Effects of Nutritional Supplements on Explosive Lower Limb Performance in Volleyball Players: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reporting Guideline and Protocol Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias and Certainty Assessment
2.7. Data Synthesis and Analysis
2.8. Sensitivity Analyses, Subgroups, and Network Meta-Regression
2.9. Patient and Public Involvement
3. Results
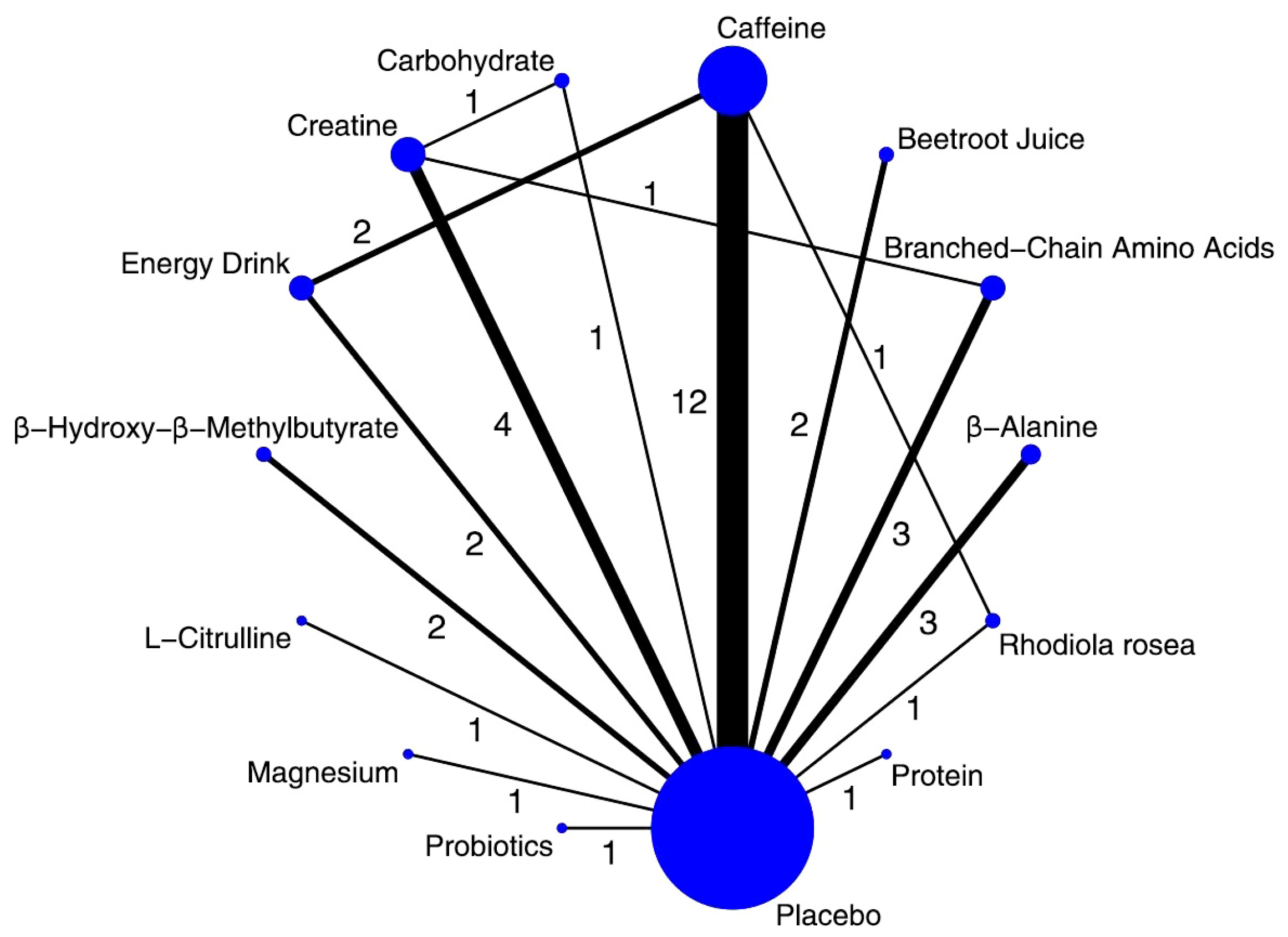
3.1. Study Selection and Characteristics
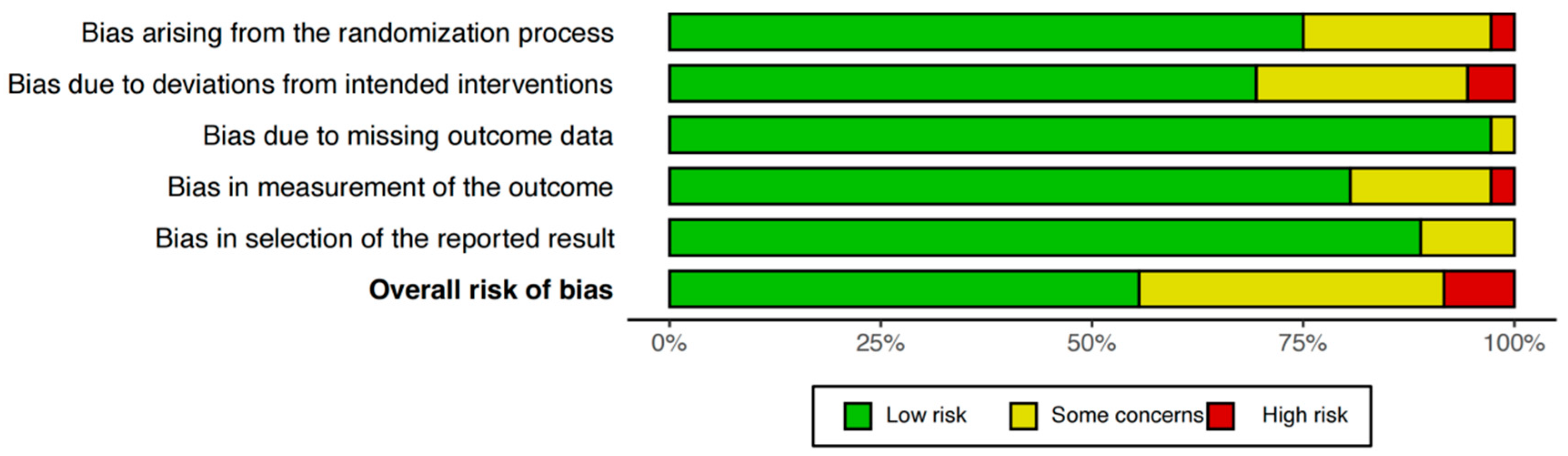
3.2. Risk of Bias, Confidence of Evidence, and Consistency
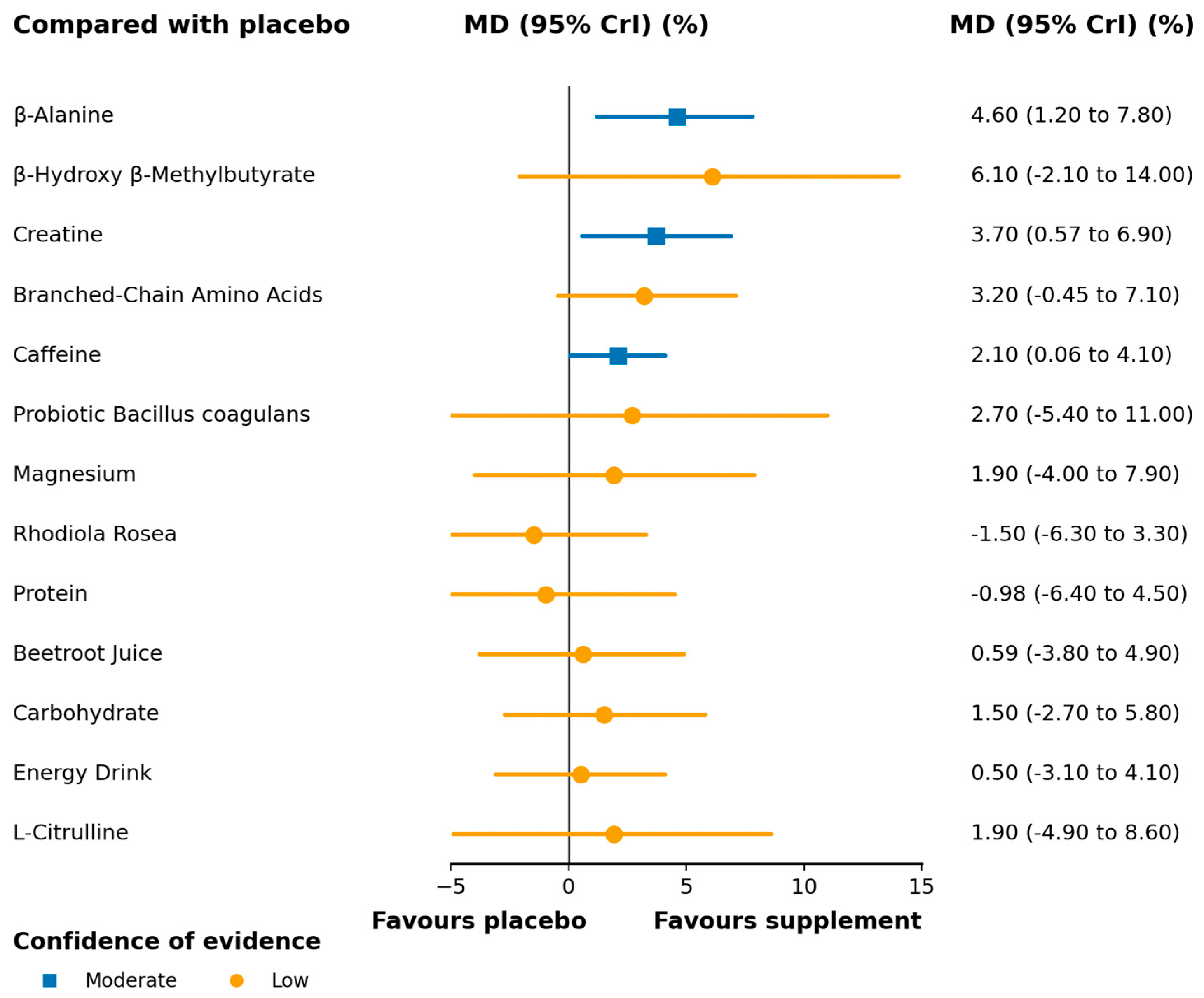
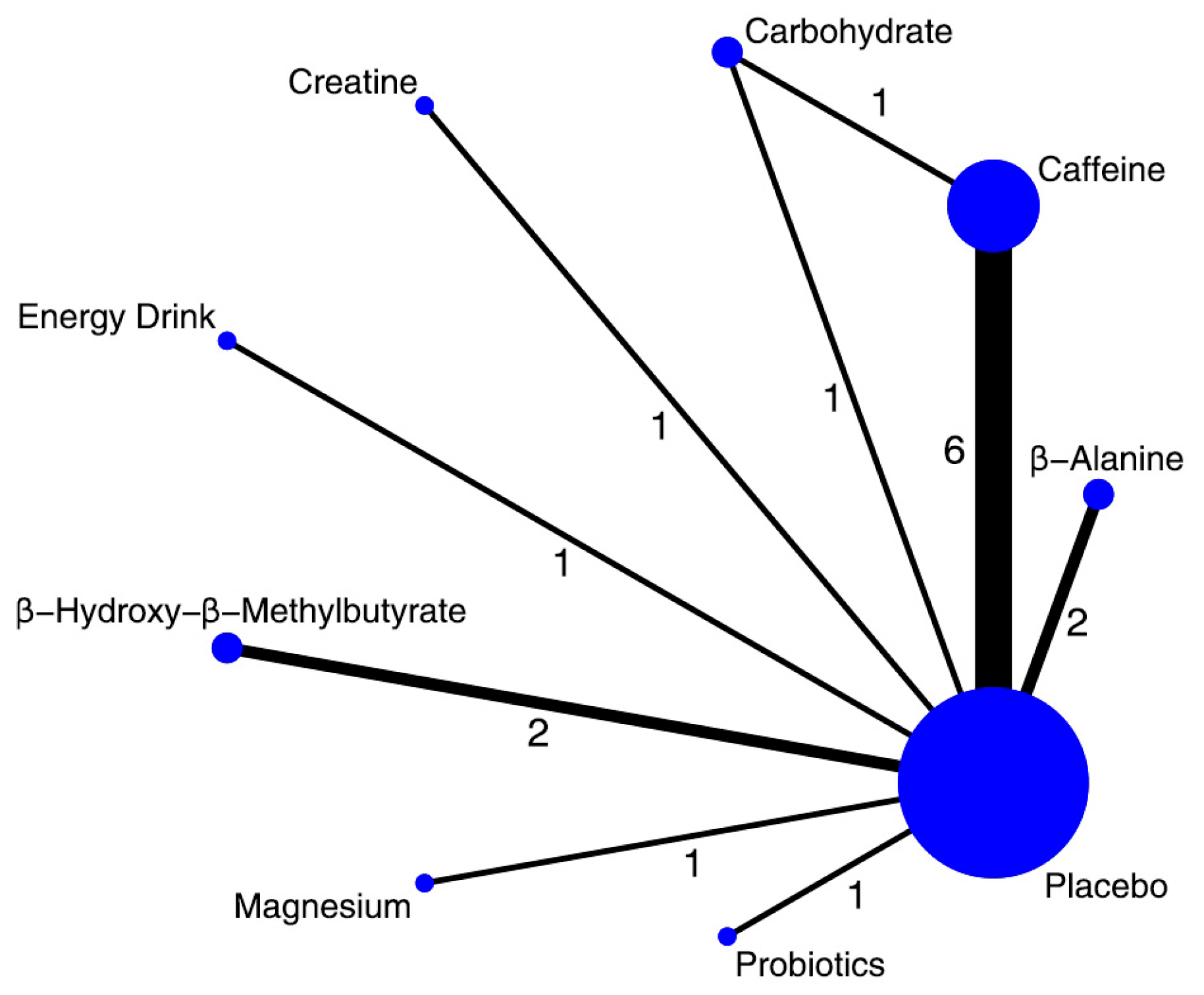
3.3. Synthesis of Results
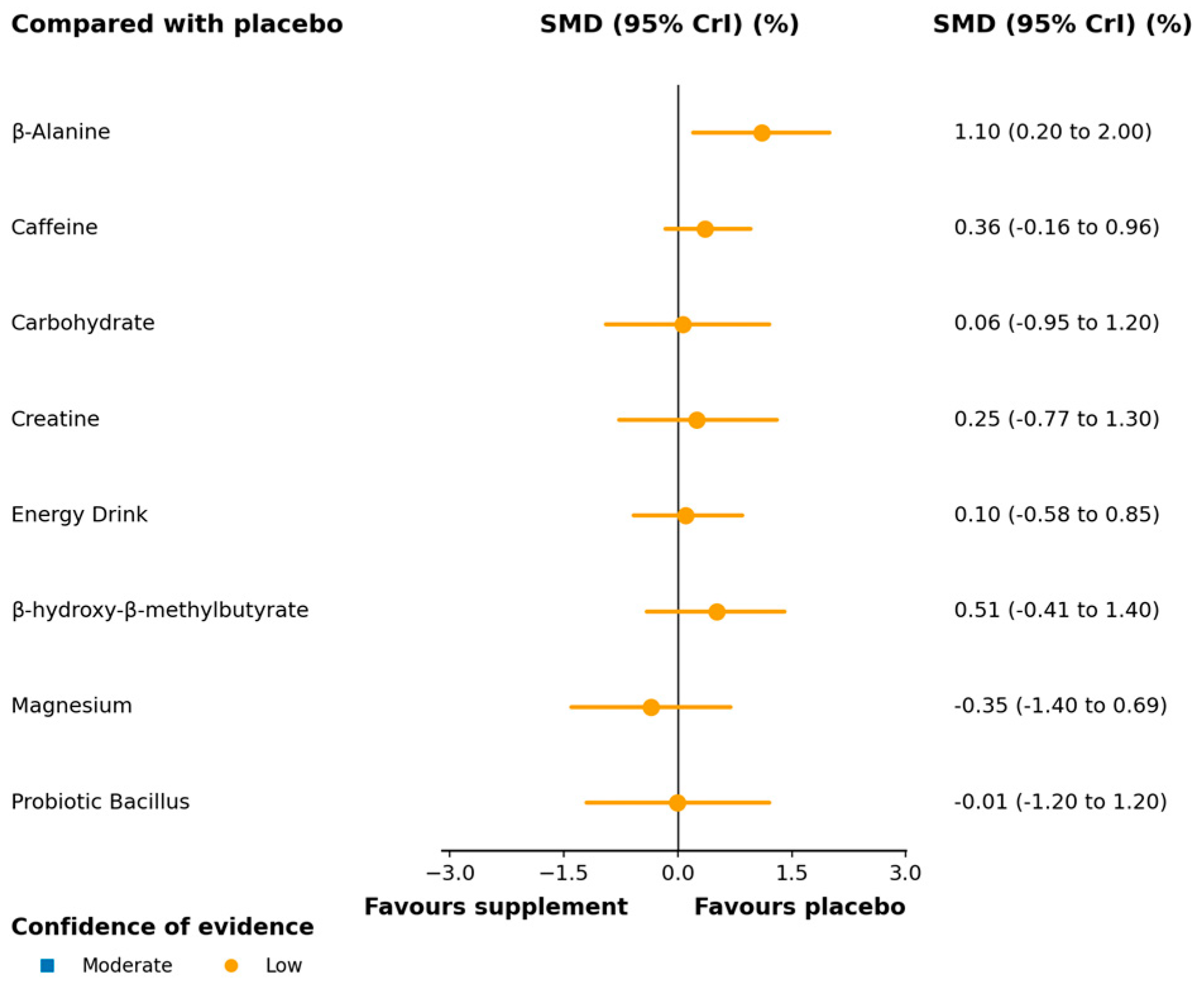
3.3.1. Vertical Jump
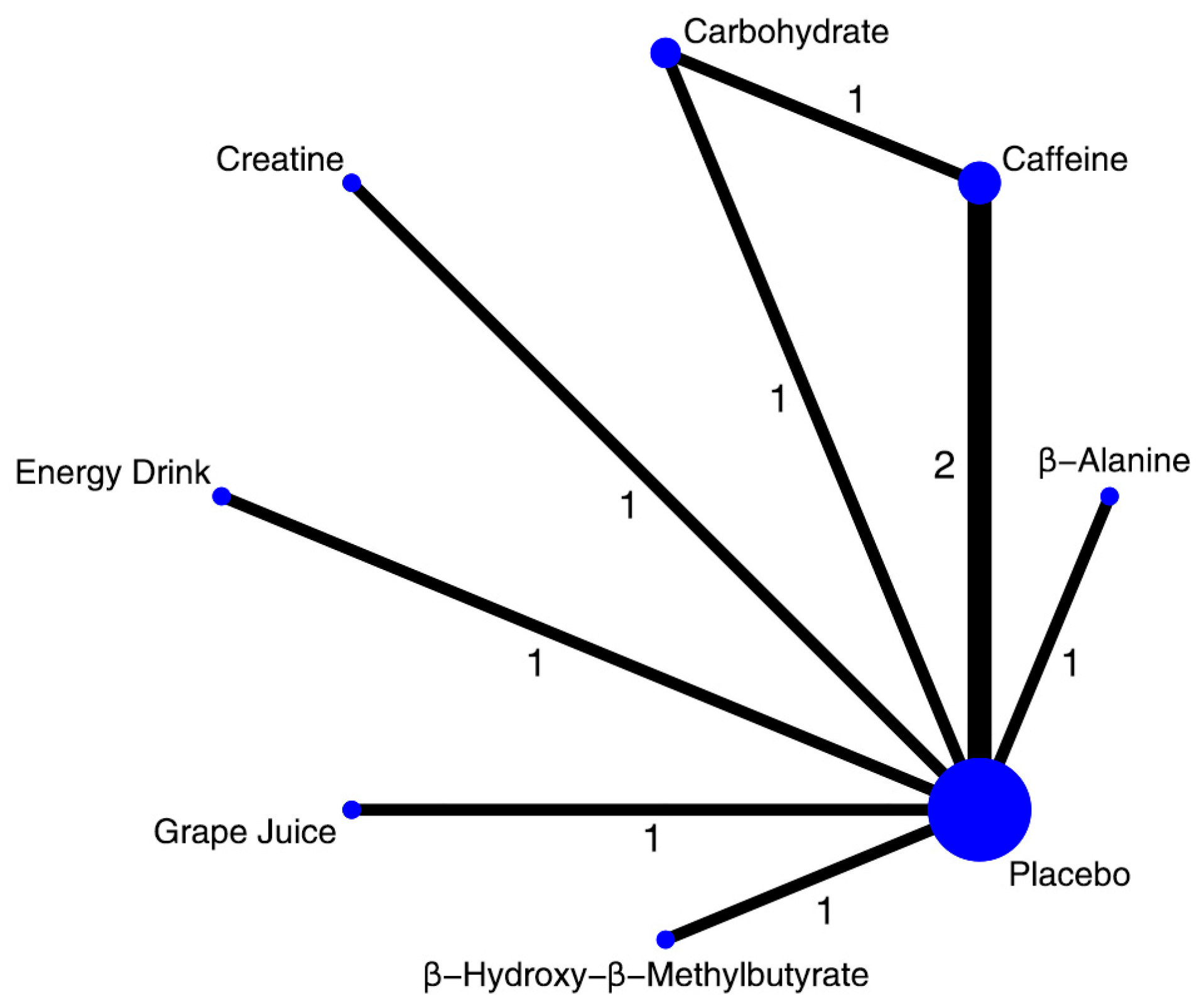
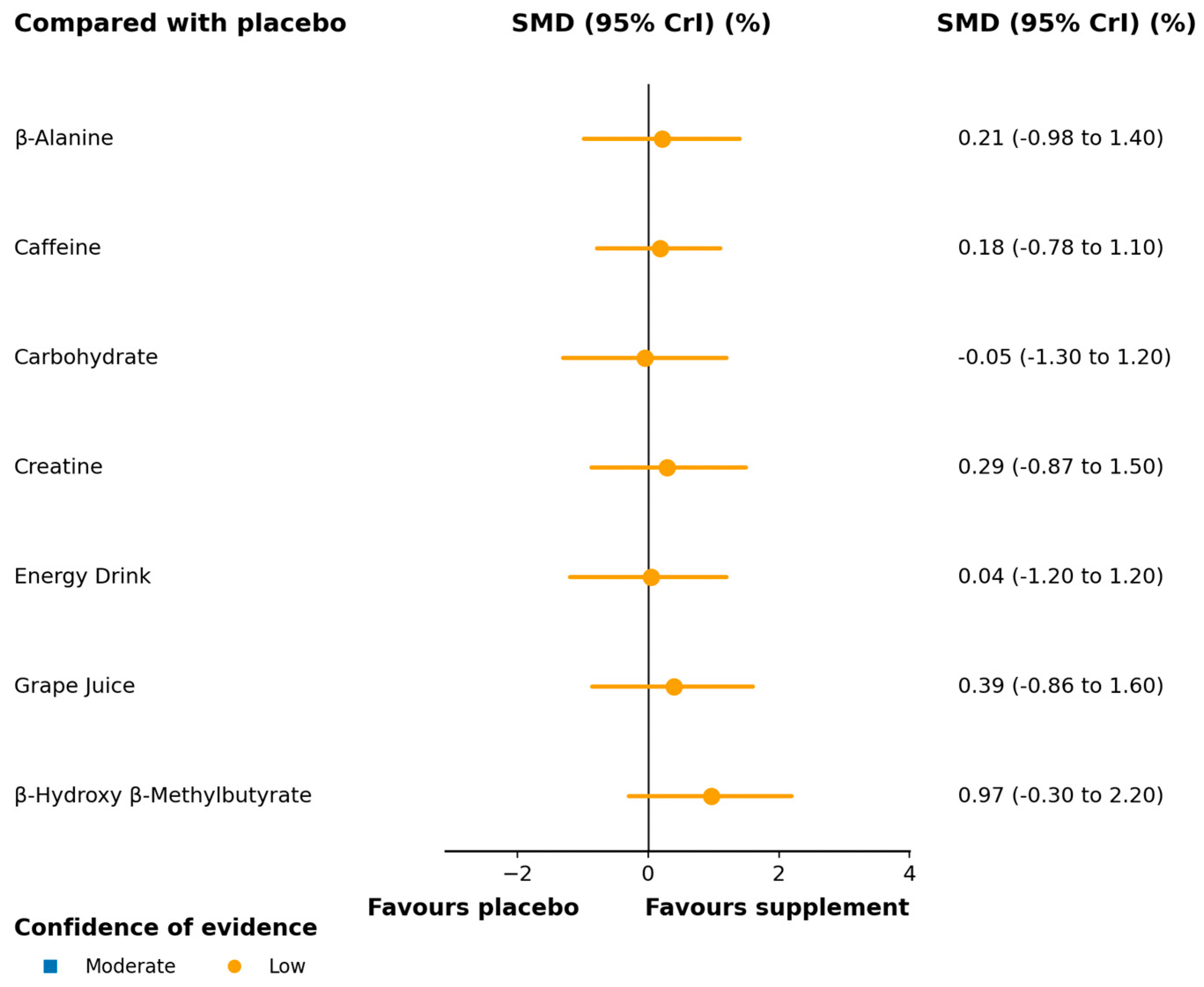
3.3.2. Lower Limb Peak Power
3.3.3. Lower Limb Mean Power
3.4. Sensitivity Analyses
3.5. Meta-Regression
3.6. Subgroup Analyses
4. Discussion
4.1. Principal Findings
4.2. General Interpretation of the Results
4.2.1. Vertical Jump
4.2.2. Lower Limb Peak Power
4.2.3. Lower Limb Mean Power
4.3. Subgroup Analysis
4.4. Practical Implications
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A1/A2A | Adenosine Receptor Subtypes A1 and A2A |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| ATP | Adenosine Triphosphate |
| BA | Beta-Alanine |
| BCAAs | Branched-Chain Amino Acids |
| CI | Confidence Interval |
| CINeMA | Confidence in Network Meta-Analysis |
| CMJ | Countermovement Jump |
| CrI | Credible Interval |
| FIVB | Fédération Internationale de Volleyball |
| HR-MRS | High-Resolution Magnetic Resonance Spectroscopy |
| HMB | Beta-Hydroxy-Beta-Methylbutyrate |
| IPD-NMA | Individual Participant Data Network Meta-Analysis |
| ISSN | International Society of Sports Nutrition |
| MD | Mean Difference |
| MCMC | Markov Chain Monte Carlo |
| mTOR | Mechanistic Target of Rapamycin |
| NA | Not Available |
| NMA | Network Meta-Analysis |
| NO | Nitric Oxide |
| PCr | Phosphocreatine |
| PH | Potential of Hydrogen |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
| RFD | Rate of Force Development |
| RAST | Running-based Anaerobic Sprint Test |
| RoB 2.0 | Risk of Bias 2.0 Tool |
| SD | Standard Deviation |
| SMD | Standardized Mean Difference |
| SJ | Squat Jump |
| SUCRA | Surface Under the Cumulative Ranking Curve |
| WADA | World Anti-Doping Agency |
| 31P-MRS PCr recovery | Phosphorus-31 Magnetic Resonance Spectroscopy—Phosphocreatine Recovery |
References
- Garcia-de-Alcaraz, A.; Ramirez-Campillo, R.; Rivera-Rodriguez, M.; Romero-Moraleda, B. Analysis of jump load during a volleyball season in terms of player role. J. Sci. Med. Sport 2020, 23, 973–978. [Google Scholar] [CrossRef]
- Sheppard, J.M.; Gabbett, T.; Taylor, K.L.; Dorman, J.; Lebedew, A.J.; Borgeaud, R. Development of a repeated-effort test for elite men’s volleyball. Int. J. Sports Physiol. Perform. 2007, 2, 292–304. [Google Scholar] [CrossRef] [PubMed]
- VanHeest, J.L. Energy demands in the sport of volleyball. In Handbook of Sports Medicine and Science: Volleyball; Wiley: Hoboken, NJ, USA, 2003; pp. 11–17. [Google Scholar]
- Andreoli, A.; Melchiorri, G.; Brozzi, M.; Di Marco, A.; Volpe, S.L.; Garofano, P.; Di Daniele, N.; De Lorenzo, A. Effect of different sports on body cell mass in highly trained athletes. Acta Diabetol. 2003, 40 (Suppl. S1), S122–S125. [Google Scholar] [CrossRef]
- Fuchs, P.X.; Mitteregger, J.; Hoelbling, D.; Menzel, H.-J.K.; Bell, J.W.; von Duvillard, S.P.; Wagner, H. Relationship between General Jump Types and Spike Jump Performance in Elite Female and Male Volleyball Players. Appl. Sci. 2021, 11, 1105. [Google Scholar] [CrossRef]
- Giatsis, G.; Drikos, S.; Lola, A. Analysis of match report indicators in men’s volleyball Olympics and world championships (2014–2021) depending on the type of final score. Int. J. Sports Sci. Coach. 2022, 18, 874–882. [Google Scholar] [CrossRef]
- Pawlik, D.; Mroczek, D. Influence of jump height on the game efficiency in elite volleyball players. Sci. Rep. 2023, 13, 8931. [Google Scholar] [CrossRef] [PubMed]
- Lidor, R.; Ziv, G. Physical and physiological attributes of female volleyball players—A review. J. Strength Cond. Res. 2010, 24, 1963–1973. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jager, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Sobiński, A.; Czerwonka, M.; Kościuszko, Z.; Kurza, K.; Ciraolo, S.; Podolec, J.; Agnieszka, K.-R.; Lesiczka-Fedoryj, K.; Wojda, J.; Walczak, A. The Impact of Creatine Supplementation on Physical Performance, Cognitive Functions, and Safety—A Literature Review. Qual. Sport 2025, 38, 58256. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef]
- Salinero, J.J.; Lara, B.; Del Coso, J. Effects of acute ingestion of caffeine on team sports performance: A systematic review and meta-analysis. Res. Sports Med. 2019, 27, 238–256. [Google Scholar] [CrossRef]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. beta-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.N.; Sakoury, M.M.; Kholif, M.A.; Albadaly, N.I.A. The role of Amino Acids in improving immunity and growth factors of Volleyball players. J. Adv. Pharm. Educ. Res. 2020, 10, 140–144. [Google Scholar] [CrossRef]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Zapolska, J.; Witczak, K.; Manczuk, A.; Ostrowska, L. Assessment of nutrition, supplementation and body composition parameters on the example of professional volleyball players. Rocz. Panstw. Zakl. Hig. 2014, 65, 235–242. [Google Scholar]
- Silva, M.; Marcelino, R.; Lacerda, D.; Vicente João, P. Match Analysis in Volleyball: A systematic review. Montenegrin J. Sports Sci. Med. 2016, 5, 35–46. [Google Scholar]
- Hernández-Landa, R.E.; Lazo, M.; Salado, D.D.; Sánchez-Almanzar, E.; Cepeda-Marte, J.L.; Zare, R.; Ali Redha, A.; Clifford, T. Dietary Supplementation Strategies for Improving Training Adaptations, Antioxidant Status and Performance of Volleyball Players: A Systematic Review. J. Sci. Sport Exerc. 2024, 1–21. [Google Scholar] [CrossRef]
- Burke, L.M. Practical Issues in Evidence-Based Use of Performance Supplements: Supplement Interactions, Repeated Use and Individual Responses. Sports Med. 2017, 47, 79–100. [Google Scholar] [CrossRef]
- Dias, S.; Caldwell, D.M. Network meta-analysis explained. In Archives of Disease in Childhood. Fetal and Neonatal Edition; BMJ Publishing Group: London, UK, 2019; Volume 104, pp. F8–F12. [Google Scholar]
- Challoumas, D.; Artemiou, A. Predictors of Attack Performance in High-Level Male Volleyball Players. Int. J. Sports Physiol. Perform. 2018, 13, 1230–1236. [Google Scholar] [CrossRef]
- Fédération Internationale de Volleyball (FIVB). Sports Regulations 2024; FIVB: Lausanne, Switzerland, 2024; Available online: https://www.fivb.com/wp-content/uploads/2024/03/FIVB-Sports-Regulations-2024_clean_website_31052024.pdf (accessed on 18 November 2025).
- Silvestre, J.; Gianoni, R.; Esteves, G.; Lambertucci, R.; Zagatto, A.; Azevedo, P. Beta-Alanine Supplementation Neither Reduce the Oxidative Stress Nor Improve Physical Performance of Volleyball Athletes. 2019. Available online: https://www.researchgate.net/publication/335563784_Beta-alanine_Supplementation_Neither_Reduce_the_Oxidative_Stress_nor_Improve_Physical_Performance_of_Volleyball_Athletes (accessed on 18 September 2025).
- Qanbar, Y.R.; Talebi-Garakani, E.; Safarzade, A. Interleukin-15 Contributes to the Benefits of Βeta-Alanine Supplementation on the Performance of Elite Volleyball Players. Ann. Mil. Health Sci. Res. 2024, 22, e143275. [Google Scholar]
- Guo, W.; Wang, S. Physiological and performance adaptations to beta alanine supplementation and short sprint interval training in volleyball players. Sci. Rep. 2024, 14, 16833. [Google Scholar] [CrossRef]
- Faiq, D.S.; Essa, S.N.A.; Muhialdeen, S.S. The Effect of Assisted Training Using Supplementation (BCAA) in Developing the Special Strength and Accuracy of Offensive Skills in Volleyball for Youth. Egypt. J. Hosp. Med. 2023, 90, 2398–2403. [Google Scholar] [CrossRef]
- Vega-Sanchez, R.; Tolentino-Dolores, M.C.; Cerezo-Rodriguez, B.; Chehaibar-Besil, G.; Flores-Quijano, M.E. Erythropoiesis and Red Cell Indices Undergo Adjustments during Pregnancy in Response to Maternal Body Size but not Inflammation. Nutrients 2020, 12, 975. [Google Scholar] [CrossRef]
- Santi, C.S.; Júnior, J.F.A.; dos Santos, A.N.; da Silva, D.P.; Leite, R.D. Effect of creatine supplementation on muscle damage markers and physical performance in volleyball athletes. J. Exerc. Physiol. Online 2020, 23, 33–42. [Google Scholar]
- Lamontagne-Lacasse, M.; Nadon, R.; Goulet, E.D. Effect of creatine supplementation on jumping performance in elite volleyball players. Int. J. Sports Physiol. Perform. 2011, 6, 525–533. [Google Scholar] [CrossRef]
- Kubota, M.; Hiruma, E.; Sasaki, H. The Effects of creatine supplementation on jumping power and endurance of volleyball players. Adv. Exerc. Sports Physiol. 2003, 9, 91–96. [Google Scholar]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of Beetroot Juice Supplementation on Muscle Soreness and Performance Recovery after Exercise-Induced Muscle Damage in Female Volleyball Players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef]
- Toohey, J.C.; Townsend, J.R.; Johnson, S.B.; Toy, A.M.; Vantrease, W.C.; Bender, D.; Crimi, C.C.; Stowers, K.L.; Ruiz, M.D.; VanDusseldorp, T.A.; et al. Effects of Probiotic (Bacillus subtilis) Supplementation During Offseason Resistance Training in Female Division I Athletes. J. Strength Cond. Res. 2020, 34, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Setaro, L.; Santos-Silva, P.R.; Nakano, E.Y.; Sales, M.M.; Pereira, G.B.; Miranda, R.A.; Colli, C. Magnesium status and its relationship with muscle damage in volleyball players. Magnes. Res. 2014, 27, 25–34. [Google Scholar]
- Portal, S.; Zadik, Z.; Rabinowitz, J.; Pilz-Burstein, R.; Adler-Portal, D.; Meckel, Y.; Cooper, D.M.; Eliakim, A.; Nemet, D. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: A prospective randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2011, 111, 2261–2269. [Google Scholar] [CrossRef]
- Sánchez-Gómez, A.; Martínez-Aranda, L.M.; Vicente-Salar, N.; Martínez-Noguera, F.J.; Alcaraz, P.E. β-hydroxy-β-methylbutyrate free acid supplementation and exercise-induced muscle damage in elite male volleyball players: A randomized, double-blind, placebo-controlled trial. Nutrients 2022, 14, 450. [Google Scholar]
- Nemati, J.; Hemmatinafar, M.; Niknam, A.; Nikahd, M.; Zeighami, N.; Imanian, B.; Safari, K.; Jahaniboushehri, N.; Suzuki, K. Effects of Different Doses of Caffeine Supplementation on Collegiate Male Volleyball Players’ Specific Performance and Skills: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Nutrients 2023, 15, 4049. [Google Scholar] [CrossRef]
- Pfeifer, D.R.; Arvin, K.M.; Herschberger, C.N.; Haynes, N.J.; Renfrow, M.S. A Low Dose Caffeine and Carbohydrate Supplement does not Improve Athletic Performance during Volleyball Competition. Int. J. Exerc. Sci. 2017, 10, 340–353. [Google Scholar] [CrossRef]
- Siquier-Coll, J.; Delgado-Garcia, G.; Soto-Mendez, F.; Linan-Gonzalez, A.; Garcia, R.; Gonzalez-Fernandez, F.T. The Effect of Caffeine Supplementation on Female Volleyball Players’ Performance and Wellness during a Regular Training Week. Nutrients 2023, 16, 29. [Google Scholar] [CrossRef]
- Zbinden-Foncea, H.; Rada, I.; Gomez, J.; Kokaly, M.; Stellingwerff, T.; Deldicque, L.; Penailillo, L. Effects of Caffeine on Countermovement-Jump Performance Variables in Elite Male Volleyball Players. Int. J. Sports Physiol. Perform. 2018, 13, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Campos, C.; Dengo, A.L.; Moncada-Jimenez, J. Acute Consumption of an Energy Drink Does Not Improve Physical Performance of Female Volleyball Players. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 271–277. [Google Scholar] [CrossRef]
- Del Coso, J.; Perez-Lopez, A.; Abian-Vicen, J.; Salinero, J.J.; Lara, B.; Valades, D. Enhancing physical performance in male volleyball players with a caffeine-containing energy drink. Int. J. Sports Physiol. Perform. 2014, 9, 1013–1018. [Google Scholar] [CrossRef]
- Filip-Stachnik, A.; Kaszuba, M.; Dorozynski, B.; Komarek, Z.; Gawel, D.; Del Coso, J.; Klocek, T.; Spieszny, M.; Krzysztofik, M. Acute Effects of Caffeinated Chewing Gum on Volleyball Performance in High-Performance Female Players. J. Human Kinet. 2022, 84, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Filip-Stachnik, A.; Spieszny, M.; Stanisz, L.; Krzysztofik, M. Does caffeine ingestion affect the lower-body post-activation performance enhancement in female volleyball players? BMC Sports Sci. Med. Rehabil. 2022, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, M.; Klocek, O.; Spieszny, M.; Filip-Stachnik, A. The Effect of Caffeinated Chewing Gum on Volleyball-Specific Skills and Physical Performance in Volleyball Players. Nutrients 2022, 15, 91. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Salinero, J.J.; Abian-Vicen, J.; Valades, D.; Lara, B.; Hernandez, C.; Areces, F.; Gonzalez, C.; Del Coso, J. Caffeinated energy drinks improve volleyball performance in elite female players. Med. Sci. Sports Exerc. 2015, 47, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, H.; Li, H.; Zhao, K.; Zhao, B.; Ma, Y.; Zhang, J.; Wu, K.; Jiang, W.; Liu, C. Effects of the Combined Supplementation of Caffeine and Rhodiola Rosea with Resistance Training on Lower Limb Explosive Power in Male Volleyball Players. Nutrients 2025, 17, 681. [Google Scholar] [CrossRef]
- Martins, N.C.; Dorneles, G.P.; Blembeel, A.S.; Marinho, J.P.; Proenca, I.C.T.; da Cunha Goulart, M.J.V.; Moller, G.B.; Marques, E.P.; Pochmann, D.; Salvador, M.; et al. Effects of grape juice consumption on oxidative stress and inflammation in male volleyball players: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 54, 102570. [Google Scholar] [CrossRef]
- Telyari, M.; Ebrahimi, M. The effect of caffeine mouth rinsing on agility, jump height and service and spike accuracy in male volleyball players. Res. Exerc. Nutr. 2022, 1, 1–9. [Google Scholar]
- Lee, C.L.; Cheng, C.F.; Astorino, T.A.; Lee, C.J.; Huang, H.W.; Chang, W.D. Effects of carbohydrate combined with caffeine on repeated sprint cycling and agility performance in female athletes. J. Int. Soc. Sports Nutr. 2014, 11, 17. [Google Scholar] [CrossRef]
- Ismail, A.; Elbattawy, K. Effect of Branched-Chain Amino Acids and Ginseng-Creatine supplementation on delayed onset muscle soreness and muscle damage in volleyball players. Assiut J. Sport Sci. Arts 2015, 2015, 309–326. [Google Scholar] [CrossRef]
- Burke, B.I.; Travis, S.K.; Gentles, J.A.; Sato, K.; Lang, H.M.; Bazyler, C.D. The Effects of Caffeine on Jumping Performance and Maximal Strength in Female Collegiate Athletes. Nutrients 2021, 13, 2496. [Google Scholar] [CrossRef]
- Hashem, E.J.; Majeed, M.H.; Al-Isawi, M. Effect of Nutritional Supplement Accompanying Functional Strength Exercises on the Special Physical Abilities and the Spiking Skill of Volleyball Players. Int. J. Disabil. Sports Health Sci. 2024, 7, 200–205. [Google Scholar] [CrossRef]
- Zhu, G.; Fu, K.; Xie, Y. Effects of progressive versus consistent dose of caffeine ingestion on volleyball players’ exercise performance adaptations following plyometric jump training. Front. Nutr. 2025, 12, 1629950. [Google Scholar] [CrossRef] [PubMed]
- López-León, I.; Moreno-Lara, J.; Sánchez-Oliver, A.J.; Rico-Saborido, E.; Muñoz-López, A.; Llerena, A.M.; Domínguez, R. Does beetroot juice supplementation affect to sport performance in high performance female volleyball players? Cult. Cienc. Deporte 2025, 20, 2235. [Google Scholar] [CrossRef]
- Vinu, W. Effect of plyometric training and plyometric training with protein suplementation on explosive power. Int. J. Adv. Educ. Res. 2018, 3, 598–600. [Google Scholar]
- Campbell, B.I.; Richmond, J.L.; Dawes, J.J. The Effects of a Commercial, Pre-exercise Energy Drink Supplement on Power, Muscular Endurance, and Repeated Sprint Speed. Int. J. Exerc. Sci. 2016, 9, 205–213. [Google Scholar] [CrossRef]
- Norozi, M.; Hejazi, K.; Marefati, H. The effect of eight weeks of high-intensity interval training combined with L-citrulline malate supplementation on muscle damage, inflammation and physical fitness factors of volleyball girls. Comp. Exerc. Physiol. 2025, 21, 369–380. [Google Scholar] [CrossRef]
- Eskici, G.; Gunay, M.; Baltaci, A.K.; Mogulkoc, R. The effect of different doses of zinc supplementation on serum element and lactate levels in elite volleyball athletes. J. Appl. Biomed. 2017, 15, 133–138. [Google Scholar] [CrossRef]
- García Verazaluce, J.J.; Vargas Corzo, M.D.C.; Aguilar Cordero, M.J.; Ocaña Peinado, F.; Sarmiento Ramírez, Á.; Guisado Barrilao, R. Effect of phlebodium decumanum and coenzyme Q10 on sports performance in professional volleyball players. Nutr. Hosp. 2015, 31, 401–414. [Google Scholar]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S. Iron supplementation prevents a decline in iron stores and enhances strength performance in elite female volleyball players during the competitive season. Appl. Physiol. Nutr. Metab. 2015, 40, 615–622. [Google Scholar] [CrossRef]
- Kartashev, V.; Medvedev, I.; Kachenkova, E. The use of the sports nutrition complex BCAA + PEPTIDECOMPLEXIPH-AGAA in representatives of team sports. Theory Pract. Phys. Cult. 2023, 460, 30–32. [Google Scholar] [CrossRef]
- Silva, A.F.; Clemente, F.M.; Lima, R.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. The Effect of Plyometric Training in Volleyball Players: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2960. [Google Scholar] [CrossRef] [PubMed]
- De Brandt, J.; Burtin, C.; Pomies, P.; Vandenabeele, F.; Verboven, K.; Aumann, J.; Blancquaert, L.; Everaert, I.; Van Ryckeghem, L.; Cops, J.; et al. Carnosine, oxidative and carbonyl stress, antioxidants, and muscle fiber characteristics of quadriceps muscle of patients with COPD. J. Appl. Physiol. (1985) 2021, 131, 1230–1240. [Google Scholar] [CrossRef]
- Baguet, A.; Everaert, I.; Hespel, P.; Petrovic, M.; Achten, E.; Derave, W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS ONE 2011, 6, e21956. [Google Scholar] [CrossRef]
- Matthews, J.J.; Artioli, G.G.; Turner, M.D.; Sale, C. The Physiological Roles of Carnosine and beta-Alanine in Exercising Human Skeletal Muscle. Med. Sci. Sports Exerc. 2019, 51, 2098–2108. [Google Scholar] [CrossRef]
- Blancquaert, L.; Everaert, I.; Missinne, M.; Baguet, A.; Stegen, S.; Volkaert, A.; Petrovic, M.; Vervaet, C.; Achten, E.; De Maeyer, M.; et al. Effects of Histidine and beta-alanine Supplementation on Human Muscle Carnosine Storage. Med. Sci. Sports Exerc. 2017, 49, 602–609. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tengku Kamalden, T.F.; Zhu, X.; Xiang, C.; Nasharuddin, N.A. Effects of different dietary supplements on athletic performance in soccer players: A systematic review and network meta-analysis. J. Int. Soc. Sports Nutr. 2025, 22, 2467890. [Google Scholar] [CrossRef]
- Deng, B.; Yan, R.; He, T.; Lin, G.; Liu, T.; Chen, W.; He, J.; Li, D. Effects of different dietary supplements combined with conditioning training on muscle strength, jump performance, sprint speed, and muscle mass in athletes: A systematic review and network meta-analysis. Front. Nutr. 2025, 12, 1636970. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health 2018, 10, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Tamilio, R.A.; Clarke, N.D.; Duncan, M.J.; Morris, R.O.; Tallis, J. How Repeatable Is the Ergogenic Effect of Caffeine? Limited Reproducibility of Acute Caffeine (3 mg.kg−1) Ingestion on Muscular Strength, Power, and Muscular Endurance. Nutrients 2022, 14, 4416. [Google Scholar] [CrossRef]
- Dodd, S.L.; Herb, R.A.; Powers, S.K. Caffeine and exercise performance. An update. Sports Med. 1993, 15, 14–23. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galan, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Abad-Colil, F.; Ramirez-Campillo, R.; Alvarez, C.; Castro, M.; Silva, S.; Izquierdo, M. Effects of beta-hydroxy-beta-methylbutyrate supplementation on physical performance of young players during an intensified soccer-training period: A short report. Human Mov. Spec. Issues 2017, 5, 91–96. [Google Scholar] [CrossRef]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Petroni, M.L.; Masala, S.; Melchiorri, G.; Pietrantuono, M.; Perriello, G.; Andreoli, A. Effect of acute and chronic branched-chain amino acids on energy metabolism and muscle performance. Diabetes Nutr. Metab. 2003, 16, 291–297. [Google Scholar] [PubMed]
- Kephart, W.C.; Wachs, T.D.; Thompson, R.M.; Brooks Mobley, C.; Fox, C.D.; McDonald, J.R.; Ferguson, B.S.; Young, K.C.; Nie, B.; Martin, J.S.; et al. Ten weeks of branched-chain amino acid supplementation improves select performance and immunological variables in trained cyclists. Amino Acids 2016, 48, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef]
- Donahue, P.T.; Wilson, S.J.; Williams, C.C.; Hill, C.M.; Garner, J.C. Comparison of Countermovement and Squat Jumps Performance in Recreationally Trained Males. Int. J. Exerc. Sci. 2021, 14, 462–472. [Google Scholar] [CrossRef]
- Nejić, K.; Stanković, M.; Rančić, D.; Jelaska, I.; Pezelj, L.; Katanić, B.; Badau, A.; Badau, D.; Masanovic, B. Associations Between Jump Performance, Speed, and COD Abilities in Young Elite Volleyball Players. Appl. Sci. 2025, 15, 9489. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M. Energy demands of volleyball. In Handbook of Sports Medicine and Science; Wiley: Hoboken, NJ, USA, 2017; pp. 1–14. [Google Scholar]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J. Appl. Physiol. (1985) 2012, 112, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Song, H.S.; Yoon, D.H.; Fukuda, D.H.; Kim, S.H.; Park, D.H. The effects of 10 weeks of beta-alanine supplementation on peak power, power drop, and lactate response in Korean national team boxers. J. Exerc. Rehabil. 2018, 14, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.; Valente-dos-Santos, J.; Pires, I.G.; Arrais, I.; Pereira, J.R.; Turner, A.N. Strength and Conditioning for Volleyball: A Review. Strength Cond. J. 2025, 47, 499–517. [Google Scholar] [CrossRef]
- Martinez, D.B. Consideration for Power and Capacity in Volleyball Vertical Jump Performance. Strength Cond. J. 2017, 39, 36–48. [Google Scholar] [CrossRef]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by beta-hydroxy-beta-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [CrossRef]
- Madeira, S.V.; Auger, C.; Anselm, E.; Chataigneau, M.; Chataigneau, T.; Soares de Moura, R.; Schini-Kerth, V.B. eNOS activation induced by a polyphenol-rich grape skin extract in porcine coronary arteries. J. Vasc. Res. 2009, 46, 406–416. [Google Scholar] [CrossRef]
- Glaister, M.; Rhodes, L. Short-Term Creatine Supplementation and Repeated Sprint Ability—A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Calleja-Gonzalez, J.; Marques-Jimenez, D.; Caballero-Garcia, A.; Cordova, A.; Fernandez-Lazaro, D. Effects of Creatine Supplementation on Athletic Performance in Soccer Players: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 757. [Google Scholar] [CrossRef]
- Grgic, J. Caffeine ingestion enhances Wingate performance: A meta-analysis. Eur. J. Sport Sci. 2018, 18, 219–225. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-Based Supplements for the Enhancement of Athletic Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef]
- Kaufman, M.W.; Roche, M.; Fredericson, M. The Impact of Supplements on Sports Performance for the Trained Athlete: A Critical Analysis. Curr. Sports Med. Rep. 2022, 21, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.K.; Erić, M.; Jovanović, G.B.; Dimitrić, G.; Čupić, M.B.; Ponorac, N. Explosive muscle power assessment in elite athletes using wingate anaerobic test. Rev. Bras. Med. Esporte 2018, 24, 107–111. [Google Scholar] [CrossRef]
- Berti Zanella, P.; Donner Alves, F.; Guerini de Souza, C. Effects of beta-alanine supplementation on performance and muscle fatigue in athletes and non-athletes of different sports: A systematic review. J. Sports Med. Phys. Fit. 2017, 57, 1132–1141. [Google Scholar] [CrossRef]
- Desbrow, B.; Burd, N.A.; Tarnopolsky, M.; Moore, D.R.; Elliott-Sale, K.J. Nutrition for Special Populations: Young, Female, and Masters Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 220–227. [Google Scholar] [CrossRef]
- Metzger, G.A.; Minneci, P.M.; Gehred, A.; Day, A.; Klingele, K.E. Creatine supplementation in the pediatric and adolescent athlete—A literature review. J. Orthop. 2023, 38, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pakulak, A.; Candow, D.G.; Totosy de Zepetnek, J.; Forbes, S.C.; Basta, D. Effects of Creatine and Caffeine Supplementation During Resistance Training on Body Composition, Strength, Endurance, Rating of Perceived Exertion and Fatigue in Trained Young Adults. J. Diet. Suppl. 2022, 19, 587–602. [Google Scholar] [CrossRef]
- Noma, H.; Tanaka, S.; Matsui, S.; Cipriani, A.; Furukawa, T.A. Quantifying indirect evidence in network meta-analysis. Stat. Med. 2017, 36, 917–927. [Google Scholar] [CrossRef]
- Culbertson, J.Y.; Kreider, R.B.; Greenwood, M.; Cooke, M. Effects of beta-alanine on muscle carnosine and exercise performance: A review of the current literature. Nutrients 2010, 2, 75–98. [Google Scholar] [CrossRef]
- Turcu, I.; Oancea, B.; Chicomban, M.; Simion, G.; Simon, S.; Negriu Tiuca, C.I.; Ordean, M.N.; Petrovici, A.G.; Nicolescu Șeușan, N.A.; Hăisan, P.L.; et al. Effect of 8-week β-alanine supplementation on CRP, IL-6, body composition, and bio-motor abilities in elite male basketball players. Int. J. Environ. Res. Public Health 2022, 19, 13700. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Waskiewicz, Z.; Poprzecki, S.; Cholewa, J. Effects of creatine and HMB supplementation on anaerobic power and body composition in basketball players. J. Hum. Kinet. 2003, 10, 95–108. [Google Scholar]
- Salleh, R.M.; Kuan, G.; Aziz, M.N.A.; Rahim, M.R.A.; Rahayu, T.; Sulaiman, S.; Kusuma, D.W.Y.; Adikari, A.M.G.C.P.; Razam, M.S.M.; Radhakrishnan, A.K.; et al. Effects of probiotics on anxiety, stress, mood and fitness of badminton players. Nutrients 2021, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Abian, P.; Del Coso, J.; Salinero, J.J.; Gallo-Salazar, C.; Areces, F.; Ruiz-Vicente, D.; Lara, B.; Soriano, L.; Muñoz, V.; Abian-Vicen, J. The ingestion of a caffeinated energy drink improves jump performance and activity patterns in elite badminton players. J. Sports Sci. 2015, 33, 1042–1050. [Google Scholar] [CrossRef]
- Rosas, F.; Ramírez-Campillo, R.; Martínez, C.; Caniuqueo, A.; Cañas-Jamet, R.; McCrudden, E.; Meylan, C.; Moran, J.; Nakamura, F.Y.; Pereira, L.A.; et al. Effects of plyometric training and beta-alanine supplementation on maximal-intensity exercise and endurance in female soccer players. J. Hum. Kinet. 2017, 58, 99–109. [Google Scholar] [CrossRef] [PubMed]
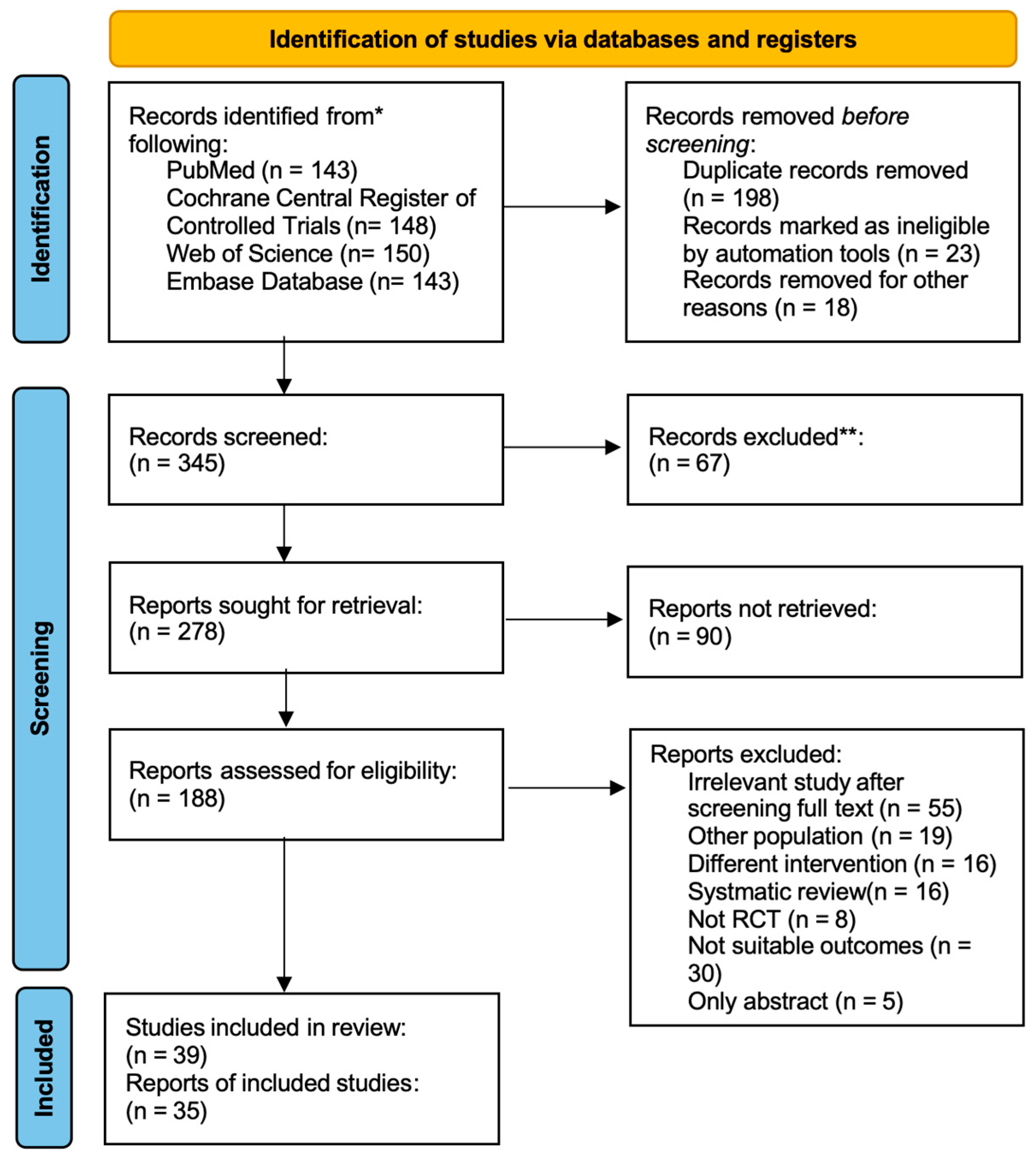
| Study | Study Design | Participants Level | Simple Size(N) | Age (Mean ± Sd), Years | Height (Mean ± Sd), Years | Weight (Mean ± Sd), Years | Sex | Study Period | Ingestion Time | Intervention | Comparator(s) | Performance Test | Side Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silvestre et al., 2019 [26]/Brazil | RCT | Elite (professional) | BA:6 PL:5 | 19 ± 0.9 20 ± 0.6 | 190 ± 10.0 180 ± 10.0 | 76.7 ± 6.1 79 ± 3.6 | Male | 8 weeks | 30 min before test | Beta-alanine supplement capsules 6.4 g/day | Placebo (maltodextrin supplement capsules) | VJ | small occurrences of paresthesia |
| Qanbar et al., 2024 [27]/Iran | RCT | Elite | BA:11 PL:11 | 24.45 ± 1.36 24.81 ± 1.32 | 1.85 ± 0.09 1.84 ± 0.07 | 66.97 ± 9.03 79.63 ± 9.77 | Male | 4 weeks | daily | Beta-alanine supplement capsules 6.4 g/day | Placebo | VJ, PP | NA |
| Guo and Wang., 2024 [28]/Korea | RCT | Non-elite (college) | BA:11 PL:11 | 24.6 ± 2.5 23.8 ± 2.7 | 182.6 ± 5.5 181.2 ± 6.7 | 81.5 ± 4.1 79.8 ± 6.9 | Male | 10 weeks | daily | Beta-alanine supplement capsules 4.8 g/day | Placebo (polydextrose capsules) | VJ, PP, MP | NA |
| Faiq et al., 2023 [29]/Iraq | RCT | Non-elite (club) | BCAA:8 PL:7 | 18.5 ± 0.29 | NA | NA | NA | 8 weeks | daily | BCAA supplementation with strength exercises | Placebo (control group using regular exercises) | VJ | NA |
| Vega-Sanchez et al., 2020 [30]/Spain | RCT | Elite (professional) | BCAA:6 PL:6 | 23.8 ± 2.2 25.3 ± 5.1 | 190.8 ± 13.0 185.7 ± 14.0 | 84.5 ± 15.1 84.9 ± 13.9 | Male | 1 week | before breakfast | BCAA supplement 7 g mixed in 500 mL of water | Placebo (500 mL of a watermelon-flavored beverage) | VJ | NA |
| Santi et al., 2020 [31]/Brazil | RCT | Elite (well-trained) | CRT:7 CHO:7 | 18 ± 0.3 19 ± 0.4 | 1.83 ± 0.04 1.80 ± 0.06 | 72.2 ± 10.7 81 ± 5.7 | NA | 11 days | daily | 0.3 g/kg/day of creatine associated with 1.2 g/kg/day of carbohydrate | Placebo (1.5 g/kg/day maltodextrin) | VJ, MP | NA |
| Lamontagne-Lacasse et al., 2011 [32]/Canada | RCT | Elite | 12 | 22 ± 1.5 | 84 ± 8 | 190 ± 7 | Male | 28 days | daily | 20 g of dextrose, 10 g of sucrose, 300 mL of water, and artificial flavor with 5 g of creatine | Placebo (20 g of dextrose, 10 g of sucrose, 300 mL of water, and artificial flavor) | VJ | NA |
| Kubota et al., 2003 [33]/Japan | RCT | Non-elite (collegiate) | CRT:11 PL:10 | 23.4 ± 4.7 22.8 ± 4.3 | 185.6 ± 5.7 184.4 ± 62 | 78.2 ± 2.7 78.5 ± 8.6 | Male | 6 days | 4 times a day | 5 g CRT and 5 g sports drink dissolved in 100 mL of water (20 g/day) | Placebo (5 g sports drink dissolved in 100 mL of water 20 g/day) | VJ, MP | Serum creatine, AST, and ALT were slightly elevated |
| Hemmatinafar et al., 2023 [34]/Iran | RCT | Non-elite (semi-professional) | 14 | 26.00 ± 3.00 | 174.08 ± 3.94 | 67.75 ± 5.14 | Female | 32 days | daily | 50 mL beetroot juice supplementation (total 400 mL over 2 days) | Placebo (matched for calories and appearance, but with negligible nitrate) | VJ | 2 digestive disorders |
| Toohey et al., 2020 [35]/USA | RCT | elite | 23 | 19.6 ± 1.0 | 170.6 ± 6.8 | 67.5 ± 7.4 | Female | 10 weeks | daily, post-workout | Probiotic Bacillus subtilis DE111 (5 billion CFU/day) | Placebo | VJ, PP | NA |
| Setaro et al., 2014 [36]/Brazil | RCT | Elite (professional) | MG:12 PL:13 | 17.42 ± 1.56 17.85 ± 0.99 | 191.4 ± 9.0 195.7 ± 8.9 | 83 ± 9.5 82.9 ± 7.8 | Male | 4 weeks | daily | Magnesium oxide capsules, 350 mg/day | Placebo (maltodextrin capsules, 500 mg/day) | VJ, PP | NA |
| Portal et al., 2011 [37]/Israel | RCT | Elite | HMB:14 PL:14 | 16.1 ± 1.3 16.2 ± 1.3 | 185.0 ± 9.6 181.9 ± 9.0 | 72.3 ± 10.3 69.9 ± 11.3 | Male and Female | 7 weeks | morning before training | HMB pills 3 g/day | Placebo pills 3 g/day | VJ, PP, MP | NA |
| Sánchez-Gómez et al., 2022 [38]/Spain | RCT | Elite (national) | 8 | 33.5 ± 8.95 | NA | HMB:80.3 ± 10.7 PL:78.8 ± 8.4 | Male and Female | 4 weeks | 60 min before test | HMB capsules 3 g/day | Placebo (sucrose capsules) | VJ, PP, MP | NA |
| Nemati et al., 2023 [39]/Iran + Japna | RCT | Non-elite (collegiate) | 15 | 20.80 ± 1.00 | 181.40 ± 4.30 | 70.22 ± 6.92 | Male | 3 weeks | 60 min before test | Caffeine capsules 3 mg/kg and 6 mg/kg | Placebo (starch capsule) | VJ | NA |
| Pfeifer et al., 2017 [40]/USA | RCT | Non-elite (collegiate) | 8 | 20 ± 1.15 | 174.8 ± 4.69 | 72.1 ± 9.94 | Female | 3 weeks | immediately before test | CHO (1.34 g/kg) and CAF (1.39 mg/kg) | Placebo (non-nutritive gel) | VJ, | blood glucose rise |
| Siquier-Coll et al., 2024 [41]/Spain | RCT | Non-elite (semi-professional) | 8 | 21 ± 2.31 | 1.63 ± 8 | 66.67 ± 4.74 | Female | 2 weeks | 60 min before test | Caffeine anhydrous powder mixed with maltodextrin-based beverage 5 mg/kg | Placebo (maltodextrin-based beverage) | VJ | NA |
| Zbinden-Foncea et al., 2018 [42]/Chile | RCT | Elite | 10 | 18.80 ± 2.00 | 1.93 ± 0.04 | 85.22 ± 10.11 | Male | 3 weeks | 60 min before test | Caffeine capsule 5 mg/kg | Placebo (dextrose capsule) | VJ, PP | NA |
| Fernández-Campos et al., 2015 [43]/USA + Costa Rica | RCT | Elite (professional) | 19 | 22.3 ± 4.9 | 171.8 ± 9.4 | 65.2 ± 10.1 | Female | 3 weeks | 30 min before test | Energy drink 6 mL/kg | Placebo (carbonated water) | VJ, PP, MP | NA |
| Del Coso et al., 2014 [44]/Spain | RCT | Non-elite (college) | 15 | 21.8 ± 6.9 | 180 ± 8 | 79.6 ± 11.0 | Male | 2 weeks | 60 min before test | Caffeine 3 mg/kg | Energy drink | VJ, MP | Insomnia |
| Filip-Stachnik et al., 2022 [45]/Spian + Poland | RCT | Elite (high-performance) | 12 | 20 ± 2 | 178 ± 6 | 69.1 ± 2.3 | Female | 1 week | 15 min before test | Caffeinated chewing gum (6.4 mg/kg) | Placebo (non-caffeinated gum) | VJ | NA |
| Filip-Stachnik et al., 2022 [46]/Poland | RCT | Non-elite (semi-professional) | 14 | 26 ± 3 | 171 ± 5 | 62.6 ± 5.6 | Female | 2 weeks | 60 min before test | Caffeinated capsule 6 mg/kg | Placebo (starch capsule) | VJ | NA |
| Kaszuba et al., 2022 [47]/Poland | RCT | Elite (high-performance) | 12 | 23 ± 3 | 188 ± 8 | 85.9 ± 11.2 | Male and Female | 2 weeks | 15 min before test | Caffeinated chewing gum (3.2 mg/kg) | Placebo (non-caffeinated gum) | VJ | NA |
| Pérez-López et al., 2015 [48]/Spain | RCT | Elite | 13 | 25.2 ± 4.8 | 174 ± 9 | 64.4 ± 7.6 | Female | 2 weeks | 60 min before test | Caffeine 3 mg/kg | Energy drink | VJ, PP | Nervousness, activeness |
| Wang et al., 2025 [49]/China | RCT | Non-elite (college) | CAF: 12 RHO: 12 CAF + RHO: 12 PL: 12 | 21 ± 1 20 ± 2 20 ± 1 20 ± 1 | 185 ± 5 183 ± 4 183 ± 4 183 ± 5 | 78 ± 5 79 ± 5 79 ± 5 78 ± 5 | Male | 4 weeks | 30 min before test | CAF: caffeine capsule 3 mg/kg RHO: RHO extract (2.4 g per day) | Placebo (capsules) | VJ | NA |
| Martins et al., 2020 [50]/Brazil | RCT | Elite (high-performance) | 12 | 16.5± 0.6 | 186.6 ± 8.4 | 77.5 ± 8.4 | Male | 2 weeks | daily | Grape juice (purple, Vitis labrusca Bordeaux, 400 mL/day) | Placebo beverage (maltodextrin, matched calories and carbohydrates, no polyphenols) | MP | NA |
| Telyari and Ebrahimi, 2022 [51]/Iran | RCT | Non-elite (semi-professional) | 12 | 21.5 ± 2.02 | 184.00 ± 3.25 | 79.33 ± 4.71 | Male | 1 week | 20 s before test | Caffeinated mouthwash (200 mg caffeine dissolved in 25 mL water) | Placebo (water with flour) | VJ, | NA |
| Lee et al., 2014 [52]/China | RCT | Non-elite (collegiate) | 11 | 21.3 ± 1.2 | 164.2 ± 5.7 | 58.6 ± 7.3 | Female | 1 week | CAF: 60 min before test; CHO: immediately before test | CAF: Caffeine capsules (6 mg/kg) CHO: Carbohydrate solution (0.8 g/kg dextrose) | Placebo (cellulose capsules); Placebo (low-calorie artificial sweetener drink) | PP, MP | anxiety, tremor, diarrhea, headache |
| Elbattawy et al., 2015 [53]/Egypt | RCT | Non-elite (club) | BCAA: 6; CRT: 6; PLA: 6 | 20.5 ± 1.5 | 187.31 ± 5.40 | 79.47 ± 9.21 | Male | 3 weeks | 60 min before test | BCAA group: 4 g, taken 3× per day (with 250 mL apple juice); Creatine group: Creatine 0.33 g/kg + Panax ginseng 1.5 g taken 3× per day (with 250 mL apple juice) | Placebo (250 mL apple juice, 3× per day) | VJ | NA |
| Burke et al., 2021 [54]/USA | RCT | Non-elite (college) | 11 | 19.7 ± 0.9 | 166.4 ± 10.2 | 67.7 ± 9.4 | Female | 1 week | 60 min before test | Caffeine anhydrous at 6 mg/kg | Placebo capsule | VJ, PP | NA |
| Hashem et al., 2024 [55]/Iraq | RCT | Non-elite (college) | 24 | 16.5 ± 0.29 | NA | NA | Male | 8 weeks | 30 min before test | Creatine 5 g | Placebo | VJ | NA |
| Zhu et al., 2025 [56]/China | RCT | Elite (high-performance) | CAF-3: 8; CAF-6: 8; CAF-3→6: 8; Placebo: 8 | 20.5 ± 1.1; 20.6 ± 1.3; 20.7 ± 1.2; 20.4 ± 1.2 | 184.4 ± 3.9 cm; 183.7 ± 4.6 cm; 185.2 ± 3.3 cm; 184.8 ± 4.1 cm | 84.2 ± 4.1 kg; 82.7 ± 5.6 kg; 83.3 ± 4.5 kg; 84.7 ± 3.9 kg | Male | 4 weeks | 45 min before test | Caffeine capsules (3 mg/kg; 6 mg/kg; 3–6 mg/kg) | Placebo capsules | VJ, PP, MP | NA |
| López-León et al., 2025 [57]/Spain + Brazil | RCT | Elite (high-performance) | 12 | 22.9 ± 3.6 | NA | NA | Female | 2 weeks | 2.5 h before testing | Beetroot juice supplementation 12.8 mmol NO3− per dose | Placebo beverage (nitrate-depleted beetroot juice, identical in flavor/appearance) | VJ | NA |
| Vinu., 2018 [58]/India | RCT | Non-elite (college) | PLA: 15; PRT: 15; PL: 15 | 20 ± 1.15 | 168.5 ± 3.8 | 67.5 ± 4.3 | Male | 12 weeks | daily | Plyometric jump training program combined with protein supplementation | Placebo (Plyometric training without any supplement) Placebo (No plyometric training, no supplement) | VJ | NA |
| Campbell et al., 2016 [59]/USA | RCT | Non-elite (college) | ED: 10; PLA: 9 | 22.4 ± 3.2 | 168.7 | 69.0 ± 12.7 | Male and Female | 1 week | 30 min before test | Energy drink (containing caffeine 2.4 mg/kg) | Placebo beverage (37 mL, non-caloric, similar taste and volume) | VJ | NA |
| Norozi et al., 2025 [60]/Iran | RCT | Non-elite (semi-professional) | LC: 10; PLA: 9 | 18.1 ± 2.8; 17.7 ± 2.9 | 167.4 ± 3.89; 166.3 ± 3.74 | 61.85 ± 14.21; 58.80 ± 10.93 | Female | 8 weeks | 60 min before training | 2 g/day L-citrulline malate (powder, dissolved in 200 mL water, Ktowa Hakko Bio Co., Japan) | Placebo (2 g/day cellulose) | VJ | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Liu, S.; Li, M.; Zhao, K.; Jiang, W.; You, T.; Wang, Z.; Zou, D.; Shu, J.; Liu, C. Effects of Nutritional Supplements on Explosive Lower Limb Performance in Volleyball Players: A Systematic Review and Network Meta-Analysis. Nutrients 2025, 17, 3702. https://doi.org/10.3390/nu17233702
Du H, Liu S, Li M, Zhao K, Jiang W, You T, Wang Z, Zou D, Shu J, Liu C. Effects of Nutritional Supplements on Explosive Lower Limb Performance in Volleyball Players: A Systematic Review and Network Meta-Analysis. Nutrients. 2025; 17(23):3702. https://doi.org/10.3390/nu17233702
Chicago/Turabian StyleDu, Haoyu, Shuning Liu, Mu Li, Kai Zhao, Wei Jiang, Ting You, Zheng Wang, Dixin Zou, Jingdan Shu, and Chang Liu. 2025. "Effects of Nutritional Supplements on Explosive Lower Limb Performance in Volleyball Players: A Systematic Review and Network Meta-Analysis" Nutrients 17, no. 23: 3702. https://doi.org/10.3390/nu17233702
APA StyleDu, H., Liu, S., Li, M., Zhao, K., Jiang, W., You, T., Wang, Z., Zou, D., Shu, J., & Liu, C. (2025). Effects of Nutritional Supplements on Explosive Lower Limb Performance in Volleyball Players: A Systematic Review and Network Meta-Analysis. Nutrients, 17(23), 3702. https://doi.org/10.3390/nu17233702






