Clinical Outcomes Associated with Parenteral Nutrition Caloric Provision in Geriatric Patients with Infectious Colitis †
Abstract
1. Introduction
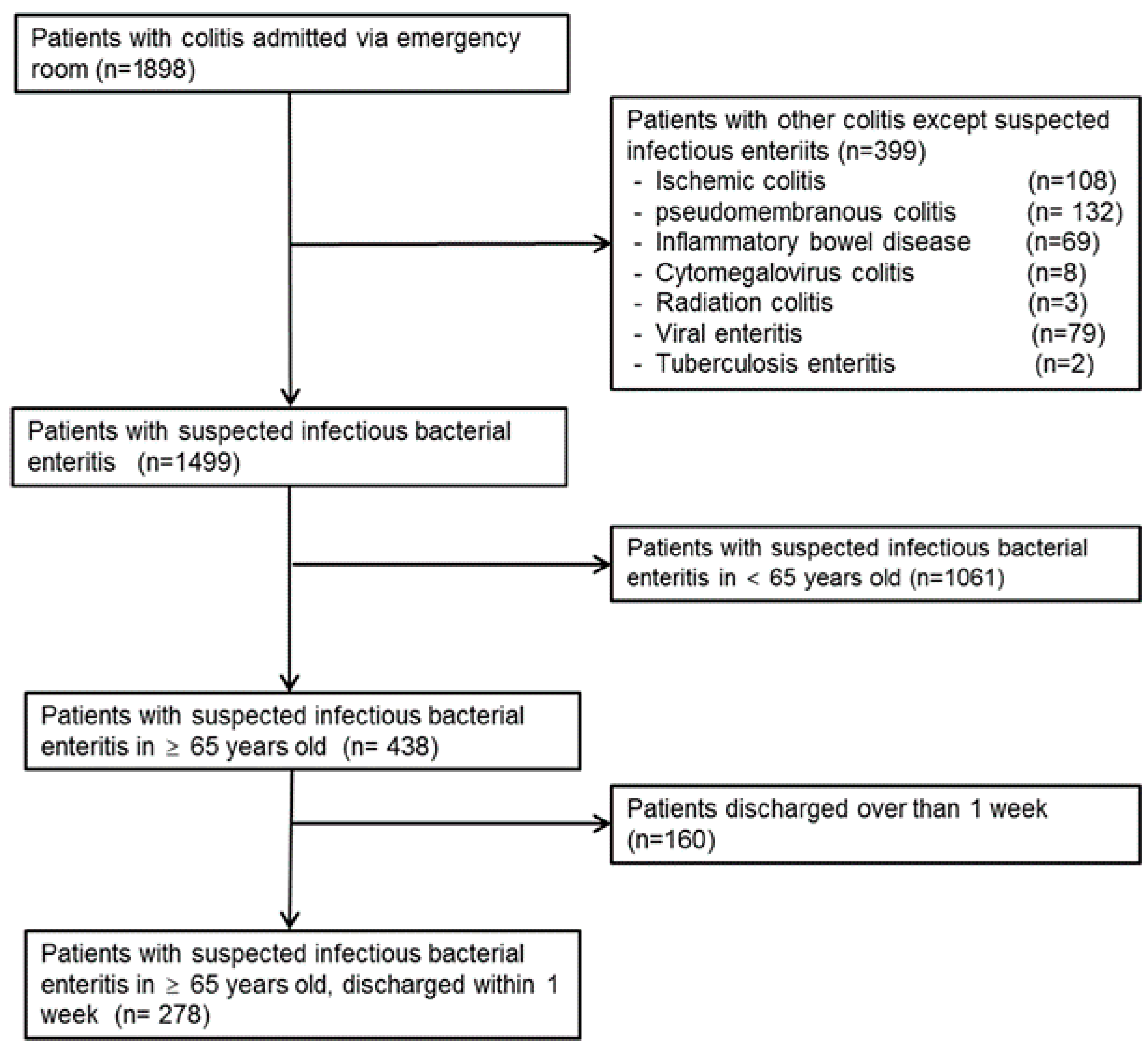
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
Analysis of Risk Factors for Prolonged Hospitalization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PN | Parenteral Nutrition |
| TPN | Total Parenteral Nutrition |
| LOS | Length of Stay |
| ESR | Erythrocyte Sedimentation Rate |
| ICU | Intensive Care Unit |
| BMI | Body Mass Index |
| CCI | Charlson Comorbidity Index |
| OR | Odds Ratio |
| CI | Confidence Interval |
| PCR | Polymerase Chain Reaction |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
References
- Berlana, D. Parenteral Nutrition Overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- Hamdan, M.; Puckett, Y. Total Parenteral Nutrition. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef]
- Azer, S.A.; Sun, Y. Colitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Berger, M.M.; Pichard, C. When is parenteral nutrition indicated? J. Intensive Med. 2022, 2, 22–28. [Google Scholar] [CrossRef]
- O’Brien, L.; Wall, C.; Wilkinson, T.J.; Gearry, R.B. Chronic diarrhoea in older adults and the role of dietary interventions. Nutr. Healthy Aging 2022, 7, 39–50. [Google Scholar] [CrossRef]
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Wanke, C.; Warren, C.A.; Cheng, A.C.; et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; DuPont, H.L. Approach to the patient with infectious colitis: Clinical features, work-up and treatment. Curr. Opin. Gastroenterol. 2021, 37, 66–75. [Google Scholar] [CrossRef]
- Papaconstantinou, H.T.; Thomas, J.S. Bacterial colitis. Clin. Colon. Rectal Surg. 2007, 20, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Elke, G.; Wang, M.; Weiler, N.; Day, A.G.; Heyland, D.K. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: Secondary analysis of a large international nutrition database. Crit. Care 2014, 18, R29. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009, 35, 1728–1737. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Hasselmann, M.; Kummerlen, C.; Kozar, R.; Kutsogiannis, D.J.; Karvellas, C.J.; Besecker, B.; Evans, D.K.; Preiser, J.C.; Gramlich, L.; et al. A randomized trial of supplemental parenteral nutrition in underweight and overweight critically ill patients: The TOP-UP pilot trial. Crit. Care 2017, 21, 142. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Cho, M.; Kim, S.; Hwang, H.; Song, M.; Yoo, S. Analysis of length of hospital stay using electronic health records: A statistical and data mining approach. PLoS ONE 2018, 13, e0195901. [Google Scholar] [CrossRef] [PubMed]
- Lucado, J.; Mohamoud, S.; Zhao, L.; Elixhauser, A. Infectious Enteritis and Foodborne Illness in the United States, 2010; NIH: Bethesda, MD, USA, 2006.
- Chen, Y.; Liu, B.C.; Glass, K.; Kirk, M.D. High incidence of hospitalisation due to infectious gastroenteritis in older people associated with poor self-rated health. BMJ Open 2015, 5, e010161. [Google Scholar] [CrossRef]
- Cho, H.; Lee, S.; Lee, J.; Lee, S.; Park, S.C. Epidemiologic and Clinical Features of Campylobacter Enteritis Before and During COVID-19 in Korea. J. Korean Med. Sci. 2023, 38, e67. [Google Scholar] [CrossRef]
- Cardemil, C.V.; Balachandran, N.; Kambhampati, A.; Grytdal, S.; Dahl, R.M.; Rodriguez-Barradas, M.C.; Vargas, B.; Beenhouwer, D.O.; Evangelista, K.V.; Marconi, V.C.; et al. Incidence, Etiology, and Severity of Acute Gastroenteritis Among Prospectively Enrolled Patients in 4 Veterans Affairs Hospitals and Outpatient Centers, 2016–2018. Clin. Infect. Dis. 2021, 73, e2729–e2738. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Dudrick, S.J. History of parenteral nutrition. J. Am. Coll. Nutr. 2009, 28, 243–251. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.C. Dextrose in Total Parenteral Nutrition. In Dietary Sugars: Chemistry, Analysis, Function and Effects; Alonso-Fernandez, J.R., Timson, D.J., Szilagyi, A., Obendorf, R.L., Abdelmalek, M.F., McGrath, M., Boukraâ, L., Gastrich, M.D., Haukioja, A., Jensen, J., Eds.; The Royal Society of Chemistry: Tokyo, Japan, 2012. [Google Scholar]
- Nguyen, V.I.; Velez, L. Dextrose (D-Glucose). In Goldfrank’s Toxicologic Emergencies, 11th ed.; Nelson, L.S., Howland, M.A., Lewin, N.A., Smith, S.W., Goldfrank, L.R., Hoffman, R.S., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Lee, J.H.; Kim, M.N.; Jung, S.T.; Lee, J.Y.; Park, K.M.; Hong, S.-B.; Kwon, K.S. Parenteral Nutrition for Infectious Colitis in Geriatric Patients. In Proceedings of the KSPEN (Korean Society for Parenteral and Enteral Nutrition) Annual Conference, Seongnam, Republic of Korea, 18 June 2019. [Google Scholar]
- Kang, M.G.; Choi, J.Y.; Yoo, H.J.; Park, S.Y.; Kim, Y.; Kim, J.Y.; Kim, S.W.; Kim, C.H.; Kim, K.I. Impact of malnutrition evaluated by the mini nutritional assessment on the prognosis of acute hospitalized older adults. Front. Nutr. 2022, 9, 1046985. [Google Scholar] [CrossRef]
- Folven, K.; Biringer, E.; Abrahamsen, J.F. Mini Nutritional Assessment Short-Form (MNA-SF) Predicts Institutionalisation in an Intermediate Post-Acute Care Setting. J. Nutr. Health Aging 2018, 22, 199–204. [Google Scholar] [CrossRef]
- Pourhassan, M.; Cederholm, T.; Donini, L.M.; Poggiogalle, E.; Schwab, U.; Nielsen, R.L.; Andersen, A.L.; Małgorzewicz, S.; Volkert, D.; Wirth, R. Severity of Inflammation Is Associated with Food Intake in Hospitalized Geriatric Patients-A Merged Data Analysis. Nutrients 2023, 15, 79. [Google Scholar] [CrossRef]
- Fatyga, P.; Pac, A.; Fedyk-Łukasik, M.; Grodzicki, T.; Skalska, A. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur. Geriatr. Med. 2020, 11, 383–391. [Google Scholar] [CrossRef]
- Contreras, N.A.; Fontana, L.; Tosti, V.; Nikolich-Žugich, J. Calorie restriction induces reversible lymphopenia and lymphoid organ atrophy due to cell redistribution. Geroscience 2018, 40, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Özşenel, E.B.; Kahveci, G.; Pekcioğlu, Y.; Güner, B.; Basat, S. The Overlooked Threat of Malnutrition: A Point Prevalence Study Based on NRS-2002 Screening in a Tertiary Care Hospital. J. Clin. Med. 2025, 14, 3976. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The Influence of Nutritional Factors on Immunological Outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, C.; Stumpf, F.; Schuetz, P. Inflammation and response to nutrition interventions. JPEN J. Parenter. Enteral Nutr. 2024, 48, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Wilson, M.; Whitney, C.; Fagnant, H.; Neumeier, W.H.; Smith, C.; Heaton, K.J.; Cho, E.; Spielmann, G.; Walsh, N.P.; et al. Supplemental Protein and a Multinutrient Beverage Speed Wound Healing after Acute Sleep Restriction in Healthy Adults. J. Nutr. 2022, 152, 1560–1573. [Google Scholar] [CrossRef]
- McCowen, K.C. Dextrose in Total Parenteral Nutrition. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2012. [Google Scholar]
- Worthington, P.; Balint, J.; Bechtold, M.; Bingham, A.; Chan, L.N.; Durfee, S.; Jevenn, A.K.; Malone, A.; Mascarenhas, M.; Robinson, D.T. When is parenteral nutrition appropriate? J. Parenter. Enter. Nutr. 2017, 41, 324–377. [Google Scholar] [CrossRef]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A. GLIM criteria for the diagnosis of malnutrition–a consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; An ad hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef] [PubMed]
| Characteristics, n (%) | ≤1000 kcal/day (n = 186) | >1000 kcal/day (n = 92) | p-Value |
|---|---|---|---|
| Age, year, mean ± SD | 74.4 ± 6.5 | 75.7 ± 7.1 | 0.1 |
| Sex | 0.74 | ||
| Male | 61 (32.8) | 32 | |
| Female | 125 (67.2) | 60 | |
| BMI kg/m2, mean ± SD | 23.7 ± 3.6 | 23.7 ± 3.6 | 0.97 |
| Long-term care facility | 8 (4.3) | 1 (1.1) | 0.15 |
| Intensive care unit | 31 (16.7) | 8 (8.7) | 0.07 |
| Shock | 25 (13.4) | 6 (6.5) | 0.08 |
| Co-existing morbidity | |||
| Diabetes | 0.83 | ||
| Uncomplicated | 60 (32.3) | 33 (35.9) | |
| Complicated | 6 (3.2) | 3 (3.3) | |
| Liver disease | 13 (7) | 1 (1.1) | 0.105 |
| Malignancy | 0.539 | ||
| Any leukemia, lymphoma, or localized solid tumor | 9 (4.8) | 2 (2.2) | |
| Metastatic solid tumor | 5 (2.7) | 2 (2.2) | |
| Chronic kidney disease | 12 (6.5) | 1 (1.1) | 0.05 |
| Congestive heart failure | 21 (11.3) | 17 (18.5) | 0.101 |
| Peripheral artery occlusive disease | 2 (1.1) | 3 (3.3) | 0.2 |
| Chronic obstructive pulmonary disease | 4 (2.2) | 3 (3.3) | 0.58 |
| Cerebral vascular attack | 26 (14.0) | 14 (15.2) | 0.78 |
| Hemiplegia | 2 (1.1) | 0 (0) | 0.31 |
| Rheumatic disease | 5 (2.7) | 2 (2.2) | 0.8 |
| Dementia | 11 (5.9) | 3 (3.3) | 0.34 |
| Peptic ulcer | 2 (1.1) | 3 (3.3) | 0.2 |
| Charlson comorbidity index | 1.1 ± 1.5 | 1.0 ± 1.2 | 0.70 |
| Laboratory exam | |||
| White blood cell, ×1000/μL, mean ± SD | 12.7 ± 22.6 | 10.9 ± 6.2 | 0.46 |
| Hemoglobin, g/dL, mean ± SD | 12.5 ± 2.2 | 13.1 ± 1.9 | 0.02 |
| Platelet, ×1000/μL, mean ± SD | 228.7 ± 102.0 | 212.7 ± 69.6 | 0.18 |
| ESR mm/h, mean ± SD | 32.6 ± 26.2 | 29.6 ± 24.3 | 0.36 |
| Lymphocyte count, /μL, mean ± SD | 1280.2 ± 1395.5 | 1249.2 ± 1004.1 | 0.85 |
| Albumin, g/dL, mean ± SD | 4.5 ± 10.5. | 3.9 ± 1.0 | 0.6 |
| Cholesterol, mg/dL, mean ± SD | 143.9 ± 51.8 | 138.4 ± 47.5 | 0.4 |
| Nutrition | |||
| Kcal/day | 316.0 ± 293.0 | 1748.0 ± 477.0 | <0.001 |
| Caloric intake, kcal/kg/day, mean ± SD | 5.7 ± 5.4 | 31.0 ± 10.3 | <0.001 |
| Protein intake, g/kg/day, mean ± SD | 0.05 ± 0.11 | 0.71 ± 0.41 | <0.001 |
| Parenteral nutrition category | <0.001 | ||
| Only dextrose | 71 (38.2) | 0 (0) | |
| Combined TPN with dextrose fluid | 77 (41.4) | 92 (100) | |
| Outcomes | |||
| Length of stay in hospital, day, mean ± SD | 4.2 ± 1.8 | 3.8 ± 1.5 | 0.03 |
| In-hospital mortality | 4 (2.2) | 1 (1.1) | 0.53 |
| Univariable Analysis | Multivariable Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | >4 day | Odd Ratio | 95% CI | p-Value | Odd Ratio | 95% CI | p-Value | ||
| Age | ≤75 years | 156 | 62 (39.7) | 1 | 0.53 | ||||
| >75 years | 122 | 44 (36.1) | 0.86 | (0.53–1.40) | |||||
| Sex | Male | 93 | 32 (34.4) | 1 | 0.36 | ||||
| female | 185 | 74 (40.0) | 1.24 | (0.74–2.08) | |||||
| Body mass index | >25 kg/m2 | 76 | 24 (31.6) | 1 | 0.023 | 1 | 0.056 | ||
| ≤25 kg/m2 | 202 | 82 (40.6) | 1.86 | (1.09–3.17) | 1.74 | (0.99–3.08) | |||
| Long-term care hospital | No | 269 | 99 (36.8) | 1 | 0.013 | 1 | 0.04 | ||
| Yes | 9 | 7 (77.8) | 5.282 | (1.19–28.57) | 5.65 | (1.11–28.87) | |||
| Intensive care unit | No | 239 | 83 (34.7) | 1 | 0.004 | 1 | 0.06 | ||
| Yes | 39 | 23 (59) | 2.60 | (1.31–5.20) | 2.2 | (0.98–4.93) | |||
| Diabetes | No | 176 | 65 (36.9) | 1 | 0.59 | ||||
| Yes | 102 | 41 (40.2) | 1.21 | (0.73–2.02) | |||||
| Liver disease | No | 264 | 101 (38.3) | 1 | 0.85 | ||||
| Yes | 14 | 5 (35.7) | 0.98 | (0.31–3.09) | |||||
| Malignancy | No | 260 | 97 (37.3) | 1 | 0.28 | ||||
| Yes | 18 | 9 (50.0) | 1.63 | (0.62–4.24) | |||||
| Chronic kidney disease | No | 265 | 100 (37.7) | 1 | 0.54 | ||||
| Yes | 13 | 6 (46.2) | 1.37 | (0.45–4.19) | |||||
| Congestive heart failure | No | 240 | 96 (40.0) | 1 | 0.11 | ||||
| Yes | 38 | 10 (26.3) | 0.52 | (0.24–1.11) | |||||
| PAOD | No | 273 | 106 (38.8) | 1 | 0.99 | ||||
| Yes | 5 | 0 (0.0) | - | - | |||||
| COPD | No | 271 | 102 (37.6) | 1 | 0.3 | ||||
| Yes | 7 | 4 (57.1) | 2.14 | (0.47–9.76) | |||||
| Cerebral vascular attack | No | 238 | 87 (36.6) | 1 | 0.19 | ||||
| Yes | 40 | 19 (47.5) | 1.51 | (0.47–9.76) | |||||
| Hemiplegia | No | 276 | 105 (38.0) | 1 | 0.73 | ||||
| Yes | 2 | 1 (50) | 1.58 | (0.10–25.52) | |||||
| Rheumatic disease | No | 271 | 103 (38) | 1 | 0.79 | ||||
| Yes | 7 | 3 (42.9) | 1.19 | (0.26–5.40) | |||||
| Dementia | No | 264 | 99 (37.5) | 1 | 0.35 | ||||
| Yes | 14 | 7 (50) | 1.61 | (0.55–4.74) | |||||
| Peptic ulcer | No | 273 | 106 (38.8) | 1 | 0.99 | ||||
| Yes | 5 | 0 (0) | - | - | |||||
| Charlson comorbidity index | ≤3 | 262 | 98 (37.4) | 1 | 0.31 | ||||
| >3 | 16 | 8 (50) | 1.62 | (0.59–4.45) | |||||
| Shock | No | 247 | 89 (36.0) | 1 | 0.04 | 1 | 0.6 | ||
| Yes | 31 | 17 (54.8) | 2.08 | (1.02–4.42) | 1.27 | (0.52–3.11) | |||
| White blood cell, /mm3 | 4000–10,000 | 126 | 43 (34.1) | 1 | 0.21 | ||||
| >10,000 or ≤4000 | 152 | 63 (41.4) | 1.44 | (0.88–2.35) | |||||
| Hemoglobin, g/dL | >12 | 185 | 70 (37.8) | 1 | 0.88 | ||||
| ≤12 | 93 | 36 (38.7) | 0.95 | (0.57–1.56) | |||||
| Platelet count, ×1000/mm3 | >15 K | 229 | 87 (38) | 1 | 0.91 | ||||
| ≤15 K | 49 | 19 (38.8) | 1.00 | (0.53–1.88) | |||||
| ESR, mm/h | ≤22 | 115 | 35 (30.4) | 1 | 0.03 | 1 | 0.04 | ||
| >22 | 163 | 71 (43.6) | 1.74 | (1.05–2.87) | 1.74 | (1.03–2.94) | |||
| Lymphocyte count, /mm3 | >1500 | 74 | 20 (27.0) | 1 | 0.02 | 1 | 0.03 | ||
| ≤1500 | 204 | 86 (42.2) | 2.05 | (1.14–3.67) | 1.95 | (1.06–3.61) | |||
| Albumin, g/dL | >3.3 | 228 | 86 (37.7) | 1 | 0.76 | ||||
| ≤3.3 | 50 | 20 (40.0) | 1.67 | (0.95–2.94) | |||||
| Calories per day, kcal/day | >1000 | 186 | 81 (43.5) | 1 | 0.002 | 1 | 0.03 | ||
| ≤1000 | 92 | 25 (27.2) | 1.86 | (1.09–3.17) | 1.9 | (1.08–3.34) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Lee, J.H.; Park, S.-H.; Park, S.; Park, S.-Y.; Yen, T.S.; Park, J.; Kwon, K.-S. Clinical Outcomes Associated with Parenteral Nutrition Caloric Provision in Geriatric Patients with Infectious Colitis. Nutrients 2025, 17, 3707. https://doi.org/10.3390/nu17233707
Kang Y, Lee JH, Park S-H, Park S, Park S-Y, Yen TS, Park J, Kwon K-S. Clinical Outcomes Associated with Parenteral Nutrition Caloric Provision in Geriatric Patients with Infectious Colitis. Nutrients. 2025; 17(23):3707. https://doi.org/10.3390/nu17233707
Chicago/Turabian StyleKang, Yuro, Jung Hwan Lee, Soo-Hyun Park, Somi Park, So-Youn Park, Tai Shun Yen, Jeongmi Park, and Kye-Sook Kwon. 2025. "Clinical Outcomes Associated with Parenteral Nutrition Caloric Provision in Geriatric Patients with Infectious Colitis" Nutrients 17, no. 23: 3707. https://doi.org/10.3390/nu17233707
APA StyleKang, Y., Lee, J. H., Park, S.-H., Park, S., Park, S.-Y., Yen, T. S., Park, J., & Kwon, K.-S. (2025). Clinical Outcomes Associated with Parenteral Nutrition Caloric Provision in Geriatric Patients with Infectious Colitis. Nutrients, 17(23), 3707. https://doi.org/10.3390/nu17233707







