Nutritional Modulation of the Gut Microbiome in Relation to Prenatal Lead-Induced Neurotoxicity: A Review
Abstract
1. Introduction
2. Literature Identification
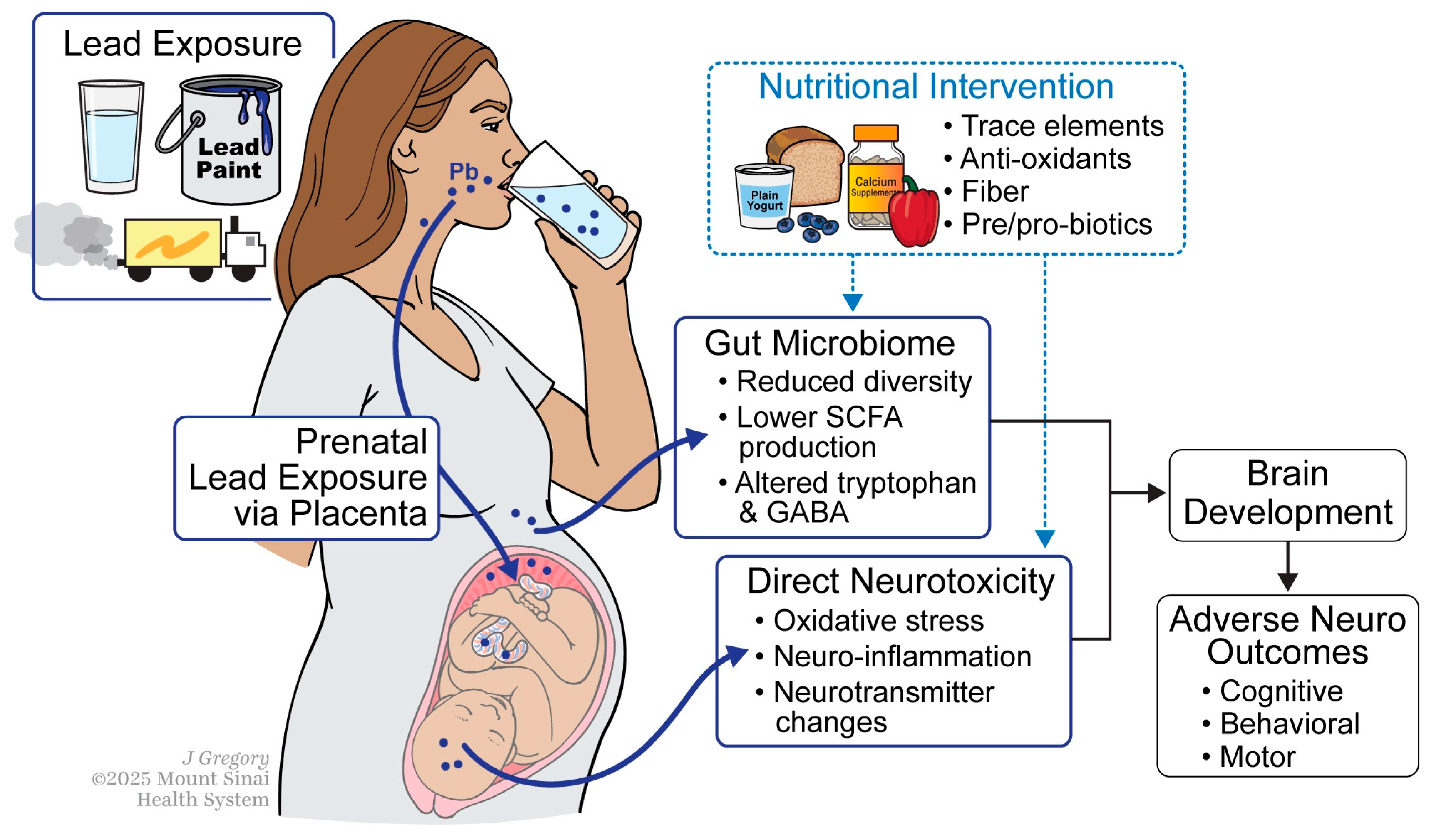
3. Mechanisms of Lead Exposure and Prenatal Neurodevelopment
4. The Gut Microbiome as a Mediator
| Citation | Population or Model | Exposure or Condition | Microbiome Findings | Neurodevelopmental or Health Implications |
|---|---|---|---|---|
| Liu et al., 2020 [22] | Carp model | Pb exposure in water | ↓ microbial diversity; ↓ Lactobacillus; ↑ Clostridium, Oscillibacter | Indicates dysbiosis and inflammatory/metabolic shifts linked to Pb toxicity |
| Wu et al., 2016 [23] | Mouse model (early-life exposure) | Prenatal Pb exposure in drinking water | Long-term microbiome alterations persisting into adulthood | Suggests developmental windows of vulnerability |
| Eggers et al., 2019 [25] | Human adults | Urinary Pb concentration (low–moderate exposure) | ↑ α- and β-diversity; ↑ Proteobacteria colonization | Proteobacteria linked to gut inflammation and dysbiosis [24] |
| Bisanz et al., 2014 [26] | Children with mixed metal exposure | Blood Pb and other metals | ↑ Gammaproteobacteria and other taxa correlated with blood Pb | Proteobacteria linked to gut inflammation and dysbiosis [24] |
| Gao et al., 2024 [27] | Human infants | Stool Pb concentrations | Altered 114 microbial metabolic pathways | Functional disruption of microbial metabolism by Pb |
| Sitarik et al., 2020 [28] | Human infants | Prenatal Pb measured in teeth | Altered bacterial and fungal communities in infants (1–6 months) | Suggests prenatal Pb disrupts early microbial colonization |
| Eggers et al., 2023 [29] | Mother–child pairs | Maternal blood Pb during pregnancy | ↓ diversity; ↓ Ruminococcus gnavus, Bifidobacterium longum at age 9–11 | Indicates lasting effects of prenatal Pb on gut composition |
| Vuong et al., 2020 [33] | Mouse model | Maternal microbiome during pregnancy | Maternal microbiome metabolites promote fetal brain development via metabolite signaling | Demonstrates maternal microbiome’s role in fetal neurodevelopment |
| Sun et al., 2023 [34] | Mother–child pairs | Prenatal maternal gut microbiome | Prenatal maternal microbiome more relevant than infant microbiome in first year | Highlights prenatal microbiome importance in neurodevelopment |
| Naspolini et al., 2025 [35] | Human children | Early-life gut microbiome (meconium and infancy) | Associations between early microbiome composition and behavioral disorders at 6 months | Links early microbial colonization to behavioral outcomes |
| Zuffa et al., 2023 [36] | Human Infants with elevated ASD risk | Early-life gut microbiome | Lower abundance of GABA-producing bacteria prior to behavioral symptoms | Suggests microbial role in ASD-related neurodevelopment |
| Moore & Townsend, 2019 [37] | Human infants | Normal microbiome development | Infant gut microbiome diversity less beneficial than adult diversity | Provides baseline context on infant microbiome |
| Carlson et al., 2018 [38] | Human infants | Gut microbiome diversity and composition | Negative association of diversity with cognitive scores at 1 year | Indicates early microbial diversity may relate to cognition |
| Midya et al., 2024 [39] | Mother–child pairs | Prenatal metals (Zn, Co) and childhood gut microbiome | Metal–microbe clique linked to higher depression scores | Shows prenatal metals and microbes jointly affect mood |
| Midya et al., 2024 [40] | Mother–child pairs | Prenatal metals and childhood A. muciniphila colonization | A. muciniphila attenuates association between metals and depressive symptoms | Suggests microbiome modulation can mitigate neurotoxicity |
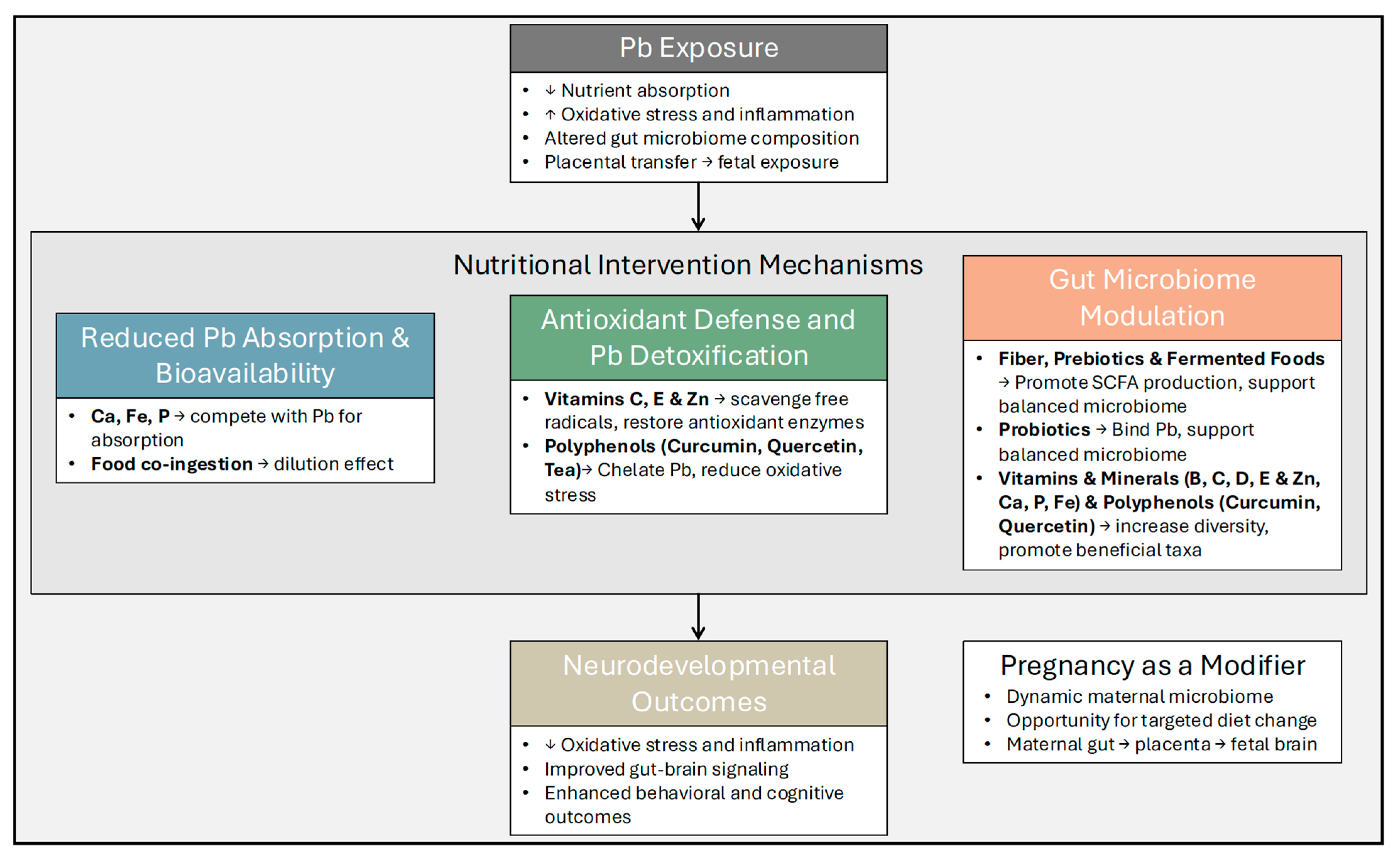
5. Nutritional Intervention Strategies
6. Research Gaps and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goyer, R.A. Transplacental Transport of Lead. Environ. Health Perspect. 1990, 89, 101–105. [Google Scholar] [CrossRef]
- Esteban-Vasallo, M.D.; Aragonés, N.; Pollan, M.; López-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Parithathvi, A.; Choudhari, N.; Dsouza, H.S. Prenatal and early life lead exposure induced neurotoxicity. Hum. Exp. Toxicol. 2024, 43, 09603271241285523. [Google Scholar] [CrossRef] [PubMed]
- Santa Maria, M.P.; Hill, B.D.; Kline, J. Lead (Pb) neurotoxicology and cognition. Appl. Neuropsychol. Child 2019, 8, 272–293. [Google Scholar] [CrossRef]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 2003, 126, 5–19. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency OoR. Development NCfEA. Integrated Science Assessment for Lead; US Environmental Protection Agency: Washington, DC, USA, 2013. [Google Scholar]
- OSHA. Lead. Occupational Safety and Health Administration (Safety and Health Topics). Available online: https://www.osha.gov/lead (accessed on 3 March 2025).
- Rodier, P.M. Developing Brain as a Target of Toxicity. Environ. Health Perspect. 1995, 103, 73–76. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Hu, H.; Hernandez-Avila, M. Dietary calcium supplementation to lower blood lead levels in pregnancy and lactation. J. Nutr. Biochem. 2007, 18, 172–178. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Kolb, B.; Mychasiuk, R.; Muhammad, A.; Li, Y.; Frost, D.O.; Gibb, R. Experience and the developing prefrontal cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 17186–17193. [Google Scholar] [CrossRef]
- Schnaas, L.; Rothenberg, S.J.; Flores, M.-F.; Martinez, S.; Hernandez, C.; Osorio, E.; Velasco, S.R.; Perroni, E. Reduced Intellectual Development in Children with Prenatal Lead Exposure. Environ. Health Perspest. 2006, 114, 791–797. [Google Scholar] [CrossRef]
- Wasserman, G.; Liu, X.; Popovac, D.; Factor-Litvak, P.; Kline, J.; Waternaux, C.; LoIacono, N.; Graziano, J. The Yugoslavia Prospective Lead Study: Contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol. Teratol. 2000, 22, 811–818. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef]
- Fruh, V.; Rifas-Shiman, S.L.; Amarasiriwardena, C.; Cardenas, A.; Bellinger, D.C.; Wise, L.A.; White, R.F.; Wright, R.O.; Oken, E.; Henn, B.C. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology 2019, 75, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.R.; Millán, M.P.; Canals, J.; Moreno, V.R.; Renzetti, S.; Arija, V. Effects of prenatal exposure to multiple heavy metals on infant neurodevelopment: A multi-statistical approach. Environ. Pollut. 2025, 367, 125647. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Warner, B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, H.; Zheng, S.; Xu, S.; Massey, I.Y.; Zhang, C.; Wang, X.; Yang, F. Pb Toxicity on Gut Physiology and Microbiota. Front. Physiol. 2021, 12, 574913. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xie, B.; Yin, N.; Zhu, H.; Gao, H.; Xu, X.; Xiao, K.; Cai, X.; Sun, G.; Sun, X.; et al. Prenatal exposure to trace elements impacts mother-infant gut microbiome, metabolome and resistome during the first year of life. Nat. Commun. 2025, 16, 5186. [Google Scholar] [CrossRef]
- Liu, H.; Fu, S.; Zhang, S.; Ding, M.; Wang, A. Lead induces structural damage, microbiota dysbiosis and cell apoptosis in the intestine of juvenile bighead carp (Hypophthalmichthys nobilis). Aquaculture 2020, 528, 735573. [Google Scholar] [CrossRef]
- Wu, J.; Wen, X.W.; Faulk, C.; Boehnke, K.; Zhang, H.; Dolinoy, D.C.; Xi, C. Perinatal Lead Exposure Alters Gut Microbiota Composition and Results in Sex-specific Bodyweight Increases in Adult Mice. Toxicol. Sci. 2016, 151, 324–333. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Eggers, S.; Safdar, N.; Sethi, A.K.; Suen, G.; Peppard, P.E.; Kates, A.E.; Skarlupka, J.H.; Kanarek, M.; Malecki, K.M. Urinary lead concentration and composition of the adult gut microbiota in a cross-sectional population-based sample. Environ. Int. 2019, 133 Pt A, 105122. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; Mwanga, J.R.; Changalucha, J.P.; Burton, J.; Gloor, G.B.; Reid, G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014, 5, e01580-14. [Google Scholar] [CrossRef]
- Gao, F.; Shen, Y.; Wu, H.; Laue, H.E.; Lau, F.K.; Gillet, V.; Lai, Y.; Shrubsole, M.J.; Prada, D.; Zhang, W.; et al. Associations of Stool Metal Exposures with Childhood Gut Microbiome Multiomics Profiles in a Prospective Birth Cohort Study. Environ. Sci. Technol. 2024, 58, 22053–22063. [Google Scholar] [CrossRef]
- Sitarik, A.R.; Arora, M.; Austin, C.; Bielak, L.F.; Eggers, S.; Johnson, C.C.; Lynch, S.V.; Park, S.K.; Wu, K.-H.H.; Yong, G.J.; et al. Fetal and early postnatal lead exposure measured in teeth associates with infant gut microbiota. Environ. Int. 2020, 144, 106062. [Google Scholar] [CrossRef]
- Eggers, S.; Midya, V.; Bixby, M.; Gennings, C.; Torres-Olascoaga, L.A.; Walker, R.W.; Wright, R.O.; Arora, M.; Téllez-Rojo, M.M. Prenatal lead exposure is negatively associated with the gut microbiome in childhood. Front. Microbiol. 2023, 14, 1193919. [Google Scholar] [CrossRef]
- Maier, K.J.; al’Absi, M. Toward a Biopsychosocial Ecology of the Human Microbiome, Brain-Gut Axis, and Health. Psychosom. Med. 2017, 79, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; E Collins, C.; Holmes, A.; Lal, R.; Duncanson, K.; Taylor, R.; Gordon, A. Maternal exposures and the infant gut microbiome: A systematic review with meta-analysis. Gut Microbes 2021, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Shen, Y.; Li, Y.; Luo, H.; Yuan, M.; Gan, J. Prenatal exposure to antibiotics and risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 1045865. [Google Scholar] [CrossRef]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Sun, Z.; Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.A.; Litonjua, A.A.; Weiss, S.T.; Liu, Y.-Y. Revealing the importance of prenatal gut microbiome in offspring neurodevelopment in humans. eBioMedicine 2023, 90, 104491. [Google Scholar] [CrossRef] [PubMed]
- Naspolini, N.F.; Natividade, A.P.; Asmus, C.I.F.; Moreira, J.C.; Dominguez-Bello, M.G.; Meyer, A. Early-life gut microbiome is associated with behavioral disorders in the Rio birth cohort. Sci. Rep. 2025, 15, 8674. [Google Scholar] [CrossRef]
- Zuffa, S.; Schimmel, P.; Gonzalez-Santana, A.; Belzer, C.; Knol, J.; Bölte, S.; Falck-Ytter, T.; Forssberg, H.; Swann, J.; Heijtz, R.D. Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder. Transl. Psychiat. 2023, 13, 257. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant Gut Microbiome Associated with Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef]
- Midya, V.; Nagdeo, K.; Lane, J.M.; Torres-Olascoaga, L.A.; Torres-Calapiz, M.; Gennings, C.; Horton, M.K.; Téllez-Rojo, M.M.; Wright, R.O.; Arora, M.; et al. Prenatal metal exposures and childhood gut microbial signatures are associated with depression score in late childhood. Sci. Total Environ. 2024, 916, 170361. [Google Scholar] [CrossRef]
- Midya, V.; Nagdeo, K.; Lane, J.M.; Torres-Olascoaga, L.A.; Gil Martínez, G.; Horton, M.K.; McRae, N.; Lopez, I.; Landero, J.; Gennings, C.; et al. Akkermansia muciniphila attenuates association between specific metal exposures during pregnancy and depressive symptoms in late childhood. iScience 2024, 27, 111335. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 2015, 7, 552–571. [Google Scholar] [CrossRef]
- Heo, S.J.; Moon, N.; Kim, J.H. Dietary interventions to reduce heavy metal exposure in antepartum and postpartum women: A systematic review. Womens Health Nurs. 2024, 30, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R. Environmental Lead Toxicity—Nutrition as a Component of Intervention. Environ. Health Perspest. 1990, 89, 75–78. [Google Scholar] [CrossRef]
- Rabinowitz, M.B.; Kopple, J.D.; Wetherill, G.W. Effect of food Intake and fasting on gastrointestinal lead absorption in humans. Am. J. Clin. Nutr. 1980, 33, 1784–1788. [Google Scholar] [CrossRef]
- Prasanthi, R.P.J.; Devi, C.B.; Basha, D.C.; Reddy, N.S.; Reddy, G.R. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int. J. Dev. Neurosci. 2010, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, K.; Muhammad Mailafiya, M.; Danmaigoro, A.; Musa Chiroma, S.; Abdul Rahim, E.B.; Bakar, A.; Zakaria, M.Z. Curcumin Attenuates Lead-Induced Cerebellar Toxicity in Rats via Chelating Activity and Inhibition of Oxidative Stress. Biomolecules 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Shao, X.; Wei, Y.; Zhang, X.; Wang, H.; Xu, F. Dietary fiber ameliorates lead-induced gut microbiota disturbance and alleviates neuroinflammation. J. Sci. Food Agric. 2022, 102, 6795–6803. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Moselhy, W.A.; Abdel-Hamed, M.I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. NeuroToxicology 2012, 33, 280–289. [Google Scholar] [CrossRef]
- Deckmann, I.; Santos-Terra, J.; Martel, F.; Vieira Carletti, J. Common pregnancy complications and polyphenols intake: An overview. Crit Rev. Food Sci. Nutr. 2024, 64, 5924–5957. [Google Scholar] [CrossRef] [PubMed]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid.-Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The Modulatory Effects of Curcumin on the Gut Microbiota: A Potential Strategy for Disease Treatment and Health Promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshi, C.; Kobori, M.; Takahashi, S.; Tomita, J.; Nishimura, M.; Nishihira, J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE 2017, 12, e0188271. [Google Scholar] [CrossRef]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut microbiome–micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Barrientos, G.; Ronchi, F.; Conrad, M.L. Nutrition during pregnancy: Influence on the gut microbiome and fetal development. Am. J. Reprod. Immunol. 2024, 91, e13802. [Google Scholar] [CrossRef]
- Li, P.; Tang, T.; Chang, X.; Fan, X.; Chen, X.; Wang, R.; Fan, C.; Qi, K. Abnormality in Maternal Dietary Calcium Intake During Pregnancy and Lactation Promotes Body Weight Gain by Affecting the Gut Microbiota in Mouse Offspring. Mol. Nutr. Food Res. 2019, 63, e1800399. [Google Scholar] [CrossRef]
- Maher, S.E.; O’Brien, E.C.; Moore, R.L.; Byrne, D.F.; Geraghty, A.A.; Saldova, R.; Murphy, E.F.; Van Sinderen, D.; Cotter, P.D.; McAuliffe, F.M. The association between the maternal diet and the maternal and infant gut microbiome: A systematic review. Brit. J. Nutr. 2023, 129, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, E.; Smithells, R.W. Folic acid metabolism and human embryopathy. Lancet 1965, 285, 1254. [Google Scholar] [CrossRef]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef]
- Zhong, H.; Xiao, R.; Ruan, R.; Liu, H.; Li, X.; Cai, Y.; Zhao, J.; Fan, X. Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism-related behaviors in a mouse model of autism. Psychopharmacology 2020, 237, 3539–3552. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.d.S.d.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Teixeira, F.C.; de Souza, A.A.; Stefanello, F.M.; Baldissarelli, J.; Gamaro, G.D.; Spanevello, R.M. Quercetin prevents alterations of behavioral parameters, delta-aminolevulinic dehydratase activity, and oxidative damage in brain of rats in a prenatal model of autism. Int. J. Dev. Neurosci. 2020, 80, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-M.; Letchumanan, V.; Tan, L.T.-H.; Hong, K.-W.; Wong, S.-H.; Ab Mutalib, N.-S.; Lee, L.-H.; Law, J.W.-F. Ketogenic diet: A dietary intervention via gut microbiome modulation for the treatment of neurological and nutritional disorders (a narrative review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Karhu, E.; Zukerman, R.; Eshraghi, R.S.; Mittal, J.; Deth, R.C.; Castejon, A.M.; Trivedi, M.; Mittal, R.; Eshraghi, A. Nutritional interventions for autism spectrum disorder. Nutr. Rev. 2020, 78, 515–531. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Zauche, L.H.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggers, S.; Nagdeo, K.P.; Sachdev, K.; Robinson, D.; Deierlein, A.L.; Lane, J.M.; Gennings, C.; Walker, R.W.; Snetselaar, L.; Nidey, N.; et al. Nutritional Modulation of the Gut Microbiome in Relation to Prenatal Lead-Induced Neurotoxicity: A Review. Nutrients 2025, 17, 3700. https://doi.org/10.3390/nu17233700
Eggers S, Nagdeo KP, Sachdev K, Robinson D, Deierlein AL, Lane JM, Gennings C, Walker RW, Snetselaar L, Nidey N, et al. Nutritional Modulation of the Gut Microbiome in Relation to Prenatal Lead-Induced Neurotoxicity: A Review. Nutrients. 2025; 17(23):3700. https://doi.org/10.3390/nu17233700
Chicago/Turabian StyleEggers, Shoshannah, Kiran P. Nagdeo, Kshitij Sachdev, Delaney Robinson, Andrea L. Deierlein, Jamil M. Lane, Chris Gennings, Ryan W. Walker, Linda Snetselaar, Nichole Nidey, and et al. 2025. "Nutritional Modulation of the Gut Microbiome in Relation to Prenatal Lead-Induced Neurotoxicity: A Review" Nutrients 17, no. 23: 3700. https://doi.org/10.3390/nu17233700
APA StyleEggers, S., Nagdeo, K. P., Sachdev, K., Robinson, D., Deierlein, A. L., Lane, J. M., Gennings, C., Walker, R. W., Snetselaar, L., Nidey, N., O’Neal, E. E., & Midya, V. (2025). Nutritional Modulation of the Gut Microbiome in Relation to Prenatal Lead-Induced Neurotoxicity: A Review. Nutrients, 17(23), 3700. https://doi.org/10.3390/nu17233700








