Bio-Assay-Guided Study of Chaenomeles japonica–Cytokine Modulation by Fruit Aqueous Extract In Vitro in Connection with Its Processing with Enzymatic and Microbial Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material Collection and Extract Preparation
2.3. Phytochemical Analysis
2.3.1. UHPLC-DAD-MS/MS Conditions
2.3.2. Total Phenol Content
2.3.3. Total Flavonoid Content
2.4. Cytokine Secretion in PMN, PBMC, and Caco-2
2.4.1. PMN Culture
2.4.2. PBMC Culture
2.4.3. Caco-2 Cell Culture
2.4.4. The Enzyme-Linked Immunosorbent Assays
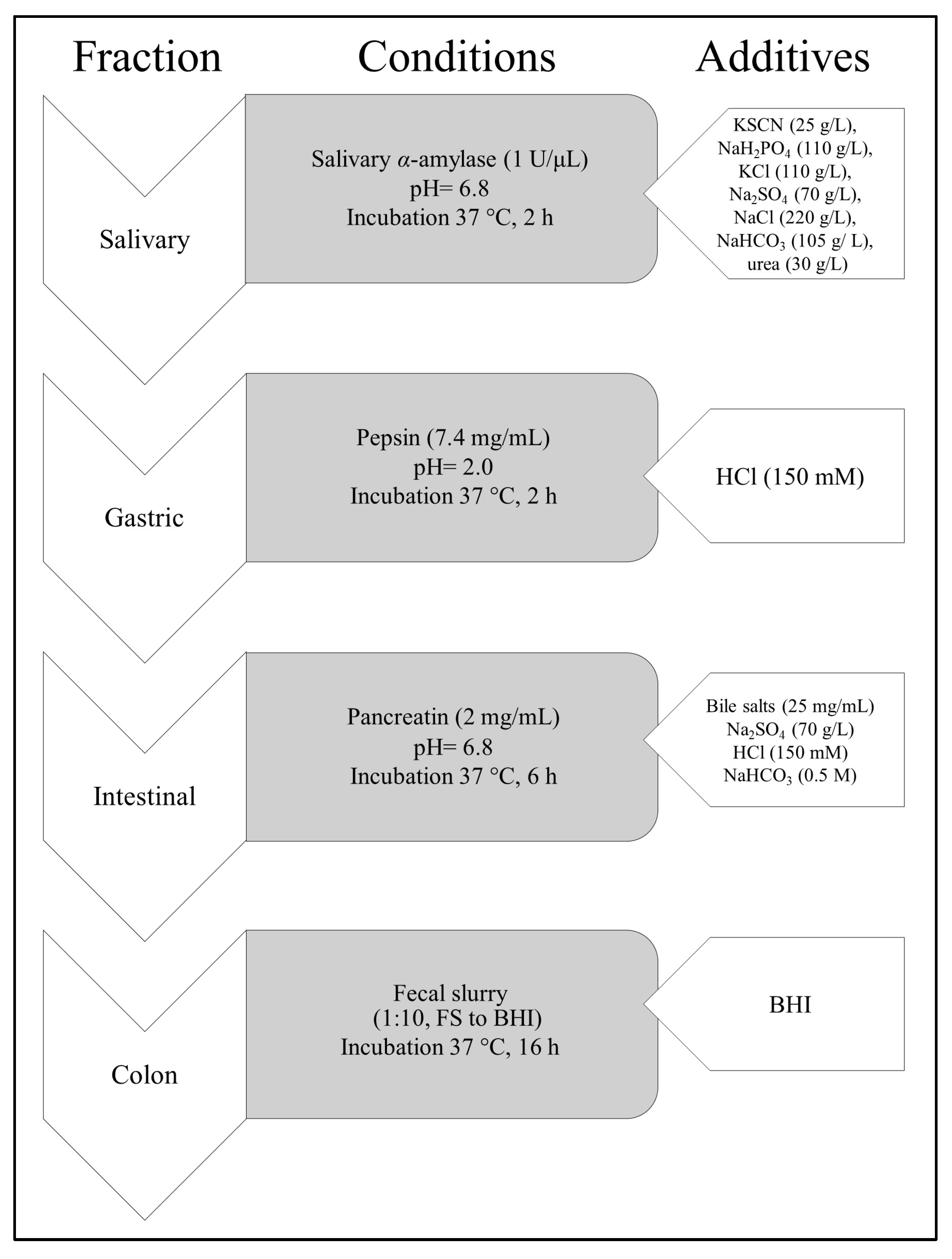
2.5. Digestion Procedure In Vitro
2.6. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of CJ Extracts
3.2. The Quantitative Analysis of Extracts
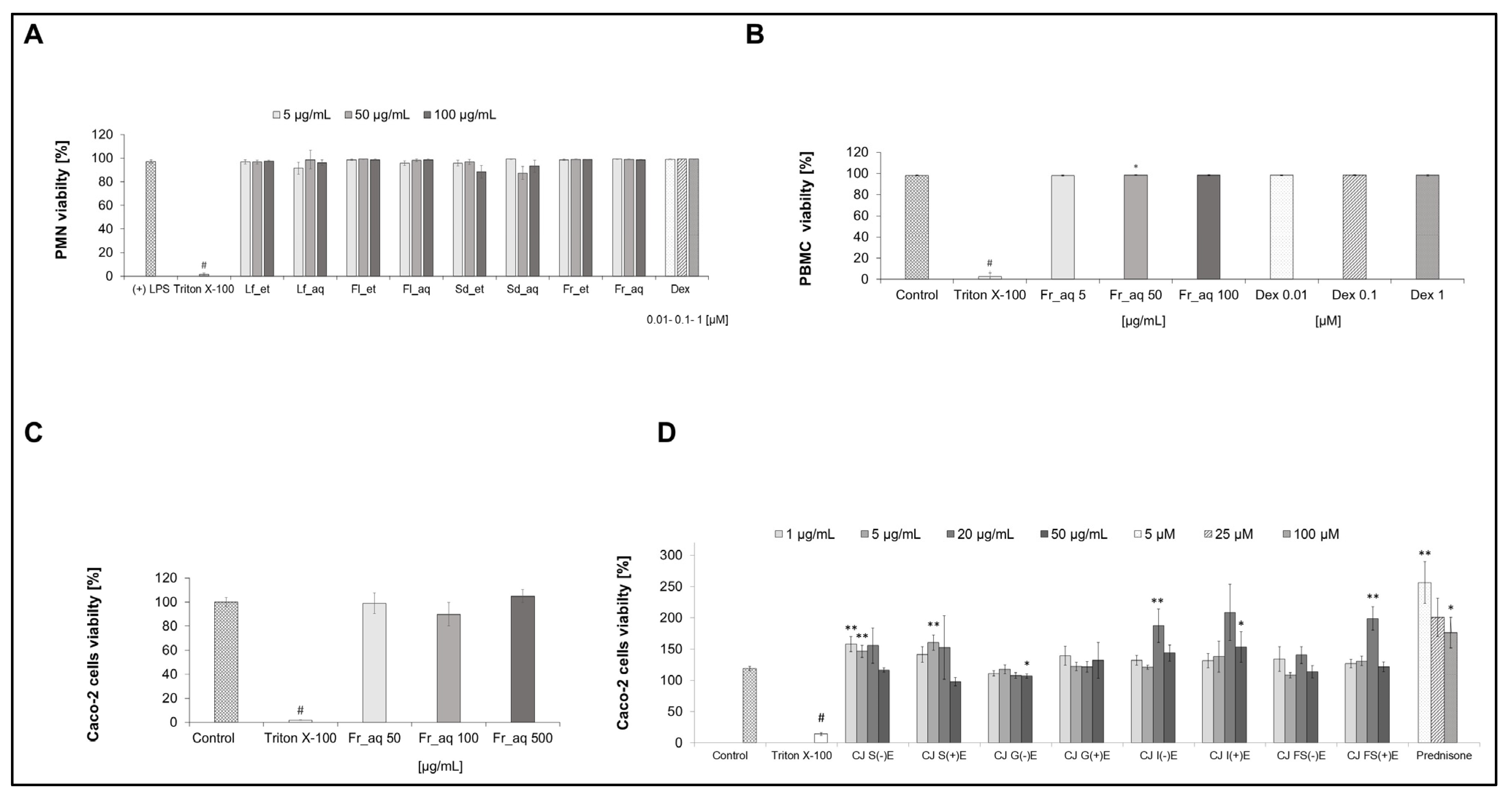
3.3. Cell Viability After Treatment with Chaenomeles japonica Extracts and GI Fractions
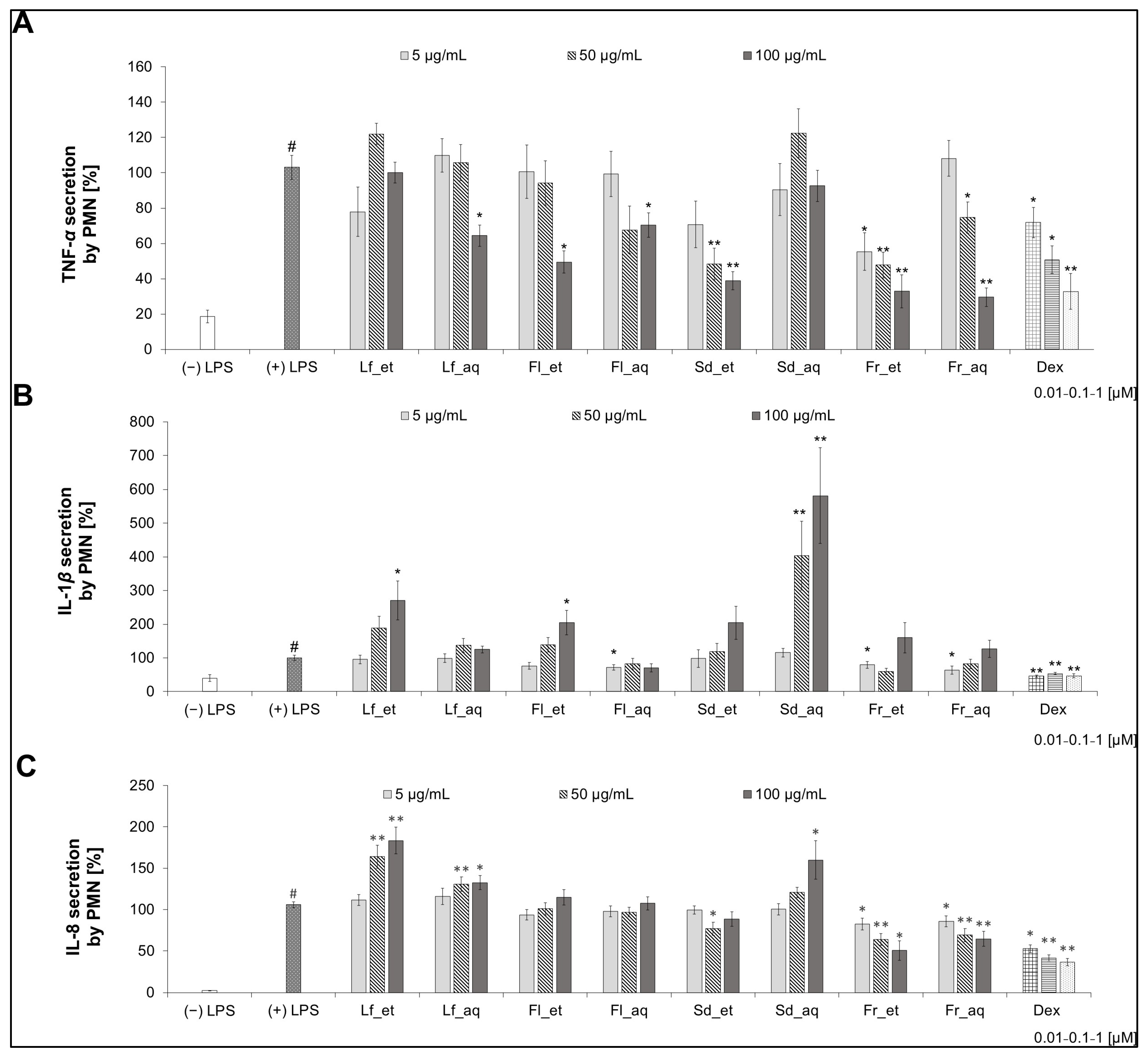
3.4. Activity of Extracts from Different Parts of CJ in PMN After Chaenomeles japonica Extract Treatment
3.4.1. TNF-α Secretion
3.4.2. IL-1β Secretion
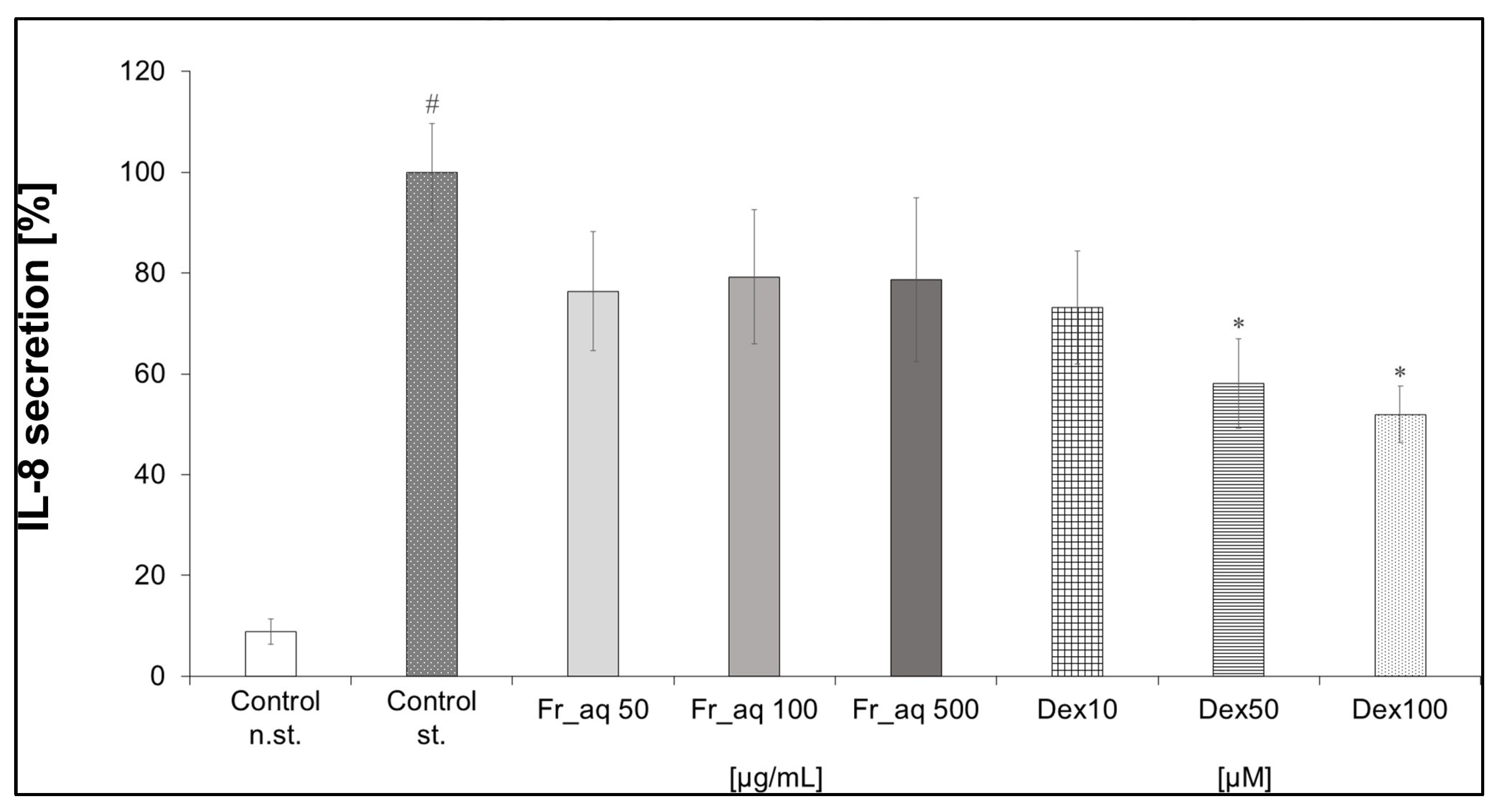
3.4.3. IL-8 Secretion
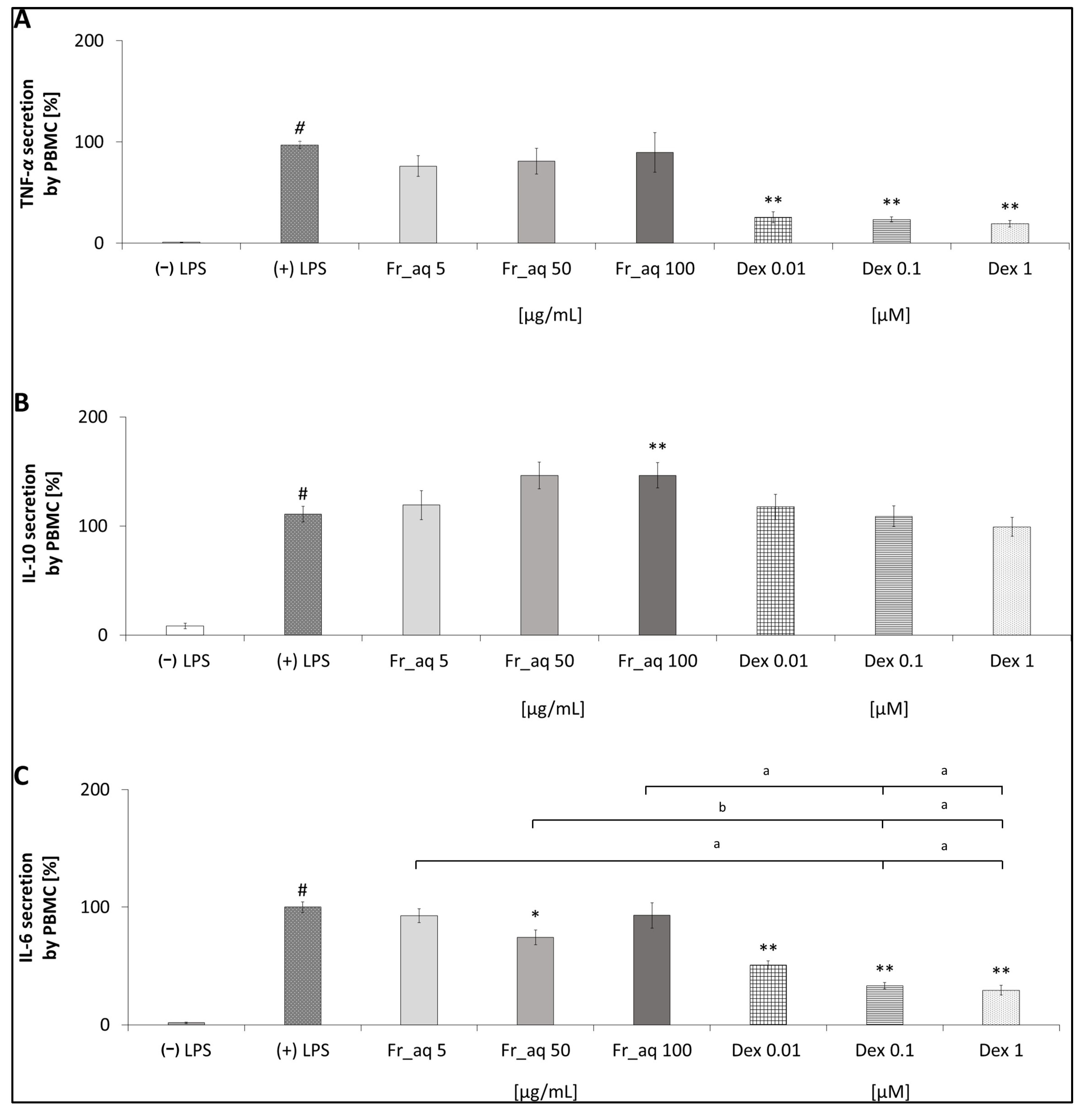
3.5. Secretion of Cytokines in PBMC After the CJ Fruit Extract Treatment
3.6. Cytokine Secretion in Caco-2 Cells After Treatment with Aqueous Fruit Extract and GI Fractions
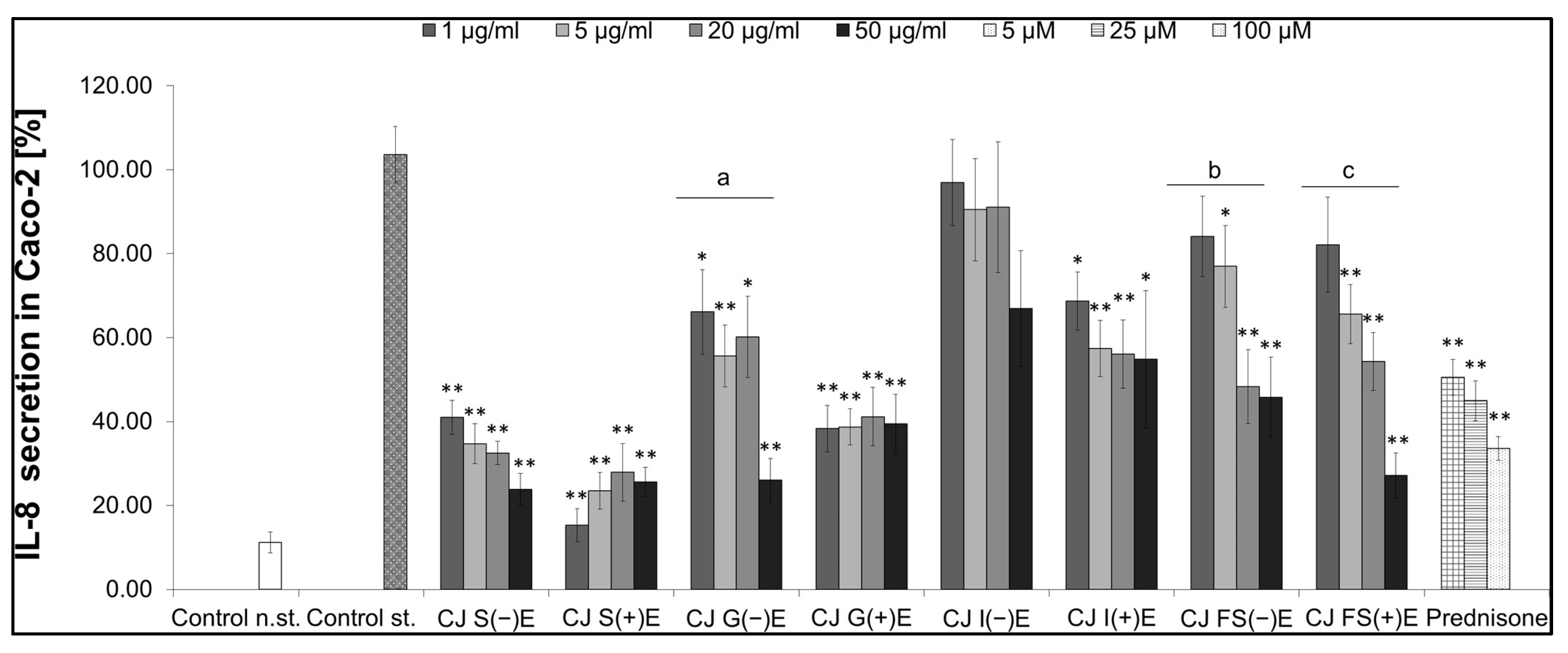
3.6.1. IL-8 Secretion
3.6.2. Activity of GI Fractions from Aqueous CJ Fruit Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CJ | Chaenomeles japonica |
| Dex | Dexamethasone |
| IBDs | Inflammatory bowel disease |
| IL-1β | Interleukin 1β |
| IL-8 | Interleukin 8 |
| LPS | Lipopolysaccharide |
| MIC | Minimal inhibitory concentration |
| NF-κβ | Nuclear factor κβ |
| PAMPs | Pathogen-associated molecular patterns |
| PBMC | Peripheral blood mononuclear cells |
| PMN | Polymorphonuclear leukocytes |
| SHIME | Simulator of the Human Intestinal Microbial Ecosystem |
| TCM | Traditional Chinese Medicine |
| TNF-α | Tumor necrosis factor α |
References
- Marat, N.; Danowska-Oziewicz, M.; Narwojsz, A. Chaenomeles Species—Characteristics of Plant, Fruit and Processed Products: A Review. Plants 2022, 11, 3036. [Google Scholar] [CrossRef] [PubMed]
- Urbanaviciute, I.; Rubinskiene, M.; Viskelis, P. The Fatty Acid Composition and Quality of Oils from Post-Industrial Waste of Quince Chaenomeles japonica. Chem. Biodivers. 2019, 16, e1900352. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses Throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Wera, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Borowitz, S.M. The Epidemiology of Inflammatory Bowel Disease: Clues to Pathogenesis? Front. Pediatr. 2022, 10, 1103713. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar]
- Olędzka, A.J.; Czerwińska, M.E. Role of Plant-Derived Compounds in the Molecular Pathways Related to Inflammation. Int. J. Mol. Sci. 2023, 24, 4666. [Google Scholar] [CrossRef]
- Dominiak, A.; Chełstowska, B.; Olejarz, W.; Nowicka, G. Communication in the Cancer Microenvironment as a Target for Therapeutic Interventions. Cancers 2020, 12, 1232. [Google Scholar] [CrossRef]
- Itoh, S.; Yamaguchi, M.; Shigeyama, K.; Sakaguchi, I. The Anti-Aging Potential of Extracts from Chaenomeles Sinensis. Cosmetics 2019, 6, 21. [Google Scholar] [CrossRef]
- Shen, T.; Hu, F.; Liu, Q.; Wang, H.; Li, H. Analysis of Flavonoid Metabolites in Chaenomeles Petals Using Uplc-Esi-Ms/Ms. Molecules 2020, 25, 3994. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Bosiacka, I.; Bosiacka, B.; Rast, J.; Gutowska, I.; Wolska, J.; Rebacz-Maron, E.; Debia, K.; Janda, K.; Korbecki, J.; Chlubek, D. Macro- and Microelement Content and Other Properties of Chaenomeles japonica L. Fruit and Protective Effects of Its Aqueous Extract on Hepatocyte Metabolism. Biol. Trace Elem. Res. 2017, 178, 327–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turkiewicz, I.P.; Wojdylo, A.; Tkacz, K.; Nowicka, P.; Golis, T.; Babelewski, P. Abts on-Line Antioxidant, Alpha-Amylase, Alpha-Glucosidase, Pancreatic Lipase, Acetyl- and Butyrylcholinesterase Inhibition Activity of Chaenomeles Fruits Determined by Polyphenols and Other Chemical Compounds. Antioxidants 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Urbanaviciute, I.; Liaudanskas, M.; Bobinas, C.; Sarkinas, A.; Rezgiene, A.; Viskelis, P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods 2020, 9, 1132. [Google Scholar] [CrossRef]
- Urbanaviciute, I.; Liaudanskas, M.; Seglina, D.; Viskelis, P. Japanese Quince Chaenomeles japonica (Thunb.) Lindl. Ex Spach Leaves a New Source of Antioxidants for Food. Int. J. Food Prop. 2019, 22, 795–803. [Google Scholar] [CrossRef]
- Chojnacka, K.; Owczarek, K.; Caban, M.; Sosnowska, D.; Kajszczak, D.; Lewandowska, U. Chemoprotective Effects of Japanese Quince (Chaenomeles japonica L.) Phenol Leaf Extract on Colon Cancer Cells through the Modulation of Extracellular Signal-Regulated Kinases/Akt Signaling Pathway. J. Physiol. Pharmacol. 2022, 73, 41–52. [Google Scholar]
- Chojnacka, K.; Owczarek, K.; Caban, M.; Sosnowska, D.; Polka, D.; Koziolkiewicz, M.; Fichna, J.; Lewandowska, U. Japanese Quince (Chaenomeles japonica) Leaf Phenol Extract as Modulator of the Inflammatory Response in Lipopolysaccharide-Triggered Murine Macrophage Raw 264.7 Cells. J. Physiol. Pharmacol. 2020, 71, 1–11. [Google Scholar]
- Owczarek, K.; Hrabec, E.; Fichna, J.; Sosnowska, D.; Koziolkiewicz, M.; Szymanski, J.; Lewandowska, U. Flavanols from Japanese Quince (Chaenomeles japonica) Fruit Suppress Expression of Cyclooxygenase-2, Metalloproteinase-9, and Nuclear Factor-Kappab in Human Colon Cancer Cells. Acta Biochim. Pol. 2017, 64, 567–576. [Google Scholar] [CrossRef]
- Sardelli, L.; Perottoni, S.; Tunesi, M.; Boeri, L.; Fusco, F.; Petrini, P.; Albani, D.; Giordano, C. Technological Tools and Strategies for Culturing Human Gut Microbiota in Engineered in Vitro Models. Biotechnol. Bioeng. 2021, 118, 2886–2905. [Google Scholar] [CrossRef]
- Siegien, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osinska, E.; Melzig, M.F.; Czerwinska, M.E. Pancreatic Lipase and Alpha-Amylase Inhibitory Activity of Extracts from Selected Plant Materials after Gastrointestinal Digestion in Vitro. Food Chem. 2021, 355, 129414. [Google Scholar] [CrossRef]
- Mainka, M.; Czerwińska, M.E.; Osińska, E.; Ziaja, M.; Bazylko, A. Screening of Antioxidative Properties and Inhibition of In-flammation-Linked Enzymes by Aqueous and Ethanolic Extracts of Plants Traditionally Used in Wound Healing in Poland. Antioxidants 2021, 10, 698. [Google Scholar] [CrossRef]
- Zulkifli, S.A.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E. Optimization of Total Phenolic and Flavonoid Contents of Defatted Pitaya (Hylocereus polyrhizus) Seed Extract and Its Antioxidant Properties. Molecules 2020, 25, 787. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, C.J.; Sherkow, J.S.; Meyer, M.N.; Zettler, P.J. Self-Experimentation, Ethics, and Regulation of Vaccines. Science 2020, 369, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Böyum, A. Isolation of Mononuclear Cells and Granulocytes from Human Blood. Isolation of Monuclear Cells by One Centrifugation, and of Granulocytes by Combining Centrifugation and Sedimentation at 1 G. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar]
- Czerwinska, M.E.; Bobinska, A.; Cichocka, K.; Buchholz, T.; Wolinski, K.; Melzig, M.F. Cornus mas and Cornus officinalis—A Comparison of Antioxidant and Immunomodulatory Activities of Standardized Fruit Extracts in Human Neutrophils and Caco-2 Models. Plants 2021, 10, 2347. [Google Scholar] [CrossRef]
- Luca, S.V.; Kulinowski, Ł.; Ciobanu, C.; Zengin, G.; Czerwińska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and Multi-Biological Characterization of Two Cynara scolymus L. Varieties: A Glance into Their Potential Large Scale Cultivation and Valorization as Bio-Functional Ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Granica, S.; Kolodziejczyk-Czepas, J.; Magiera, A.; Czerwinska, M.E.; Nowak, P.; Rutkowska, M.; Wasinski, P.; Owczarek, A. Variability of Sinapic Acid Derivatives During Germination and Their Contribution to Antioxidant and Anti-Inflammatory Effects of Broccoli Sprouts on Human Plasma and Human Peripheral Blood Mononuclear Cells. Food Funct. 2020, 11, 7231–7244. [Google Scholar] [CrossRef]
- Tenore, G.C.; Campiglia, P.; Giannetti, D.; Novellino, E. Simulated Gastrointestinal Digestion, Intestinal Permeation and Plasma Protein Interaction of White, Green, and Black Tea Polyphenols. Food Chem. 2015, 169, 320–326. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic Characterization and Antioxidant Activity of Malus domestica and Prunus domestica Cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P. Uplc/Esi-Q-Tof-Ms Analysis of (Poly)Phenols, Tocols and Amino Acids in Chaenomeles Leaves Versus in Vitro Anti-Enzyme Activities. Ind. Crops Prod. 2022, 181, 114829. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdylo, A.; Tkacz, K.; Lech, K.; Michalska-Ciechanowska, A.; Nowicka, P. The Influence of Different Carrier Agents and Drying Techniques on Physical and Chemical Characterization of Japanese Quince (Chaenomeles japonica) Microencapsulation Powder. Food Chem. 2020, 323, 126830. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, G.; Wood, E.; Rogel Castillo, C.; Mitchell, A.E. Quantification of Amygdalin in Nonbitter, Semibitter, and Bitter Almonds (Prunus dulcis) by Uhplc-(Esi)Qqq Ms/Ms. J. Agric. Food Chem. 2013, 61, 7754–7759. [Google Scholar] [CrossRef]
- Kikowska, M.; Włodarczyk, A.; Stochmal, A.; Żuchowski, J.; Thiem, B. Pentacyclic Triterpenoids and Polyphenols Accumulation in Cell Suspension Culture of (Thunb.) Lindl. Ex Spach. Herba Pol. 2019, 65, 1–11. [Google Scholar] [CrossRef]
- Chojnacka, K.; Sosnowska, D.; Polka, D.; Owczarek, K.; Gorlach-Lira, K.; Oliveira de Verasa, B.; Lewandowska, U. Comparison of Phenolic Compounds, Antioxidant and Cytotoxic Activity of Extracts Prepared from Japanese Quince (Chaenomeles japonica L.) Leaves. J. Physiol. Pharmacol. 2020, 71, 2–10. [Google Scholar]
- Cha, J.M.; Kim, D.H.; Subedi, L.; Khan, Z.; Choi, S.U.; Kim, S.Y.; Kim, C.S. Chemical Constituents of Chaenomeles sinensis Twigs and Their Biological Activity. Beilstein J. Org. Chem. 2020, 16, 3078–3085. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zou, L.; Cao, H.; Ni, X.; Xiao, J. Dietary Proanthocyanidins on Gastrointestinal Health and the Interactions with Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2022, 63, 6285–6308. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.; Pisciottano, M.N.; Amorim, E.L.; Albuquerque, U.P. Which Approach Is More Effective in the Selection of Plants with Antimicrobial Activity? Evid. Based Complement. Altern. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated Strategies for Enzyme Assisted Extraction of Bioactive Molecules: A Review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic Acid and the Pathogenesis of Obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. [Google Scholar] [CrossRef]
- Kamińska, W.; Neunert, G.; Siejak, P.; Polewski, K.; Tomaszewska-Gras, J. Cold Pressed Oil from Japanese Quince Seeds (Chaenomeles japonica): Characterization Using Dsc, Spectroscopic, and Monolayer Data. Molecules 2025, 30, 477. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of Inflammation and Oxidative Stress in Ophthalmic Disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Šola, I.; Poljuha, D.; Mikulic-Petkovsek, M.; Davosir, D.; Pinterić, M.; Bilić, J.; Veberic, R.; Hudina, M.; Rusak, G. Biopotential of Underutilized Rosaceae Inflorescences: Lc-Dad-Ms Phytochemical Profiles Associated with Antioxidant, Antidiabetic, Anti-Inflammatory and Antiproliferative Activity in Vitro. Plants 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, I.P.; Tkacz, K.; Nowicka, P.; Wojdylo, A. Investigating in Vitro Anticholinergic Potential (Anti-Ache and Anti-Buche) of Chaenomeles Leaves Extracts and Its Phytochemicals Including Chlorophylls, Carotenoids and Minerals. Sci. Rep. 2024, 14, 23132. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Boland, M. Human Digestion—A Processing Perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef]
- Gouseti, O.; Larsen, M.E.; Amin, A.; Bakalis, S.; Petersen, I.L.; Lametsch, R.; Jensen, P.E. Applications of Enzyme Technology to Enhance Transition to Plant Proteins: A Review. Foods 2023, 12, 2518. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Białas, W.; Grajek, W. Integrated Approach for Obtaining Bioactive Peptides from Whey Proteins Hydrolysed Using a New Proteolytic Lactic Acid Bacteria. Food Chem. 2020, 312, 126035. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Van den Abbeele, P.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (Shime(®)). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 305–317. [Google Scholar]
- Mendes, T.M.N.; Murayama, Y.; Yamaguchi, N.; Sampaio, G.R.; Fontes, L.C.B.; Torres, E.A.F.d.S.; Tamura, H.; Yonekura, L. Guaraná (Paullinia cupana) Catechins and Procyanidins: Gastrointestinal/Colonic Bioaccessibility, Caco-2 Cell Permeability and the Impact of Macronutrients. J. Funct. Foods 2019, 55, 352–361. [Google Scholar] [CrossRef]
- Lee, H.A.; Song, Y.R.; Park, M.H.; Chung, H.Y.; Na, H.S.; Chung, J. Catechin Ameliorates Porphyromonas gingivalis-Induced Inflammation Via the Regulation of Tlr2/4 and Inflammasome Signaling. J. Periodontol. 2020, 91, 661–670. [Google Scholar] [CrossRef]
- Song, D.Q.; Liu, J.; Wang, F.; Li, X.F.; Liu, M.H.; Zhang, Z.; Cao, S.S.; Jiang, X. Procyanidin b2 Inhibits Lipopolysaccharide-Induced Apoptosis by Suppressing the Bcl-2/Bax and Nf-Κb Signalling Pathways in Human Umbilical Vein Endothelial Cells. Mol. Med. Rep. 2021, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Yu, Z.; Zhang, X.; Li, W.; Gao, D.; Wang, J.; Ma, X.; Nie, X.; Wang, W. Epicatechin Alleviates Inflammation in Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting the P38 Mapk Signaling Pathway. Int. Immunopharmacol. 2019, 66, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Ordóñez, J.L.; Ludwig, I.; Gaillet, S.; Mena, P.; Del Rio, D.; Rouanet, J.M.; Bindon, K.A.; Moreno-Rojas, J.M.; Crozier, A. Development and Validation of an Uhplc-Hrms Protocol for the Analysis of Flavan-3-Ol Metabolites and Catabolites in Urine, Plasma and Feces of Rats Fed a Red Wine Proanthocyanidin Extract. Food Chem. 2018, 252, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, X.; Liu, H.; Peng, Y.; Wang, X.; Xu, Y. Interaction Behavior between Five Flavonoids and Pepsin: Spectroscopic Analysis and Molecular Docking. J. Mol. Struct. 2021, 1223, 128978. [Google Scholar] [CrossRef]
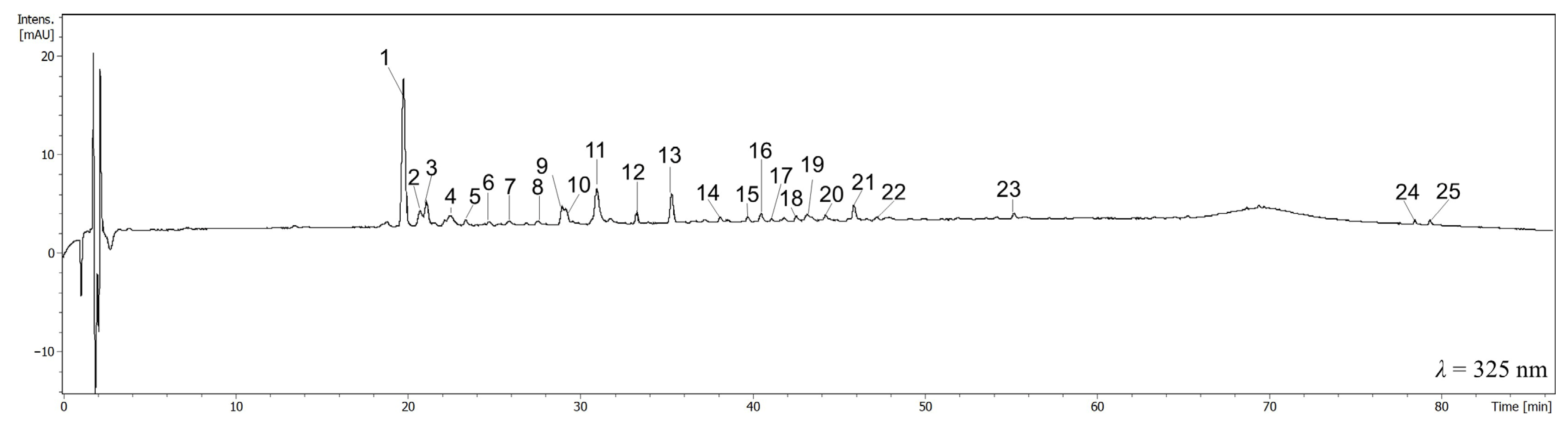
| No. | Compound | Retention Time [min] | λmax [nm] | [M-H]− m/z | MS/MS |
|---|---|---|---|---|---|
| 1 | p-Coumaric acid O-hexoside | 20.0 | 319 | 325 | 163 |
| 2 | Apigenin pentoside | 20.6 | 270, 320, 372 | 447 * | 401, 269 |
| 3 | p-Coumaric acid O-hexoside | 21.4 | 265, 320 | 325 | 163 |
| 4 | Caffeoyl-di-O-galloyl-hexoside | 21.8 | 270, 320 | 645 | 627, 528, 493, 451, 289, 193 |
| 5 | Galloyl derivative of catechin | 23.1 | 270, 320 | 673 | 719, 627, 557, 521, 383, 289, 221 |
| 6 | Procyanidin B2 | 26.2 | 276 | 577 | 425 |
| 7 | Epicatechin | 28.1 | 275 | 289 | 377, 245, 205 |
| 8 | Unidentified | 29.1 | 276 | 567 | 451, 331 |
| 9 | Caffeoyl acid derivative | 29.7 | 275 | 565 | 403, 223, 179 |
| 10 | Unidentified | 31.3 | 276 | 385 | 341, 217 |
| 11 | Procyanidin trimer | 33.6 | 254, 354 | 865 | 663, 521, 401, 289 |
| 12 | Feruloylquinic acid | 35.6 | 276 | 367 | 191 |
| 13 | Procyanidin derivative | 40.5 | 280 | 720 | 575, 489, 451, 207 |
| 14 | Unidentified | 41.8 | 276 | 415 | 369, 225, 179 |
| 15 | Procyanidin dimer | 43.2 | 276 | 577 | 425 |
| 16 | Unidentified hexoside | 45.9 | 277, 354 | 577 | 431 |
| 17 | Unidentified | 55.8 | 276 | 441 | 395, 305, 225, 179, 161 |
| Plant Material | Lf_et | Lf_aq | Fl_et | Fl_aq | Sd_et | Sd_aq | Fr_et | Fr_aq |
|---|---|---|---|---|---|---|---|---|
| TPC [mg/g] | 43.72± 2.61 | 60.26 ± 3.27 | 39.97 ± 7.24 | 39.00 ± 2.41 | 2.94 ± 0.72 | 9.58 ± 4.96 | 24.90 ± 0.72 | 19.11 ± 1.11 |
| Plant Material | Lf_et | Lf_aq | Fl_et | Fl_aq | Sd_et | Sd_aq | Fr_et | Fr_aq |
|---|---|---|---|---|---|---|---|---|
| TFC [mg/g] | 26.53± 2.88 | 15.15 ± 1.09 | 7.46 ± 2.05 | 4.78 ± 0.71 | n.d. | 7.17 ± 2.05 | n.d. | n.d. |
| Cell Line | TNF-α | IL-1β | IL-8 | IL-6 | IL-10 |
|---|---|---|---|---|---|
| PMN | + | +++ | ++ | ||
| PBMC | + | ++ | * | ||
| Caco-2 | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olędzka, A.J.; Sirak, A.; Hovtvian, D.; Koshovyi, O.; Czerwińska, M.E. Bio-Assay-Guided Study of Chaenomeles japonica–Cytokine Modulation by Fruit Aqueous Extract In Vitro in Connection with Its Processing with Enzymatic and Microbial Additives. Nutrients 2025, 17, 3716. https://doi.org/10.3390/nu17233716
Olędzka AJ, Sirak A, Hovtvian D, Koshovyi O, Czerwińska ME. Bio-Assay-Guided Study of Chaenomeles japonica–Cytokine Modulation by Fruit Aqueous Extract In Vitro in Connection with Its Processing with Enzymatic and Microbial Additives. Nutrients. 2025; 17(23):3716. https://doi.org/10.3390/nu17233716
Chicago/Turabian StyleOlędzka, Agata J., Aleksandra Sirak, Dariia Hovtvian, Oleh Koshovyi, and Monika E. Czerwińska. 2025. "Bio-Assay-Guided Study of Chaenomeles japonica–Cytokine Modulation by Fruit Aqueous Extract In Vitro in Connection with Its Processing with Enzymatic and Microbial Additives" Nutrients 17, no. 23: 3716. https://doi.org/10.3390/nu17233716
APA StyleOlędzka, A. J., Sirak, A., Hovtvian, D., Koshovyi, O., & Czerwińska, M. E. (2025). Bio-Assay-Guided Study of Chaenomeles japonica–Cytokine Modulation by Fruit Aqueous Extract In Vitro in Connection with Its Processing with Enzymatic and Microbial Additives. Nutrients, 17(23), 3716. https://doi.org/10.3390/nu17233716








