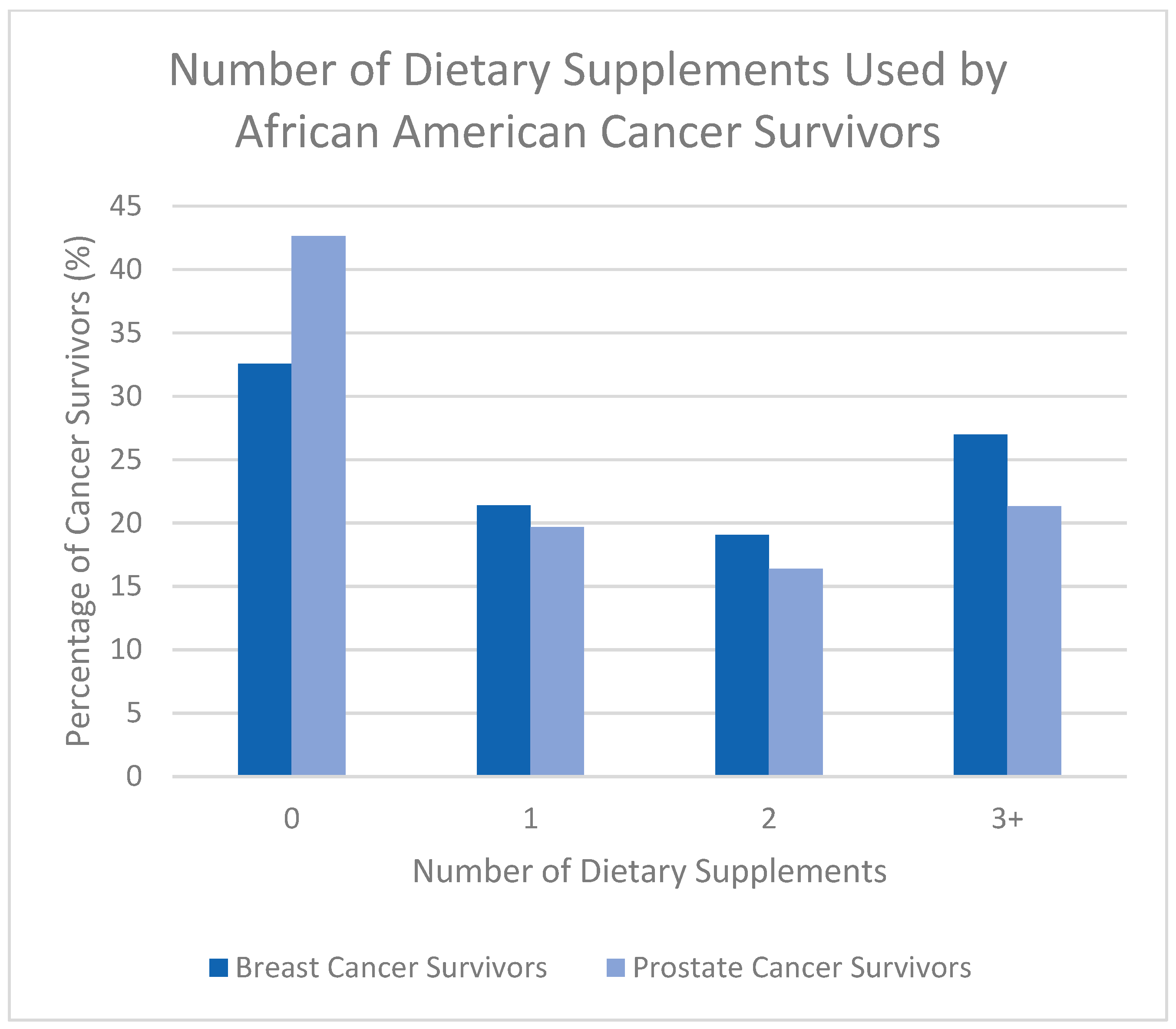
3.2. Prevalence of Dietary Supplement Use
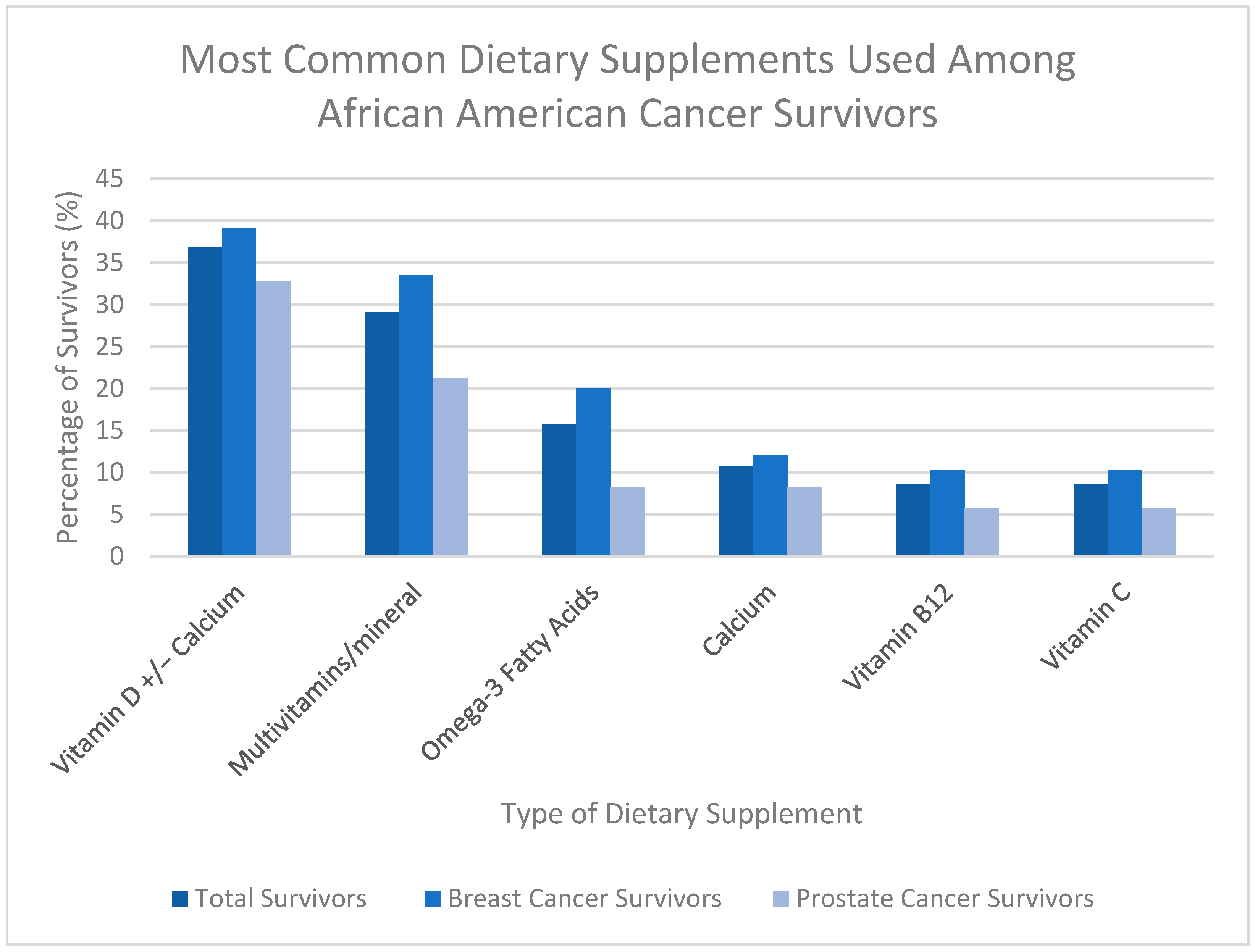
3.3. Types of Dietary Supplements Used
3.4. Predictors of Dietary Supplement Use
3.4.1. Prostate Cancer Survivors
3.4.2. Breast Cancer Survivors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | American Cancer Society |
| AIC | Akaike Information Criterion |
| BC | Breast cancer |
| BMI | Body mass index |
| CI | Confidence Interval(s) |
| cm | Centimeter(s) |
| DGA | Dietary Guidelines for Americans |
| DS | Dietary supplement(s) |
| DSHEA | Dietary Supplement Health and Education Act |
| FDA | Food and Drug Administration |
| HEI | Healthy Eating Index |
| Kg | Kilogram(s) |
| m2 | Meters squared |
| MF | Moving Forward |
| MMF | Men Moving Forward |
| N | Number |
| NHANES | National Health and Nutrition Examination Survey |
| OR | Odds ratio(s) |
| PC | Prostate cancer |
| PSA | Prostate-specific antigen |
| SD | Standard deviation |
| U.S. | United States of America |
Appendix A
| Dietary Supplement | Overall, N = 376 1 | MF, N = 246 1 | MMF, N = 130 1 |
|---|---|---|---|
| Multivitamin | 63 | 44 | 19 |
| B complex | 8 | 6 | 2 |
| Alive Multivitamin | 2 | 2 | 0 |
| Hair & Nails Multivitamin | 1 | 1 | 0 |
| Other multivitamin type | 8 | 7 | 1 |
| Unknown multivitamin type | 47 | 31 | 16 |
| Multivitamins with multiminerals | 41 | 33 | 8 |
| Centrum | 20 | 14 | 6 |
| GNC brand | 3 | 3 | 0 |
| One A Day | 19 | 17 | 2 |
| Other multivitamins with multiminerals type | 1 | 1 | 0 |
| Unknown multivitamins with multiminerals type | 1 | 1 | 0 |
| Biotin | 8 | 8 | 0 |
| Calcium | 36 | 26 | 10 |
| Calcium acetate | 1 | 0 | 1 |
| Calcium carbonate | 2 | 2 | 0 |
| Calcium citrate | 1 | 1 | 0 |
| Oyster shell calcium | 1 | 1 | 0 |
| Other calcium type | 1 | 1 | 0 |
| Unknown calcium type | 31 | 22 | 9 |
| Folic acid | 7 | 4 | 3 |
| Iron | 18 | 12 | 6 |
| Ferrous sulfate | 8 | 5 | 3 |
| Other iron type | 1 | 1 | 0 |
| Unknown iron type | 9 | 6 | 3 |
| Magnesium | 8 | 4 | 4 |
| Potassium | 18 | 13 | 5 |
| Potassium phosphate (KPhos) | 1 | 0 | 1 |
| Potassium chloride (KCI) | 8 | 6 | 2 |
| Other potassium type | 1 | 1 | 0 |
| Unknown potassium type | 9 | 7 | 2 |
| Selenium | 1 | 1 | 0 |
| Trace mineral combination | 3 | 3 | 0 |
| GTF chromium | 1 | 1 | 0 |
| Chromium picolinate | 1 | 1 | 0 |
| Unknown trace mineral combination type | 1 | 1 | 0 |
| Vitamin B6/pyridoxine | 2 | 2 | 0 |
| Vitamin B12/cobalamin | 29 | 22 | 7 |
| Vitamin C | 29 | 22 | 7 |
| Vitamin D | 92 | 55 | 37 |
| Calcitriol (D3) | 1 | 0 | 1 |
| Cholecalciferol (D3) | 37 | 17 | 20 |
| Ergocalciferol (D2) | 1 | 0 | 1 |
| Unknown Vitamin D type | 53 | 38 | 15 |
| Vitamin E | 5 | 4 | 1 |
| Zinc | 3 | 1 | 2 |
| Vitamin–mineral combination | 36 | 32 | 4 |
| Calcium/Vitamin D | 33 | 30 | 3 |
| Other vitamin–mineral combination type | 3 | 2 | 1 |
| Vitamin K | 1 | 1 | 0 |
| Omega-3 fatty acids | 53 | 43 | 10 |
| Flaxseed | 5 | 3 | 2 |
| Fish oil (EPA or DHA) | 29 | 23 | 6 |
| Mega Red | 2 | 2 | 0 |
| Cod liver oil | 3 | 3 | 0 |
| Other omega-3 fatty acids type | 3 | 2 | 1 |
| Unknown omega-3 fatty acids type | 17 | 15 | 2 |
| Cinnamon | 5 | 3 | 2 |
| Curcumin | 3 | 2 | 1 |
| Curamed | 1 | 0 | 1 |
| Other curcumin type | 1 | 1 | 0 |
| Unknown curcumin type | 1 | 1 | 0 |
| Garlic | 2 | 2 | 0 |
| Green tea | 1 | 1 | 0 |
| Neem Leaves Tea | 1 | 0 | 1 |
| Turmeric | 4 | 0 | 4 |
| Amino acids | 4 | 2 | 2 |
| Arginine | 1 | 0 | 1 |
| Other amino acids type | 4 | 2 | 2 |
| Cherry extract | 1 | 0 | 1 |
| Uric Acid Support | 1 | 0 | 1 |
| Papaya enzyme | 1 | 1 | 0 |
| Raspberry ketone | 2 | 2 | 0 |
| Probiotics | 4 | 4 | 0 |
| Prebiotics | 1 | 1 | 0 |
| Garcinia Cambogia | 1 | 0 | 1 |
| OxyElite Pro | 1 | 1 | 0 |
| Meal replacement | 1 | 1 | 0 |
| Fruit and Veggie Pills | 1 | 1 | 0 |
| Collagen | 1 | 1 | 0 |
| Antacids | 1 | 1 | 0 |
| Tums | 1 | 1 | 0 |
| Coenzyme Q10 | 7 | 4 | 3 |
| Fiber | 6 | 2 | 4 |
| Metamucil | 2 | 1 | 1 |
| MiraLAX | 3 | 0 | 3 |
| Unknown fiber type | 1 | 1 | 0 |
| Glucosamine chondroitin | 10 | 7 | 3 |
| Sodium bicarbonate | 3 | 0 | 3 |
| Body Balance | 1 | 1 | 0 |
| Raw Enzymes | 3 | 3 | 0 |
| Immune Balance Sinus | 1 | 1 | 0 |
| Remifemin | 1 | 1 | 0 |
| Aspen Silymarin | 1 | 1 | 0 |
| St Johns Wort | 1 | 1 | 0 |
| Ocuvite | 1 | 1 | 0 |
| Gingko Biloboa | 1 | 1 | 0 |
| Coffee extract | 1 | 1 | 0 |
| Echinacea root | 1 | 1 | 0 |
| Cleanse Smart | 1 | 1 | 0 |
| Focus Factor | 2 | 2 | 0 |
| Melatonin | 4 | 1 | 3 |
| Chia seeds | 1 | 1 | 0 |
| Emergen-C | 1 | 1 | 0 |
| Coconut oil | 1 | 1 | 0 |
| Spirulina | 1 | 1 | 0 |
| Aloe Vera | 1 | 1 | 0 |
| Ginger | 1 | 0 | 1 |
| Specialty vitamins | 1 | 0 | 1 |
| Vitamin D Nature Made | 1 | 0 | 1 |
| Purge (Cherry/Celery) | 1 | 0 | 1 |
| Tart Cherry | 1 | 0 | 1 |
| Glucomannan | 1 | 0 | 1 |
| Vitaeyes | 1 | 0 | 1 |
| Moringa | 1 | 0 | 1 |
| Black Seed Oil | 1 | 0 | 1 |
| Golden Seal | 1 | 0 | 1 |
| Elderberry | 1 | 0 | 1 |
| Oil of oregano | 1 | 0 | 1 |
| Vitamin C and rose hips | 1 | 0 | 1 |
References
- National Cancer Institute. Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 24 September 2025).
- Definitions|Division of Cancer Control and Population Sciences (DCCPS). Available online: https://cancercontrol.cancer.gov/ocs/definitions (accessed on 24 September 2025).
- American Cancer Society. Key Statistics for Prostate Cancer|Prostate Cancer Facts. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 24 September 2025).
- Centers for Disease Control and Prevention. Breast Cancer Risk Factors. CDC. Available online: https://www.cdc.gov/breast-cancer/risk-factors/index.html (accessed on 22 September 2025).
- Saka, A.H.; Giaquinto, A.N.; McCullough, L.E.; Tossas, K.Y.; Star, J.; Jemal, A.; Siegel, R.L. Cancer Statistics for African American and Black People, 2025. CA A Cancer J. Clin. 2025, 75, 111–140. [Google Scholar] [CrossRef]
- Hamblen, A.J.; Bray, J.W.; Hingorani, M.; Saxton, J.M. Physical Activity and Dietary Considerations for Prostate Cancer Patients: Future Research Directions. Proc. Nutr. Soc. 2023, 82, 298–304. [Google Scholar] [CrossRef]
- Khalifa, A.; Guijarro, A.; Nencioni, A. Advances in Diet and Physical Activity in Breast Cancer Prevention and Treatment. Nutrients 2024, 16, 2262. [Google Scholar] [CrossRef]
- Stolley, M.R.; Sharp, L.K.; Fantuzzi, G.; Arroyo, C.; Sheean, P.; Schiffer, L.; Campbell, R.; Gerber, B. Study Design and Protocol for Moving Forward: A Weight Loss Intervention Trial for African-American Breast Cancer Survivors. BMC Cancer 2015, 15, 1018. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society Nutrition and Physical Activity Guideline for Cancer Survivors. CA A Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. CA A Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Velicer, C.M.; Ulrich, C.M. Vitamin and Mineral Supplement Use among US Adults after Cancer Diagnosis: A Systematic Review. J. Clin. Oncol. 2008, 26, 665–673. [Google Scholar] [CrossRef]
- Conway, R.E.; Rigler, F.V.; Croker, H.A.; Lally, P.J.; Beeken, R.J.; Fisher, A. Dietary Supplement Use by Individuals Living with and beyond Breast, Prostate, and Colorectal Cancer: A Cross-Sectional Survey. Cancer 2021, 128, 1331–1338. [Google Scholar] [CrossRef]
- National Institute of Health. Vitamin C. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 24 September 2025).
- Chen, Q.-Y.; Kim, S.; Lee, B.; Jeong, G.; Lee, D.H.; Keum, N.; Manson, J.E.; Giovannucci, E.L. Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis. Nutrients 2022, 14, 3418. [Google Scholar] [CrossRef]
- Kanellopoulou, A.; Riza, E.; Samoli, E.; Benetou, V. Dietary Supplement Use after Cancer Diagnosis in Relation to Total Mortality, Cancer Mortality and Recurrence: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 16–30. [Google Scholar] [CrossRef]
- Thomson, C.A.; Aragaki, A.K.; Prentice, R.L.; Stefanick, M.L.; Manson, J.E.; Wactawski-Wende, J.; Watts, N.B.; Van Horn, L.; Shikany, J.M.; Rohan, T.E.; et al. Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women. Ann. Intern. Med. 2024, 177, 428–438. [Google Scholar] [CrossRef]
- Atoum, M.F.; Alzoughool, F.E.; Al-Mazaydeh, Z.A.; Rammaha, M.S.; Tahtamouni, L.H. Vitamin B12 Enhances the Antitumor Activity of 1,25-Dihydroxyvitamin D 3 via Activation of Caspases and Targeting Actin Cytoskeleton. Tumor Biol. 2022, 44, 17–35. [Google Scholar] [CrossRef]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Jaén, C.R.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer. JAMA 2022, 327, 2326. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire: Validity Evidence Supporting Its Use for Classifying Healthy Adults into Active and Insufficiently Active Categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Du, M.; Luo, H.; Blumberg, J.B.; Rogers, G.; Chen, F.; Ruan, M.; Shan, Z.; Biever, E.; Zhang, F.F. Dietary Supplement Use among Adult Cancer Survivors in the United States. J. Nutr. 2020, 150, 1499–1508. [Google Scholar] [CrossRef]
- Mishra, S.; Gahche, J.J.; Ogden, C.L.; Dimeler, M.; Potischman, N.; Ahluwalia, N. Dietary Supplement Use in the United States: National Health and Nutrition Examination Survey, 2017–March 2020; National Health Statistics Reports; no 183; National Center for Health Statistics: Hyattsville, MD, USA, 2023. [CrossRef]
- Abdel-Rahman, O. Dietary Supplements Use among Adults with Cancer in the United States: A Population-Based Study. Nutr. Cancer 2020, 73, 1856–1863. [Google Scholar] [CrossRef]
- Ferrucci, L.M.; McCorkle, R.; Smith, T.; Stein, K.D.; Cartmel, B. Factors Related to the Use of Dietary Supplements by Cancer Survivors. J. Altern. Complement. Med. 2009, 15, 673–680. [Google Scholar] [CrossRef]
- Kaur, H.; Hoenemeyer, T.; Parrish, K.B.; Demark-Wahnefried, W. Dietary Supplement Use among Older Cancer Survivors: Socio-Demographic Associations, Supplement Types, Reasons for Use, and Cost. Nutrients 2022, 14, 3402. [Google Scholar] [CrossRef]
- Miller, P.E.; Vasey, J.J.; Short, P.F.; Hartman, T.J. Dietary Supplement Use in Adult Cancer Survivors. Oncol. Nurs. Forum 2009, 36, 61–68. [Google Scholar] [CrossRef]
- Miller, P.; Demark-Wahnefried, W.; Snyder, D.C.; Sloane, R.; Morey, M.C.; Cohen, H.; Kranz, S.; Mitchell, D.C.; Hartman, T.J. Dietary Supplement Use among Elderly, Long-Term Cancer Survivors. J. Cancer Surviv. 2008, 2, 138–148. [Google Scholar] [CrossRef]
- Friedman, J.; Birstler, J.; Love, G.D.; Kiefer, D. Diagnoses Associated with Dietary Supplement Use in a National Dataset. Complement. Ther. Med. 2019, 43, 277–282. [Google Scholar] [CrossRef]
- Barnard, N.D.; Kahleova, H.; Becker, R. The Limited Value of Multivitamin Supplements. JAMA Netw. Open 2024, 7, e2418965. [Google Scholar] [CrossRef]
- Jung, A.Y.; Cai, X.; Thoene, K.; Obi, N.; Jaskulski, S.; Behrens, S.; Flesch-Janys, D.; Chang-Claude, J. Antioxidant Supplementation and Breast Cancer Prognosis in Postmenopausal Women Undergoing Chemotherapy and Radiation Therapy. Am. J. Clin. Nutr. 2019, 109, 69–78. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Gkiouras, K.; Papageorgiou, S.Τ.; Myrogiannis, I.; Mykoniatis, I.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. Dietary Factors and Supplements Influencing Prostate-Specific Antigen (PSA) Concentrations in Men with Prostate Cancer and Increased Cancer Risk: An Evidence Analysis Review Based on Randomized Controlled Trials. Nutrients 2020, 12, 2985. [Google Scholar] [CrossRef]
- Dwyer, J.; Coates, P.; Smith, M. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Assadourian, J.N.; Peterson, E.D.; Navar, A.M. Label Statements and Perceived Health Benefits of Dietary Supplements. JAMA Netw. Open 2025, 8, e2533118. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.; Brown, L.L.; Costello, R.B.; Deuster, P.A. Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review. Nutrients 2022, 14, 4604. [Google Scholar] [CrossRef] [PubMed]
- Veatch-Blohm, M.E.; Chicas, I.; Margolis, K.; Vanderminden, R.; Gochie, M.; Lila, K. Screening for Consistency and Contamination within and between Bottles of 29 Herbal Supplements. PLoS ONE 2021, 16, e0260463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Liu, J.; Hébert, J.R.; Giovannucci, E.; Zhang, X.; Steck, S.E. Trends in Dietary Supplement Use among U.S. Adults between 2011 and 2023. Eur. J. Nutr. 2025, 64, 304. [Google Scholar] [CrossRef]
| Characteristic | Overall, N = 376 1 | BC Survivors (MF), N = 246 | PC Survivors (MMF), N = 130 |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 60.33 (9.96) | 57.46 (10.09) | 65.76 (7.06) |
| Unknown | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Highest grade or year of school completed | |||
| Eighth grade or less | 3 (0.81%) | 0 (0.00%) | 3 (2.38%) |
| Some high school | 28 (7.53%) | 12 (4.88%) | 16 (12.70%) |
| High school graduate or GED | 77 (20.70%) | 47 (19.11%) | 30 (23.81%) |
| Associates degree or 2-year certificate | 49 (13.17%) | 39 (15.85%) | 10 (7.94%) |
| Some college | 95 (25.54%) | 54 (21.95%) | 41 (32.54%) |
| College graduate | 61 (16.40%) | 47 (19.11%) | 14 (11.11%) |
| Graduate or professional degree | 58 (15.59%) | 47 (19.11%) | 11 (8.73%) |
| Other | 1 (0.27%) | 0 (0.00%) | 1 (0.79%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| Combined family income for the last 12 months | |||
| Less than $20,000 | 74 (19.89%) | 58 (23.58%) | 16 (12.70%) |
| $20,000–$39,999 | 77 (20.70%) | 56 (22.76%) | 21 (16.67%) |
| $40,000–$59,999 | 63 (16.94%) | 48 (19.51%) | 15 (11.90%) |
| $60,000–$79,999 | 49 (13.17%) | 33 (13.41%) | 16 (12.70%) |
| $80,000 or more | 90 (24.19%) | 50 (20.33%) | 40 (31.75%) |
| Prefer not to answer/Refused | 19 (5.11%) | 1 (0.41%) | 18 (14.29%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| Marital status | |||
| Single, never married | 86 (23.18%) | 66 (26.94%) | 20 (15.87%) |
| Married or living with a partner | 162 (43.67%) | 95 (38.78%) | 67 (53.17%) |
| Separated | 18 (4.85%) | 13 (5.31%) | 5 (3.97%) |
| Divorced | 79 (21.29%) | 52 (21.22%) | 27 (21.43%) |
| Widowed | 26 (7.01%) | 19 (7.76%) | 7 (5.56%) |
| Unknown | 5 (1.33%) | 1 (0.41%) | 4 (3.08%) |
| Godin Score | |||
| Mean (SD) | 18.72 (18.64) | 16.87 (18.78) | 22.36 (17.88) |
| Unknown | 5 (1.33%) | 0 (0.00%) | 5 (3.85%) |
| Godin Score | |||
| <14: Insufficiently active (low benefits) | 216 (58.22%) | 175 (71.14%) | 41 (32.80%) |
| 14–23: Moderately active (some benefits) | 54 (14.56%) | 24 (9.76%) | 30 (24.00%) |
| ≥ 24: Active (substantial benefits) | 101 (27.22%) | 47 (19.11%) | 54 (43.20%) |
| Unknown | 5 (1.33%) | 0 (0.00%) | 5 (3.85%) |
| HEI score | |||
| Mean (SD) | 64.46 (11.30) | 65.08 (11.09) | 63.36 (11.64) |
| Unknown | 30 (7.98%) | 25 (10.16%) | 5 (3.85%) |
| HEI score | |||
| <51: Low diet quality | 43 (12.43%) | 23 (10.41%) | 20 (16.00%) |
| 51–80: Moderate diet quality | 269 (77.75%) | 174 (78.73%) | 95 (76.00%) |
| >80: High diet quality | 34 (9.83%) | 24 (10.86%) | 10 (8.00%) |
| Unknown | 30 (7.98%) | 25 (10.16%) | 5 (3.85%) |
| BMI | |||
| Mean (SD) | 34.31 (6.43) | 36.13 (6.25) | 30.72 (5.15) |
| Unknown | 6 (1.60%) | 0 (0.00%) | 6 (4.62%) |
| Total comorbidities | |||
| Mean (SD) | 2.38 (1.66) | 2.34 (1.64) | 2.48 (1.71) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| High blood pressure | 234 (62.90%) | 145 (58.94%) | 89 (70.63%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| Arthritis/back problems | 155 (41.67%) | 121 (49.19%) | 34 (26.98%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| High cholesterol | 153 (41.13%) | 93 (37.80%) | 60 (47.62%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| Diabetes | 94 (25.27%) | 56 (22.76%) | 38 (30.16%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| Sleep apnea | 68 (18.28%) | 42 (17.07%) | 26 (20.63%) |
| Unknown | 4 (1.06%) | 0 (0.00%) | 4 (3.08%) |
| Characteristic | Overall, N = 376 1,2 | BC Survivors (MF), N = 246 1,2 | PC Survivors (MMF), N = 130 1,2 |
|---|---|---|---|
| Cancer survivors taking at least 1 DS | |||
| 0 | 122 (36.20%) | 70 (32.56%) | 52 (42.62%) |
| 1+ | 215 (63.80%) | 145 (67.44%) | 70 (57.38%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Average number of DSs used | |||
| Mean (SD) | 1.68 (2.03) | 1.87 (2.15) | 1.36 (1.76) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Number of DSs used | |||
| 0 | 122 (36.20%) | 70 (32.56%) | 52 (42.62%) |
| 1 | 70 (20.77%) | 46 (21.40%) | 24 (19.67%) |
| 2 | 61 (18.10%) | 41 (19.07%) | 20 (16.39%) |
| 3+ | 84 (24.93%) | 58 (26.98%) | 26 (21.31%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Vitamin D or combination of Vitamin D and calcium use | 124 (36.80%) | 84 (39.07%) | 40 (32.79%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Multivitamin or multivitamin with multiminerals | 98 (29.08%) | 72 (33.49%) | 26 (21.31%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Calcium | 36 (10.68%) | 26 (12.09%) | 10 (8.20%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Omega-3 fatty acids | 51 (15.73%) | 43 (20.00%) | 10 (8.20%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| Vitamin B12/Cobalamin | 29 (8.63%) | 22 (10.28%) | 7 (5.74%) |
| Unknown | 40 (10.64%) | 32 (13.01%) | 8 (6.15%) |
| Vitamin C | 29 (8.61%) | 22 (10.23%) | 7 (5.74%) |
| Unknown | 39 (10.4%) | 31 (12.60%) | 8 (6.15%) |
| (a) | |||
| Characteristic | OR 2 | 95% CI 2 | p-Value 3 |
| Age | 1.06 | 0.997, 1.13 | 0.067 |
| Education | |||
| Graduate or professional degree | — | — | |
| Eighth grade or less/some high school | 0.12 | 0.02, 0.72 | 0.025 |
| High school graduate or GED | 0.80 | 0.15, 4.11 | 0.794 |
| Associates degree or 2-year certificate | 0.51 | 0.06, 4.60 | 0.540 |
| Some college | 0.38 | 0.07, 1.76 | 0.230 |
| College graduate | 0.84 | 0.11, 6.48 | 0.861 |
| Other | 0.00 | 0.00, Inf | 0.990 |
| HEI Score | |||
| <51: Low diet quality | — | — | |
| 51–80: Moderate diet quality | 0.42 | 0.11, 1.41 | 0.177 |
| >80: High diet quality | 0.05 | 0.01, 0.32 | 0.003 |
| Total Comorbidities | 1.51 | 1.16, 2.03 | 0.004 |
| (b) | |||
| Characteristic | OR 2 | 95% CI 2 | p-Value 3 |
| Education | |||
| Graduate or professional degree | — | — | |
| Eighth grade or less/some high school | 1.06 | 0.23, 5.89 | 0.939 |
| High school graduate or GED | 1.29 | 0.47, 3.63 | 0.623 |
| Associates degree or 2-year certificate | 0.40 | 0.15, 1.01 | 0.055 |
| Some college | 0.90 | 0.36, 2.25 | 0.823 |
| College graduate | 1.90 | 0.70, 5.46 | 0.218 |
| HEI Score | |||
| <51: Low diet quality | — | — | |
| 51–80: Moderate diet quality | 2.62 | 0.99, 6.88 | 0.050 |
| >80: High diet quality | 2.25 | 0.63, 8.38 | 0.213 |
| Missing | 0.91 | 0.26, 3.11 | 0.879 |
| Total Comorbidities | 1.30 | 1.07, 1.60 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranjac, C.A.; Sheean, P.; Banerjee, A.; Teng, B.Q.; O'Connell, K.; Tovar, M.; Alonso, E.; Snider, Z.; Stolley, M. Exploring Dietary Supplement Utilization Patterns Among African American Survivors of Prostate and Breast Cancer: A Cross-Sectional Analysis. Nutrients 2025, 17, 3724. https://doi.org/10.3390/nu17233724
Kranjac CA, Sheean P, Banerjee A, Teng BQ, O'Connell K, Tovar M, Alonso E, Snider Z, Stolley M. Exploring Dietary Supplement Utilization Patterns Among African American Survivors of Prostate and Breast Cancer: A Cross-Sectional Analysis. Nutrients. 2025; 17(23):3724. https://doi.org/10.3390/nu17233724
Chicago/Turabian StyleKranjac, Carlene A., Patricia Sheean, Anjishnu Banerjee, Bi Qing Teng, Kathleen O'Connell, Margaret Tovar, Estefania Alonso, Zoe Snider, and Melinda Stolley. 2025. "Exploring Dietary Supplement Utilization Patterns Among African American Survivors of Prostate and Breast Cancer: A Cross-Sectional Analysis" Nutrients 17, no. 23: 3724. https://doi.org/10.3390/nu17233724
APA StyleKranjac, C. A., Sheean, P., Banerjee, A., Teng, B. Q., O'Connell, K., Tovar, M., Alonso, E., Snider, Z., & Stolley, M. (2025). Exploring Dietary Supplement Utilization Patterns Among African American Survivors of Prostate and Breast Cancer: A Cross-Sectional Analysis. Nutrients, 17(23), 3724. https://doi.org/10.3390/nu17233724







